Developing a Reproducible Procedure for Optimal Utilization of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Aesthetic Treatments: Efficacy Evaluation Using Ultrasound Imaging—A Single-Center Prospective Open-Label Randomized Study
Abstract
Objective. This single-center, prospective, open-label randomized study aimed to produce a repeatable procedure for isolating platelet-rich plasma (PRP), maximizing platelet recovery and concentration parameters from the collected material, and to assess the effectiveness of autologous platelet-rich plasma (concentrated platelet rich plasma C-PRP and platelet rich plasma low centrifugation concept PRP LCC) and platelet-rich fibrin (injectable platelet rich fibrin I-PRF and fluid platelet rich fibrin F-PRF) injections on skin density and thickness in facial aesthetic treatments. Methods. Twenty participants aged 30–60 underwent a series of three treatments (intervals: 4–6 weeks). The study encompassed the following two stages: a laboratory and a clinical one. In the first stage, the aim was to optimize centrifugation parameters (time and speed) of whole blood to produce the highest quality product for use in aesthetic treatments. Double centrifugation of blood produced the following four types of plasma fractions with different parameters: platelet-rich plasma (C-PRP and PRP LCC) and platelet-rich fibrin (I-PRF and F-PRF) each with two different platelet concentrations (202% in the first centrifugation and 148% in the second centrifugation). The total PLT recovery in the procedure was 76%, with an average of 32% in the first centrifugation and that of 44% in the second one. In the clinical stage, treatments involved C-PRP, PRP LCC, I-PRF, and F-PRF injections in the forehead, lower eyelid, and cheek areas. In ultrasound skin examination with randomly assigned measurements for each patient, a series of acoustic density values directly proportional to tissue density was obtained. Thickness of the skin was also determined (µm) by summation of the epidermis and dermis thickness. Measurements were made in the lateral forehead, lower eyelids, and cheek regions. Results. Statistical analysis elucidates the effectiveness of blood-derivatives therapy in the introduced regiment. The increase in skin density was statistically significant after the following treatment: three sessions in 4–6 week intervals, each consisting of 1 mL of C-PRP injections in the forehead area, 1 mL of I-PRF injections in the lower eyelid area, and 3.5 mL of PRP LCC and 3.5 mL of F-PRF injections in the cheek area. Furthermore, significant improvements in skin density were recorded in the cheek and forehead areas (F(3, 24) = 4.5170 with p = 0.011971 for cheeks and F(3, 24) = 9.2327 with p = 0.000305 for forehead). Lower eyelid skin density and thickness also increased significantly (F(3, 24) = 3.2653, p = 0.038881). No significant changes were noted in skin thickness in the forehead and cheek areas (F(3, 6) = 1.438771, p = 0.321616 for cheeks; F(3, 6) = 2.383248, p = 0.168172 for forehead). Patient satisfaction was high, as evidenced by a GAIS average score of 2.75. Conclusion. The study confirms the efficacy of C-PRP, PRP LCC and I-PRF, and F-PRF injections in increasing skin density and, to a lesser extent, skin thickness in specific facial areas. As a result of this research, ready-to-use kits (PLASMOO) for obtaining PRP and PRF were developed and put into production. These findings underscore the potential of autologous plasma and fibrin formulations in aesthetic medicine, which offer substantial improvements in skin quality and patient satisfaction. This trial is registered with ISRCTN10538865.
1. Introduction
Various types and preparations of platelet-rich plasma and fibrin, according to different protocols, are used in many medical fields. In the 1990s, Marx [1] demonstrated that properly prepared, concentrated plasma with a high platelet concentration, administered to surgical sites, initiates tissue repair by releasing biologically active substances such as cytokines, growth factors, and adhesive proteins. These factors play a critical role in stimulating the healing process, promoting revascularization, and the formation of new connective tissue through initiating collagenesis and elastogenesis. PRP mixtures contain supraphysiological concentrations of growth factors, thereby significantly accelerating healing after various types of surgical procedures and injuries [2, 3]. According to the PRP classification by Ehrenferst [4], the preparation protocols for various plasma and fibrin types (pure platelet-rich plasma P-PRP, leucocyte-rich PRP LR-PRP, pure platelet-rich PRF P-PRF, and leucocyte-rich PRF or L-PRF) were either based on fully assembled systems/kits or manually performed.
