Mathematical Modeling of Brucellosis Transmission Dynamics and Its Age-Specific Applications
Abstract
Brucellosis remains a significant public health and economic burden, especially in developing nations where livestock–human interactions are frequent. This paper presents a partial differential equation (PDE) model to capture the transmission dynamics of brucellosis, with a focus on age-specific impacts in humans and domestic animals. Using the integrated semigroup approach, the positivity and boundedness of the model were established ensuring biologically meaningful solutions. The basic reproduction number (R0) was derived through the Lotka–Sharpe–McKendrick integral equation, and the disease-free equilibrium (DFE) was proven to be locally asymptotically stable when R0 < 1. Global sensitivity analysis was performed using the PAWN method to identify key parameters influencing disease dynamics. Results revealed that humans aged 0–72 years are carriers of the disease, while domestic animals, particularly those aged 0–5 and 11–15 years, act as significant reservoirs. Furthermore, environmental contamination was found to be a critical driver of transmission, emphasizing the urgent need for improved sanitation and hygiene measures. This study underscores the importance of targeting specific age groups and addressing environmental factors to enhance brucellosis control strategies, providing valuable insights for policymakers and public health practitioners.
1. Introduction
Brucellosis, also known as Malta fever, is a contagious disease of livestock that poses significant economic burdens globally [1]. It affects various animals, including cattle, swine, sheep, goats, camels, equines, and dogs, and can even extend to humans and marine mammals. The primary causative agents of brucellosis are different species of the Brucella family, B. abortus in cattle, B. melitensis in sheep and goats, B. suis in swine, and B. canis in dogs, as identified by the World Organization for Animal Health (WOAH) [2]. Transmission occurs through direct or indirect contact with infected animals, ingestion of contaminated dairy products, or inhalation of aerosols from infected animal secretions, making it a zoonotic concern for both animals and humans [3].
To mitigate the transmission of brucellosis, several interventions have been implemented, including vaccination, treatment, culling of seropositive animals, promoting proper environmental hygiene and sanitation, and personal protection [4–8]. However, these measures have proven challenging in some regions. For instance, a brucellosis control program in the Middle East showed limited implementation, with only 7% success in applying quarantine protocols [9]. While treatments have been introduced with the hope of eliminating Brucella from small ruminants and cattle, the bacteria often persist in tissues, such as lymph nodes, despite seemingly successful interventions. Additionally, the lack of extensive evaluation of published treatment regimens further complicates disease control, leading experts to generally discourage treatment [10].
Brucellosis remains a major global health concern, with an estimated 2.1 million human cases annually. However, its actual burden is often underestimated due to underreporting and diagnostic limitations [11]. While precise global prevalence and mortality rates are challenging to determine, the disease leads to significant morbidity, with a case fatality rate of ~1% [12]. In Tanzania’s Kilimanjaro Region, the reported annual incidence rates were 35 cases per 100,000 people in 2007–2008, 33 cases per 100,000 in 2012–2014, and 52 cases per 100,000 in 2020–2023, indicating its persistent occurrence. It remains endemic across Africa, with varying incidence rates, yet it is frequently underdiagnosed due to limited awareness and inadequate diagnostic infrastructure [13]. These patterns underscore the urgent need for enhanced surveillance systems, improved diagnostic tools, and strengthened public health interventions to effectively control brucellosis in endemic areas [14].
In efforts to understand and control the transmission of brucellosis, various mathematical models have been developed [6, 10, 15, 16]. These include models focusing on sheep brucellosis transmission [17], animal–human transmission dynamics in Mongolia [18], optimal control strategies [7], and time series analyses of human brucellosis [19]. Researchers have explored transmission mechanisms such as direct and indirect contact [20], vertical transmission [21], and seasonal influences [22, 23]. However, a significant limitation of many existing models lies in their assumption of homogeneous mixing populations, which restricts their applicability. In reality, individual contact probabilities and transmission risks are inherently heterogeneous. The spread of brucellosis is closely tied to host individuals’ heterogeneity, as factors like age in children, adults, or the elderly strongly influence exposure risks, clinical outcomes, and transmission pathways [14]. Integrating age-specific components into models maximize the likelihood of accurately understanding transmission dynamics and designing targeted interventions.
