Modeling and Stability Analysis of the Dynamics of Malaria Disease Transmission with Some Control Strategies
Abstract
In this study, we proposed and analyzed a nonlinear deterministic mathematical model of malaria transmission dynamics. In addition to the previous approaches, we incorporated the class of aware people and other control measures. We established the wellposedness of the model, and the asymptotic behavior of the solutions is rigorously studied depending on the basic reproduction number R0. The model system admits two equilibrium points: disease-free and disease-persistent equilibrium points. The analytical result of the model system revealed that the disease-free equilibrium point is both locally as well as globally asymptotically stable whenever R0 < 1 while the disease-persistence equilibrium point is globally asymptotically stable whenever R0 > 1. Moreover, the forward bifurcation phenomenon of the model system for R0 = 1 was analyzed by using center manifold theory. A sensitivity analysis of the basic reproduction number was performed to identify parameters that will cause to trigger the transmission of malaria disease and should be targeted by control strategies. Then, the model was extended to the optimal control problem, with the use of three time-dependent controls, namely, preventive measures(treated bednets and indoor residual spraying), continuous awareness campaigns to susceptible individuals, and treatment for infected individuals. By using Pontryan’s maximum principle, necessary conditions for the transmission of malaria disease were derived. Numerical simulations are illustrated by using MATLAB ode45 to validate the theoretical results of the model. The numerical findings of the optimal model suggested that integrated control strategies are better than a sole intervention to eliminate malaria disease.
1. Introduction
Nowadays, developing countries are challenging with a lot of problems, such as climatic change, racism, terrorism, unemployment, poverty, and public health crises. Among them, malaria disease is continued as a killer death and a major public health issue [1]. It is a contagious illness and spreads between people through the bites of infected female Anopheles mosquitoes [2, 3]. The most common species that can cause to human illness are Plasmodium vivax, plasmodium falciparum, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowles, and their distribution may vary from place to place [1]. The most common symptoms of malaria include high fever, joint pains, abdominal pains, headaches, vomiting, nausea, diarrhea, and extreme fatigue [3, 4, 5, 6, 7]. Approximately 241 million cases and 627,000 deaths were reported globally in 2020, with 95% of cases occurring in the African Region [8]. Utilizing bed nets that have been treated, antimalarial drugs, and indoor residual spraying are among the most effective malaria prevention measures [9].
A thorough understanding of the disease’s transmission process and its control is necessary to create effective intervention measures and mitigations. Mathematical models help in describing the evolution of infectious diseases [10, 11, 12, 13, 14, 15, 16, 17, 18]. The first published paper on the dynamics of malaria that demonstrates the life cycle of the malaria parasite dates back to Sir Ross [19], which is SIR type. Recently, various scholars have studied the dynamics of malaria transmission using mathematical modeling approaches to combat it. For example, in 2022, Collins and Duffy [20] proposed and analyzed the dynamics of the malaria transmission model in Nigeria that takes into account drug resistance, treatment, and the use of mosquito nets as preventative measures. Their findings confirmed that unless better control strategies are put into place, the disease is likely to remain endemic in Nigeria. In 2021, Keno et al. [21] developed and analyzed a mathematical model of malaria transmission with optimal controls. Their study showed that the most cost-effective ways to reduce malaria transmission are through the combination of control measures, such as treatment and indoor residual spraying. In 2020, Ghosh et al. [17] formulated and analyzed SEIRS for human populations and SEI for vector populations with optimal control and relapse. Their result revealed that treated bed nets and indoor residue springs are the best strategies to eliminate malaria transmission. In 2018, Leiton et al. [22] presented an SEIRS-SI mathematical model for malaria transmission with optimal control in Colombia. Their finding concluded that to prevent malaria transmission the integrated control strategies must hold effectively. In 2022, Keno et al. [23] presented a nonlinear mathematical model of malaria transmission with three control strategies, namely treated bed nets, treatment, and indoor residual spraying. Both forward and backward bifurcation were present in their model, and (treatment and treated bed nets) are the best ways to reduce the burden of malaria transmission among control measures. In 2022, Gautam et al. [24] developed a mathematical model for malaria transmission dynamics that included cross-border travel between a low-endemic country (Nepal) and a high-endemic country (India) with optimal control. They reaffirmed that their study offers crucial insights into a potential barrier that cross-border mobility may pose to malaria elimination programs. Even though most countries have policies or strategies that are being made to eradicate malaria disease, it is continued as a challenge, especially for developing countries. For the best of our knowledge, mathematical models that deal with the impact of awareness creation for susceptible individuals are very rare. Thus, we are motivated to fill this gap and consider other alternative control measures.