This paper presents a mathematical model of brucellosis transmission dynamics with a focus on age-specific factors. It examines the role of age in both the transmission and progression of brucellosis, offering insights distinct from previous models [3, 5–7, 9, 15], which did not incorporate age as a component. Unlike prior studies [2, 16, 18, 20, 23], this work employs partial differential equations (PDEs) as the mathematical framework, diverging from the more commonly used ordinary differential equations (ODEs). Although some studies have integrated age structures into their models [24, 25], the influence of age-specific variations on brucellosis transmission remains insufficiently explored in the literature. This study addresses this critical gap by developing a PDE-based age-structured model that more comprehensively captures the distinct roles of age in brucellosis transmission. Understanding age-related variations is essential, as susceptibility, transmission dynamics, and disease progression differ across age groups. For instance, children, adults, and the elderly exhibit varying exposure risks and clinical outcomes, with younger individuals more likely to experience acute symptoms and older individuals at greater risk of chronic complications. Similarly, domestic animals, as the primary reservoirs of brucellosis, display age-dependent differences young animals tend to be more susceptible to infection, while older ones shed pathogens more efficiently. By identifying these age-specific dynamics, this model provides a more refined understanding of brucellosis transmission and informs targeted public health interventions. Age-specific vaccination strategies, education campaigns, and testing programs can be optimized to enhance disease control efforts and improve resource allocation.
2. Model Formulation
The model consist of domestic animals, human, and reservoir of pathogens. At any given time t, human population are subdivided into susceptible individuals Sh(a, t), acute individuals Ah(a, t), and chronic individuals Ch(a, t) in a specific age group a ≥ 0. The total population is given by Nh(a, t) = Sh(a, t) + Ah(a, t) + Ch(a, t). The susceptible human is recruited into a population at a rate of πh(a) > 0 by birth. They acquire the disease from infected animals and contaminated environment and transitioning into the acute class with a force of infection λh. In the acute stage, symptoms manifest, and treatment with antibiotics is possible and those who fail progress to chronic class at a rate of b(a). The probability of a human becoming infected is age-dependent and represented by αh(a). Natural death occurs for all humans at a rate μh(a), while chronic individuals may also experience disease-induced death at the rate φh(a) due to complications of blood donating or bone marrow plantation. Notably, the model neglect recovery class with assumption that human who recover from the infection do not develop long-term immunity or the immunity is temporary, meaning they can become susceptible again soon after recovery.
For domestic animals, the model is subdivided into susceptible Sd(a, t) and infectious class Id(a, t) at any given time t in specific age groups a. The total animal populations is Nd(a, t) = Sd(a, t) + Id(a, t). The susceptible domestic animals are introduced into the population through birth and migration at a rate πd(a) > 0. Susceptible domestic animals acquire the disease from infected animal through direct contact and contaminated environment and move to the infectious class with the force of infection λd. It is assumed that the probability of domestic animal to infect is αd(a). All domestic animals experience natural death at a rate μd(a), and infected animals can also experience disease induced death at the rate φd(a) due to severe reproductive failures and other health issues. The model assume the domestic animal recovery rate is sufficiently small compared to the infection rate; the number of recovered animals remains negligible.
Infected domestic animals release pathogens into the environment at a rate ρ(a) contributing to the environmental reservoir of pathogens E(t). Pathogens decay at a rate ε(a), but Brucella species are known to survive in both environment and in animals for extended periods, allowing for long-term persistence of the bacteria [26]. It assumed that their immunity grow age-wise. The flow diagram of the model is shown in Figure 1, and the parameters used are in Table 1.
| Parameter | Description | Values (1) | Values (2) | Source |
|---|---|---|---|---|
| πh(a) | Human birth rate | 0.001–0.005 | 0.005 | [15] |
| αh(a) | Human probability of getting infection | 0.5–0.9 | 0.9 | [27] |
| b(a) | Human transfer rate from acute class to chronic class | 0.5–0.8 | 0.5 | [12] |
| φh(a) | Human-induced death rate | 0.5–0.9 | 0.3 | [28] |
| μh(a) | Human death rate | 0.1–0.9 | 0.4 | [29] |
| πd(a) | Domestic animal recruitment rate | 0.0025–0.01 | 0.01 | [3] |
| αd(a) | Domestic animal probability of getting infection | 0.6–1 | 1 | [30] |
| φd(a) | Domestic animal-induced death rate | 0.5–0.6 | 0.4 | [18] |
| ρ(a) | Rate of concentration of bacteria | 0.7–1 | 1 | [31] |
| ɛ(a) | Rate of bacteria decay | 0.3–0.6 | 0.5 | [32] |
| μd(a) | Domestic animal mortality rate | 0.1–0.9 | 0.4 | [33] |
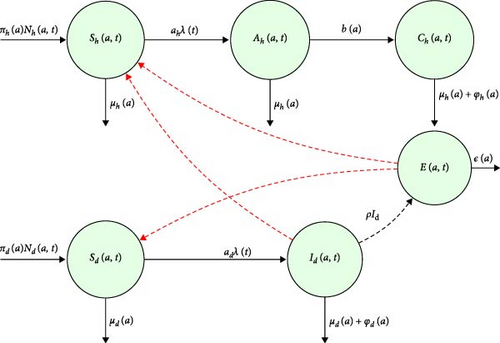
2.1. Model Equations
3. Model Analysis
3.1. Normalization of the System
Consider the following total population for humans and domestic animals.
3.2. Existence of Positive Solution and Boundedness
The model system shown in Equation (2) shows positive solutions, and its boundedness is shown using the integrated semigroup approach [34]. Reformulate the system shown in Equation (2) into a more concise representation, commonly referred to as an abstract Cauchy problem. Consider whichdenote the set of all real-valued functions defined on an open subset such that . Also, assume X be a Banach space, defined by equipped with the norm for .
Assume Ω = {(sh, ah, ch, sd, id, e) ∈ X+ | 0 ≤ sh, ah, ch, sd, id, e ≤ 1}, where . Consider B be a linear operator defined by .
According to Lotka and Sharpe and Lotka [35–37], the Cauchy problem shown in Equation (2) was formulated, and it has a solution; therefore, Equation (3) is well-posed.
3.3. Disease-Free Equilibrium (DFE)
3.4. Basic Reproduction Number and Local Stability of DFE
3.4.1. Basic Reproduction Number
3.4.2. Local Stability of the DFE Point
Theorem 1. The DFE is locally asymptotically stable if R0h < 1 and unstable if R0h > 1.
Proof. , , and . Equation (12) has a unique negative real solution ω∗ if and only if Mh(0) < 1; hence, R0h < 1. Also, Equation (12) has a unique positive (zero) real solution if R0h > 1. To depict that ω∗ is the dominant real part of the roots of Mh(ω), we let ω = r + iq be an arbitrary complex solution to Equation (12); thus, 1 = Mh(ω) = |Mh(r + iq)| ≤ Mh(r), indicating that R0h(ω) ≤ ω∗. Then, it follows that the DFE is locally asymptotically stable if R0h < 1 and unstable if R0h > 1.
Theorem 2. The DFE is locally asymptotically stable if R0d < 1 and unstable if R0d > 1.
Proof. , , and . Equation (13) has a unique negative real solution ω∗ if and only if Md(0) < 1, and then R0d < 1. In addition, Equation (13) has a unique positive (zero) real solution if R0d > 1. To depict that ω∗ is the dominant real part of the root of Md(ω), let ω = x + iy be an arbitrary complex solution to Equation (13); thus, 1 = Md(ω) = |Md(x + iy)| ≤ Md(x), indicating that R0d(ω) ≤ ω∗. Then, it follows that the DFE is locally asymptotically stable if Rod < 1 and unstable if Rod > 1.
3.4.3. Global Stability of the DFE Point
Assume ∃ a positive constant K for convenience:
Theorem 3. The DFE is globally asymptotically stable if K · Roh < 1 and K · Rod < 1 and otherwise.