The remaining parts of this study are outlined as follows: The model description and underlying assumptions are covered in Section 2. The basic properties of the model are discussed in Section 3. An analysis of the model is given in Section 4. The optimal control problem is presented in Section 5. Numerical simulations and discussions of the model are covered in Section 6. The conclusion is provided in Section 7.
2. Mathematical Model Formulation and Description
- (i)
We assumed that all newly recruited humans and mosquitoes are not carriers of malaria infection.
- (ii)
We considered only female anapheles mosquitoes in our recent study.
- (iii)
In addition to natural death, we assumed that other human activities contribute for the mortality of mosquitoes.
- (iv)
We assumed that mosquitoes feed only human blood, and vertical transmission of the infection between them is not under consideration.
- (v)
We assumed that infected mosquitoes remain infectious until they die.
Keeping the above discussions in mind, the new model is portrayed in Figure 1, and state variables and parameters are presented in Tables 1 and 2, respectively.
| Notation | Description |
|---|---|
| Sh(t) | The class of susceptible human population at given time t |
| Ah(t) | The class of aware human population at a given time t |
| Ih(t) | The class of infectious human population at a given time t |
| Rh(t) | The class of recovered human population at a given time t |
| Sm(t) | The class of susceptible female Anopheles mosquitoes at a given time t |
| Im(t) | The class of infectious female Anopheles mosquitoes at a given time t |
| Notation | Description |
|---|---|
| Λh | The recruitment rate of susceptible individuals |
| Λm | The recruitment rate of susceptible female Anopheles mosquitoes population |
| τ | The progression rate of susceptible individuals into the class of aware individuals |
| βh | The rate in which susceptible humans get infected by infected mosquitoes |
| βm | The rate in which susceptible mosquitoes get infected by infectious individuals |
| μ | The natural death rate of individuals |
| α | The natural death rate of mosquitoes |
| σ | The disease induced-death rate of infectious individuals |
| ω | The progression rate of recovered individuals to the class of aware individuals |
| γ | The recovery rate of infectious individuals |
| δ | The mortality rate of susceptible mosquitoes population due to human activities |
| ϕ | The mortality rate of infected mosquitoes population due to human activities |
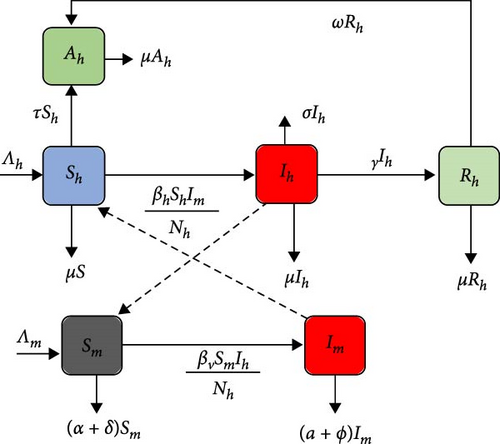
3. Basic Properties of the Model
Lemma 1 (Existence and Uniqueness of Model Solutions). The unique solution in , is admissible for the model system (1) with initial condition (2).
Proof. we can rewrite the system (1) in the form of , where
It is straightforward to see that all the right-hand-side components of the function f (x) are continuously differentiable almost everywhere in , which implies that f (x) is of a class C1 on for all t ≥ 0. Hence, by Picard–Lindelôf theorem [26], the model system (1) has a unique solution locally in for all t ≥ 0. This completes the proof.