Proof. To show that
Integrating the system as in Equation (2) along the characteristics line (t − a > 0) gives the following:
Substituting Equation (19) into Equation (20) gives the following:
Substituting Equation (22) into Equation (23) gives the following:
Putting Equations (24), (25), and (22) into the last equation of the system as shown in Equation (2) gives Equations (26) and (27). λ(t) is for human and domestic animal, respectively:
Taking the limit supremum of λ(t) as t → ∞, it can be easily shown that
It results in and . Thus, and , since we have and , which completes the proof.
3.5. Endemic Equilibrium
Assume the right-hand side of Equations (37) and (38) is denoted by R(η), respectively. If R(η) > 1, there exists an endemic equilibrium.
3.5.1. Local Stability of Endemic Equilibrium
The initial conditions are .
Assume , , , , , and .
It follows that P (σ) is a decreasing function such that if the perturbations introduced at the endemic steady state are small. Setting σ = 0 in Equations (50) and (51), we notice that the first integral is equal to 1. From Equation (45), if the difference of , and from Equation (42), is positive if the difference of , which shows that the second integral is positive if is greater than zero and if is greater than zero. Thus, with the previous considerations, we have P(0) < 1. It follows that a unique positive real solution of P(σ = 1) exists, and as consequence [38], we have the following theorem: The endemic equilibrium state is locally asymptotically stable if R0d > 1 and R0h > 1.
4. Sensitivity Analysis
A 95% confidence interval is estimated through bootstrapping. We consider four key classes acute, chronic, infected, and environmental due to their significant impact on model outputs. Figures 2–7 indicate that μh is the most influential parameter for the acute, chronic, and infected classes, while μd is the most influential for the environmental class, as shown in Figures 8 and 9. This implies that brucellosis progression and prevalence in humans are highly sensitive to the mortality rate. Specifically, disease-related mortality significantly influences the size and behavior of the acute, chronic, and infected human subpopulations. Meanwhile, the death rate of domestic animals (μd) directly affects pathogen load in the environment, as animal carcasses and waste products serve as significant reservoirs for Brucella bacteria. A higher μd could lead to increased environmental contamination.
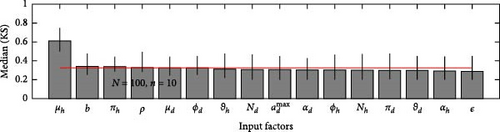
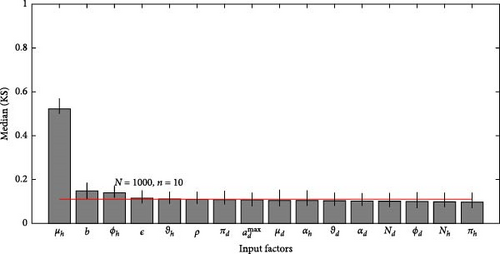
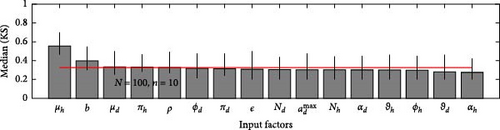
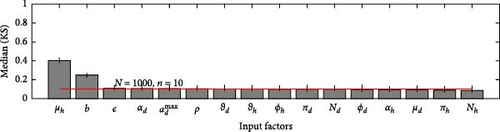
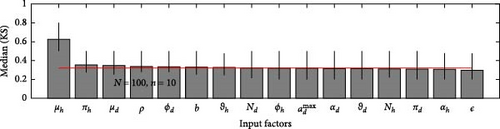
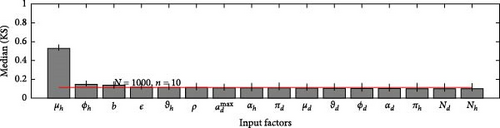
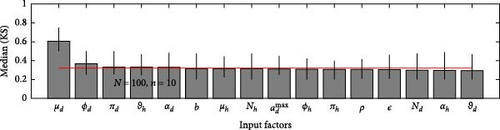
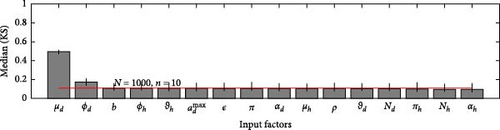