Since we are dealing with populations, it is necessary to ensure the nonnegativity and boundedness of model solutions.
Lemma 2 (Nonnegativity of Model Solutions). The solution set {Sh(t), Ah(t), Ih(t), Rh(t), Sm(t), Im(t)} of model system (1) remains nonnegative for all future time, t ≥ 0 under the initial condition (2) in the closed region .
Proof. Consider the 1st equation in the system (1),
Likewise, consider the 5th equation in the system (1)
Lemma 3 (Boundedness of Model Solutions). The malaria model (1) with initial condition (2) in is positively invariant in the feasible region , where
Proof. Adding all the model equations of (1), we obtain the following:
.
Similarly, the time derivative of the total mosquito population, Nm(t) along model solutions (1), is obtained and given in compact form by the following:
4. Analytical Analysis of the Model
4.1. Malaria Disease Free-Equilibrium (DFE) Point
To obtain the DFE, we set the right-hand sides of model system (1) to zero at Ih = Im = 0. Thus, the DFE point of the model is denoted by Df and given by the following:
4.2. Basic Reproduction Number
4.3. Malaria Disease Persistent Equilibrium Point of the Model
4.4. Stability Analysis of Model Equilibrium Points
In the subsequent theorems, we will examine the obtained equilibria, which are Df and Dp of model system (1), both in local and global sense.
Theorem 1. The malaria-free equilibrium point of model system (1), is locally asymptotically stable whenever R0 < 1 otherwise unstable.
Proof. The Jacobian matrix of the system (1) from DFE, Df, is given by the following:
Theorem 2. The DFE point of model system (1) is globally asymptotically stable whenever R0 < 1 otherwise unstable.
Proof. To evaluate the global stability of Df of model system (1), we formulate a suitable Lyapunov function
Theorem 3. The disease persistent equilibrium point of the model system (1) is globally asymptotically stable on Δ for R0 > 1.
Proof. To prove this theorem, we employ the Dulac’s criterion [30] by taking as the candidate of Dulac’s function for model system (1) and
Γ = (Sh, Ah.Ih, Rh, Sm, Im).
Since ϱ(Ih, Im) > 0, ∀Ih, Im > 0, we have that
4.5. Bifurcation Analysis of the Model
4.6. Sensitivity Analysis of the Basic Reproductive Number
To ascertain the model’s robustness to parameter values, we conducted a sensitivity analysis on the model’s basic reproductive number. Mathematically, the normalized forward sensitivity index of a variable to a parameter is a differentiable function of the parameter and is defined using partial derivatives based on the classical definition given in [32].
Definition 1. The sensitivity and elasticity of quantity R0 with respect to the parameter v is given by the following:
Here, for example means, increasing (or decreasing) the value of v by 10%, always result to increase (or decrease) the value of R0 by 10%, whereas means increasing (or decreasing) the value of v by 10%, always result to decrease (or increase) R0 by 10%.
According to the sensitivity analysis index, parameters with positive indexes βh, βv, and Λv have an adverse effect on the ability to control the spread of malaria, whereas parameters with negative indexes τ, δ, ϕ, and γ have an advantageous effect. Table 3 provides the summarize of the sensitivity analysis of model system (1).
| Parameter | Elasticity index |
|---|---|
| βh | +ve |
| βm | +ve |
| Λm | +ve |
| τ | −ve |
| γ | −ve |
| δ | −ve |
| ϕ | −ve |
4.6.1. Interpretation of Sensitivity Indices
It is noted from Table 3 that when the values of βh, βv, and Λv rise while the values of the other parameters remain constant, the value of R0 rises. By increasing the values of these parameters, the secondary infection, R0, will also increase, which means malaria transmission will revive within the society. On the other hand, while the other parameters remain constant, increasing the values of γ, ϕ, τ, and δ causes the value of the secondary infection, R0, to decrease. This indicates a reduction in the transmission of malaria as a result of the population’s overall infection rate falling as these parameters’ values are raised. As a result, the model’s sensitivity analysis showed that in order to stop the transmission of malaria among the population, authorities, and health practitioners should increase parameters with negative indices and decrease those with positive indices.
5. Extension of the Model into Optimal Control
- (i)
c1(t) stands the efforts intended for susceptible individuals to create awareness through all media outlets.
- (ii)
c2(t) stands the initiatives aimed at providing adequate control measures of malaria.
- (iii)
c3(t) stands the efforts made to assist infected people with continuous treatment.
5.1. Existence of an Optimal Control
Theorem 4. There exists an optimal control that minimizes the objective functional J(c1, c2, c3), subject to the control system (49).
Proof. To show the existence of optimal control, we denote the right-hand sides of system (49) by . Then, to prove the existence of optimal control we followed the classical result of [36, 37]. To achieve this, the following conditions must met.
- (i)
z is of class C1 and there exists a constant φ such that
() - (ii)
The admissible set of all solutions to system (49) with corresponding control in Cad is nonempty,
- (iii)
There exist functions a and b such that ,
- (iv)
The control set C = [0, 1] × [0, 1] × [0, 1] is closed, convex and compact,
- (v)
The integrand of the objective function is convex in C.
To verify condition (1), rewrite
Moreover, consider
A unique solution to system (49) for a constant control exists in accordance with condition (i), which also implies that condition (ii) is satisfied.
Furthermore, we have the following:
Hence, the proof of the theorem is completed.
5.2. The Hamiltonian and Optimality System
Theorem 5. Suppose we have optimal controls , and and Sh, Ah, Ih, Rh, Sm and Im solutions of the respective state system that minimizes the objective functional (50) over C, there exist costate variables, ξ1, …, ξ6 satisfying
Proof. Differentiating the Hamiltonian, H with respect to state variables provides the following:
5.3. Analysis of the Control Reproductive Number
6. Numerical Results and Discussions
In this section, we carried out some numerical results of model (1) to establish the validity of the theoretical results using MATLAB ode45 solver by using initial conditions: Sh0 = 100, Ih0 = 10, Sm0 = 1, 000, Im0 = 20 taken in [40] and assumptions Ah0 = 20, Rh0 = 10. It is important to notice that parameter values used in the simulations are for illustrative purposes only and may not be epidemiologically accurate.