According to Figure 3, the second most influential parameters are φd, b, φh, Nd, αh, and ɛ. These parameters form a secondary group that plays a crucial role in refining the understanding of brucellosis transmission dynamics and guiding the design of specific, secondary-level interventions to complement efforts targeting the primary influential factors. The remaining parameters exhibit average sensitivity values close to the dummy parameter, suggesting they may be uninfluential. Further analysis reveals that larger sample sizes improve the identification of highly influential parameters. For instance, Figure 5 ranks b as the second most influential parameter in the chronic class, while Figure 7 ranks φh and b as the second most influential parameters in the infected class. Similarly, Figure 9 identifies φd as the second most influential parameter in the environmental class. Together, these parameters provide valuable insights into the broader ecological and epidemiological dynamics of brucellosis. Their grouping suggests a collective influence on key factors such as human–animal interactions, the force of infection, and the basic reproduction number (R0). This has significant implications for intervention strategies: while the first group of parameters requires immediate attention due to its overarching impact, the second group highlights specific areas for targeted intervention.
4.1. Result and Discussions
A numerical simulation was conducted using MATLAB 2016b software, employing the ODE15s solver to integrate the PDE model along characteristic lines. The ODE15s solver, which uses a combination of Euler–Riemann and backward Euler–Riemann methods, was chosen due to its robustness in handling the stiffness inherent in differential equations. To generate the initial conditions for the human (Sh, Ah, and Ch) and animal (Sd and Id) compartments, parameter values in Equation (2) from Table 1 were applied, with the total human and domestic animal populations set at Nh = 100 and Nd = 50, respectively. The age groups investigated include 0–5, 6–10, 11–15, and 0–15 years for domestic animals and 0–18, 19–36, 37–54, 54–72, and 0–72 years for the human population.
4.1.1. Animal Population Dynamics
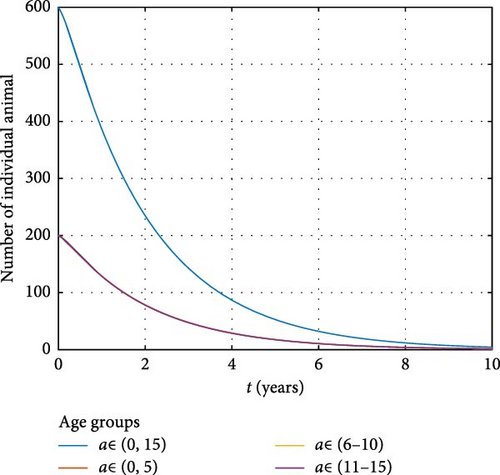
Figure 10 illustrates the total number of animals in different age groups over time. A notable decrease in the susceptible animal population is observed, which can be attributed to the high rate of Brucella pathogen release from infected animals. This pathogen contaminates the environment, contributing to further disease transmission. These findings align with previous studies that highlight the significant role of environmental contamination in the persistence of brucellosis in animal populations. For instance Ahmed et al. [41] demonstrated that environmental reservoirs of Brucella play a critical role in maintaining the infection cycle within animal populations, leading to a sustained decrease in healthy animal numbers.