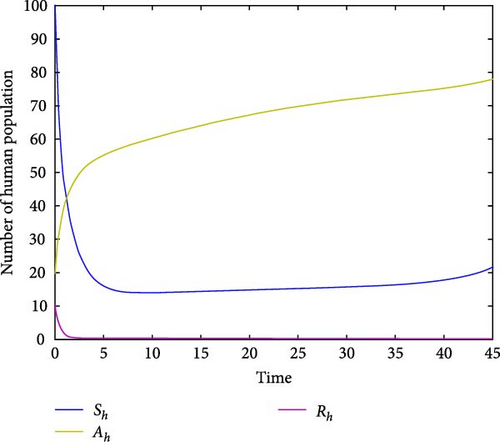
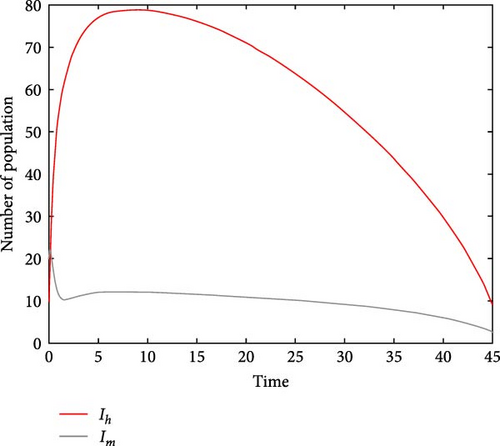
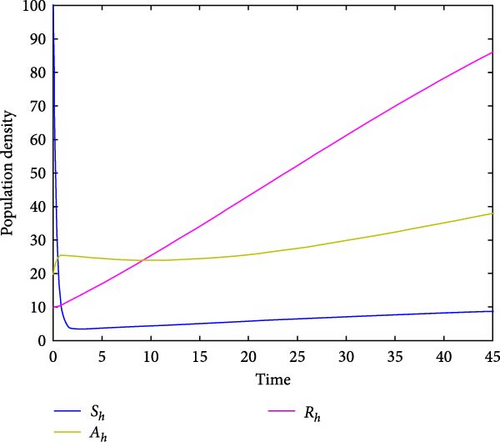
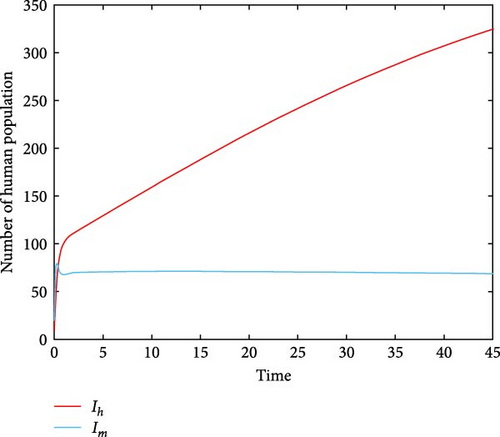
For parameter values: Λh = 10, βh = 5.9, Λm = 500, βm = 0.955, σ = 0.051, μ = 0.05, α = 1/15, γ = 0.00991, ω = 1.9, τ = 0.2, δ = 9.91, ϕ = 1.89, π = 0.28, the numerical simulation of model system (1) is illustrated. Figures 2(a) and 2(b) show for R0 = 0.3812, the class of aware population Ah(t) and susceptibles populations Sh(t) are increasing, while recovered populations are decreasing. At the same condition, infected human and vector populations are decreasing but not sufficient to eradicate malaria disease. This finding supports the analytical result that the DFE point is locally asymptotically stable for R0 < 1 as stated in Theorem 1. This result indicates that malaria disease will gradually decrease or eliminate from the population. Figures 3(a) and 3(b) shows that all solution curves approaches almost to non-zero components or to their endemic equilibrium point components for parameter values Λh = 10, βh = 5.99, Λv = 600, βv = 9.05, σ = 0.051, μ = 0.05, α = 1, γ = 0.01991, ω = 0.0075, τ = 0.1892, δ = 11.99, ϕ = 1.89, π = 0.18. This result revealed that the global asymptotic stability of the malaria disease persistent equilibrium point, which occurs when R0 = 2.9713 > 1, indicates that the disease will persist in the population.
The simulation of sensitivity analysis is displayed in Figure 4(a). The baseline parameter values with positive index will result a major effect on the frequency of the ailment spread, while parameters with negative index help to reduce the disease. In Figure 4(b), for R0 < 1, the model system (1) has stable DFE, and it has no endemic equilibrium point. While for R0 > 1, a stable endemic equilibrium appeared, and a stable disease-free became unstable. Hence, the transcritical bifurcation arises.