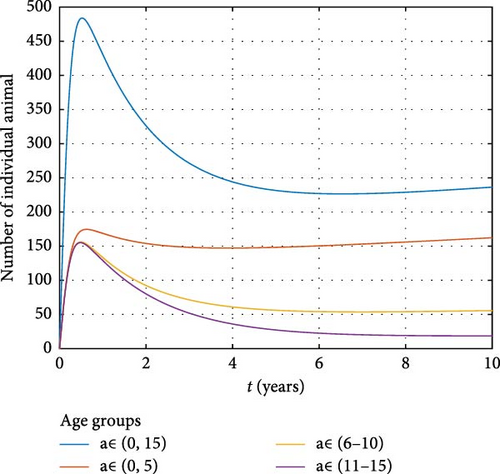
4.1.2. Infection Dynamics in Animals
Figure 11 shows a notable increase in the number of infected animals, particularly in the newborn cohort. The high susceptibility of young animals, specifically those aged 0–5 years, makes this age group a vulnerable and high-risk population for brucellosis transmission. This persistence of brucellosis among young animals is consistent with the finding of Salmon et al. [42], who observed that younger animals are more prone to brucellosis infection due to their immature immune systems, which fail to mount a sufficient defense against the pathogen. Furthermore, the high reproductive rates among infected animals contribute to the continued transmission of the disease, as infected newborns serve as new reservoirs for the pathogen (vertical transmission).
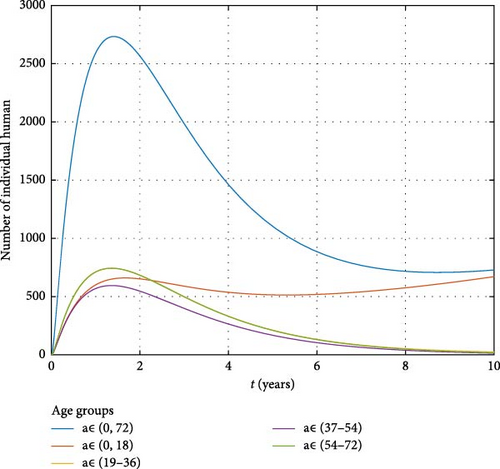
4.1.3. Chronic Stage of Human Infections
Figure 12 presents the dynamics of human populations at different stages of infection, with a specific focus on those in the chronic stage. It reveals that a significant number of individuals in the age group 0–18 are progressing to chronic infection, indicating that many are carrying the Brucella bacteria from a young age into maturity. This pattern underscores the long-term public health burden posed by brucellosis, as individuals with chronic infections can remain carriers of the pathogen for extended periods. Mangal et al. [43] similarly found that chronic brucellosis cases often stem from early-life exposure, leading to lifelong complications and the potential for transmission to new generations. The persistence of carriers in the population highlights the challenge of eradicating brucellosis and reinforces the need for early interventions and continued monitoring.
5. Conclusion
The paper presents a mathematical modeling of brucellosis transmission dynamics and its age-specific applications. A PDE model was formulated; the existence of positivity and boundedness was shown using the integrated semigroup approach. The basic reproduction number, R0, was derived using the Lotka–Sharpe–McKendrick integral equation, and the DFE was found to be locally asymptotically stable when R0 < 1. Global sensitivity analysis was conducted using the PAWN method, which identified the most influential parameters affecting disease transmission. Humans aged 0–72 years are identified as carriers of the disease, while domestic animals, particularly those aged 0–5 and 11–15 years, serve as critical reservoirs and transmitters of brucellosis. This highlights the need for targeted interventions in these specific age groups to control disease spread. Additionally, environmental contamination plays a significant role in sustaining transmission, emphasizing the importance of improving sanitation and hygiene practices. These results underscore the necessity for comprehensive public health measures, including enhanced testing protocols, age-specific interventions, and environmental management, to effectively mitigate the transmission of brucellosis.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Innocent Sosoma: conceptualization, formal analysis, methodology, software, writing–original draft, writing–review and editing. Eunice Mureithi: conceptualization, formal analysis, methodology, visualization, writing–review and editing. Nyimvua Shaban Mbare: conceptualization, formal analysis, methodology, writing–review and editing.
Funding
This work has been supported by the Mathematics for Sustainable Development (MATH4SDG) project, which is a research and development project running in the period 2021–2026 at Makerere University, Uganda; University of Dar es Salaam, Tanzania; and the University of Bergen, Norway, funded through the NORHED II program under the Norwegian Agency for Development Cooperation (NORAD) (Project No. 68105).
Open Research
Data Availability Statement
This study did not involve the collection of primary data. Instead, it utilized data from various secondary sources, which are appropriately cited within the manuscript. Readers may refer to the cited sources for access to the original datasets.