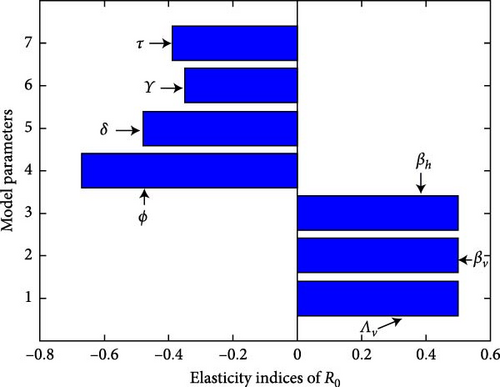
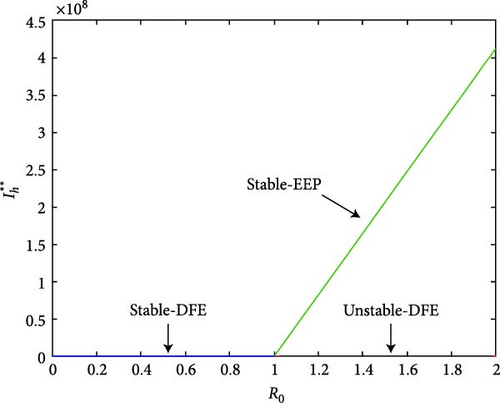
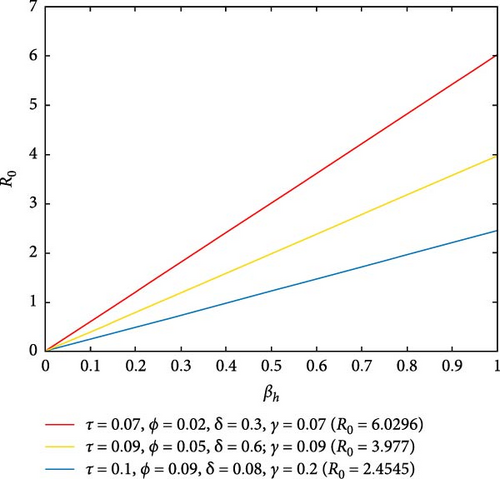
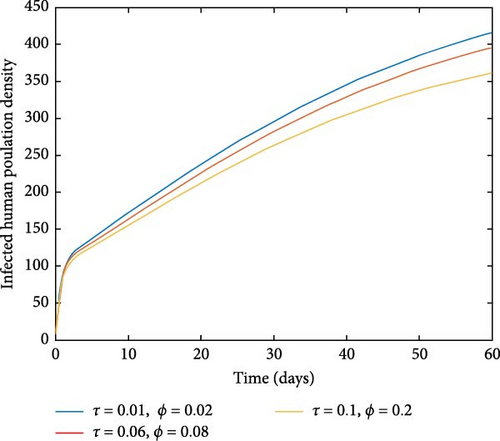
Figure 5(a) depicts that a large value of τ or γ or δ or ϕ in the presence of βh can lead to a small value of R0. That is to say, if we increase the awareness creation, recovery rate of infected individuals and environmental cleaning (chemical spraying), the spread of malaria disease will decrease in the population. Figure 5(b) reflects that a large value of τ or ϕ leads to a small value of R0. Therefore, by increasing public awareness creation and chemical spraying, we can reduce the spread of malaria disease.
6.1. Numerical Results of the Optimal Control Model
In this section, we examine the effects of various control measures on the eradication of malaria disease through some numerical results.
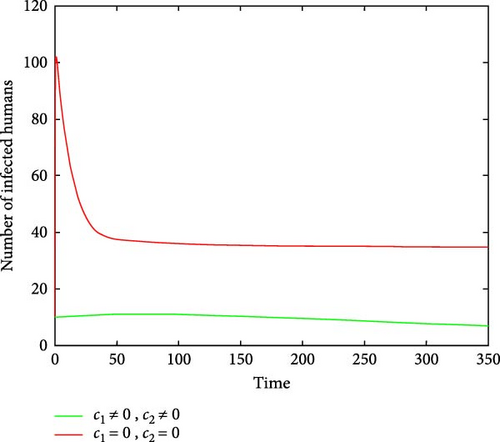
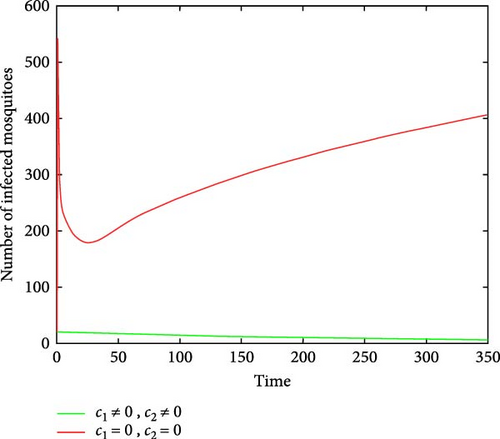
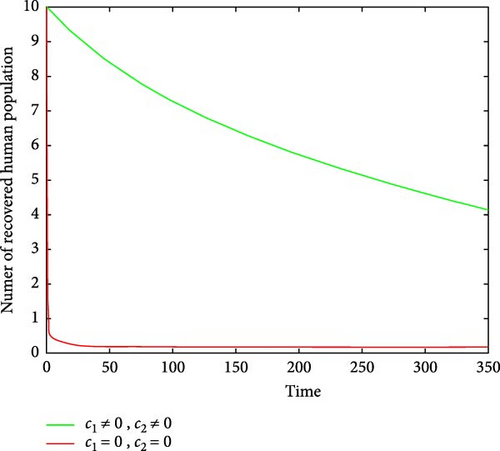
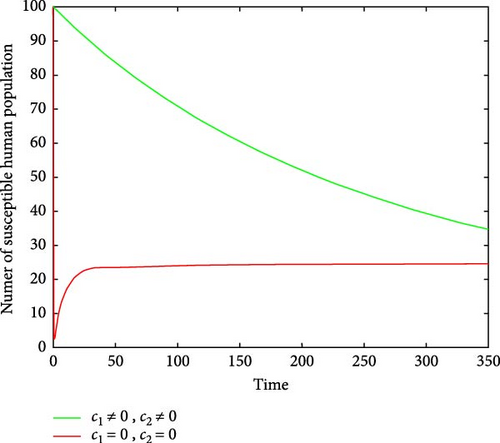
Figures 6(a) and 6(b) show the effectiveness of public awareness in the reduction of both infected human and mosquitoes population. As we observed, in the absence of a public awareness campaign, the number of infected mosquitoes increases and, in turn, this leads to an increase in the numbers of the infected human population, while in the presence of a public awareness campaign, the numbers of infected mosquitoes dramatically decrease and also infected human populations decrease at the same time. Therefore, in order to stop the spread of malaria, policymakers must focus on raising public awareness to the fullest extent possible and ensuring that there are enough preventive controls available. Figures 7(a) and 7(b) show that the number of recovered human populations is higher under the control strategy than in the case without control, while the number of susceptible mosquito populations is lower under the control strategy than in the case without control. Therefore, in order to stop the spread of malaria in the community, policymakers and the government must place a high priority on providing ongoing treatment for those who are infected.
7. Conclusion
In this study, a nonlinear deterministic mathematical model of malaria transmission is formulated and analyzed, dividing susceptible into another aware human population. Under the given domain, the mathematical as well as epidemiological welposedness of the model is proved. Malaria disease reproduction number (threshold) of the model was obtained and provided the scenarios to stick or eradicate in the population. The stability of the disease-free and endemic equilibrium points was examined, and the DFE point was found to be asymptotically stable when R0 < 1 and the existence of bifurcation at R0 = 1 was analyzed, and it was shown that when R0 = 1 the model undergo forward bifurcation phenomenon. We found that R0 < 1 is the sufficient condition to eradicate malaria disease.
The sensitivity analysis of the basic reproduction number of the model revealed that parameters with negative indices are likely to decrease the disease while parameters with positive indices are likely to increase the expansion of malaria disease in the society. The numerical simulation of the model revealed that the numbers of infected human populations decrease when the biting rate of susceptible humans by infected mosquitoes decrease. The qualitative study proved the existence and characterization of an optimal control solution. Also, various control strategies were tested and showed that the combination of continuous public awareness creation and access to adequate control measures are more effective in the control of malaria disease transmission.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors would like to thank Hawassa University for its Wi-Fi contribution.
Open Research
Data Availability
The data used to supplement the findings of this study are from previously published articles and cited in relevant places.