Growth factors, cytokines, and regulatory proteins present in PRP concentrates stimulate target cells through auto-, endo-, and paracrine processes, which accelerate the healing process [5]. Everts [6] emphasized the importance of leukocytes in such preparations. The main benefit of platelet-rich plasma preparations is their safety, biocompatibility, and effectiveness in a wide range of medical indications [7, 8], as well as lack of serious side effects, which distinguishes this therapy from other approaches. Unfortunately, to date, there is no consensus or clear regulations regarding the quality of plasma preparations, which would translate into the quantity of platelets, leukocytes, growth factors, fibrin, or other biologically active factors, and erythrocytes [9]. In recent years, the Scientific Standardization Committee introduced a classification of plasma preparations for regenerative medicine (International Society on Thrombosis and Hemostasis classification) [10]. However, a consensus in this matter seems distant. Continuous development of therapies and technologies using PRP suggests that, in the future, separate preparation protocols for PRP or PRF will be developed to tailor specific medical therapies. This idea was commercialized by producing PLASMOO kits (Innmedis, Poland) for simultaneous acquisition of PRP and PRF.
Platelet-rich plasma (PRP) is a fraction of blood, appropriately centrifuged, which contains a much higher platelet concentration than that of whole blood [1, 2]. It is obtained by collecting a certain amount of peripheral blood on the day of the procedure. PRP preparations thus prepared usually contain various types of anticoagulants. Platelet degranulation and release of biologically active substances are signalled by contact with collagen or endogenous thrombin upon PRP injection into the skin (nonactivated systems). Systems with an activator usually contain calcium gluconate, calcium chloride, and exogenous thrombin. PRF (platelet-rich fibrin) preparations do not contain anticoagulants. The objective here is based on obtaining a fibrin-rich preparation. After injection, the preparation undergoes clotting, forming a fibrin matrix, within which the platelets are suspended. This fibrin network extends the lifespan and effectiveness of the preparation for several days after injection of the product [11]. PRP is responsible for differentiation, proliferation, and migration of cells, acting through growth factors, which are released from the platelets about 10 minutes post activation. From the perspective of platelet-rich plasma activity on the skin, the most important growth factors include PDGF, VEGF, EGF, and FGF. In addition, anti-inflammatory cytokines such as IL-1ra, sTNF-R I, IL-4, IL-10, and IL-13 are released during the process [11].
2. Aim
This single-center, prospective, open-label randomized study aimed to produce a repeatable procedure for isolating platelet-rich plasma (PRP), maximizing platelet recovery and concentration parameters from the collected material, and to assess the effectiveness of autologous platelet-rich plasma (concentrated platelet rich plasma C-PRP and platelet rich plasma low centrifugation concept PRP LCC) and platelet-rich fibrin (injectable platelet rich fibrin I-PRF and fluid platelet rich fibrin F-PRF) injections on skin density and thickness in facial aesthetic treatments. After optimization of this process and determination of optimal centrifugation parameters, we administered the preparation to female patients in the form of mesotherapy injections into the facial skin for bioregeneration. The study included 20 female volunteers aged between 30 and 60 years seeking facial skin rejuvenation, who were treated at no cost. All participants had Fitzpatrick skin types I–III and facial wrinkles classified as Glogau class II or higher. Exclusion criteria for the study were pregnancy or breastfeeding, blood or platelet disorders, facial surgery or semipermanent dermal fillers in the past year, genetic conditions affecting fibroblasts or collagen, a history of herpes simplex infection, active skin diseases or infections in the treatment area, a tendency to hypertrophic scars or keloids, immunosuppressive disorders or treatments, and a history of skin cancer. In addition, those who had used topical or oral tretinoin, have undergone botulinum toxin and/or dermal filler injections, chemical peelings or laser treatments for facial rejuvenation in the past six months, or planned such treatments in the next six months were excluded.
After obtaining approval from the Bioethics Committee of the Jagiellonian University in Krakow (approval number 1072.6121.11.2020), the research commenced. The study protocol adhered to the guidelines of the Helsinki Declaration.
3. Materials and Methods
3.1. Study Design
The study consisted of the following two stages: a laboratory and a clinical one. Laboratory tests and platelet count in PRP preparations were carried out at the University Children’s Hospital Laboratory in Kraków, Poland.
In the laboratory phase, 20 volunteers participated. According to the recommendations of the Bioethics Committee, the amount of blood drawn from each participant could not exceed 50 ml. Blood samples were collected from all volunteers, with each person providing blood into 5 tubes. It was assumed that the centrifugation parameters would follow the low-speed centrifugation concept (LSCC), with the primary goal being to obtain products with the highest concentration and recovery of platelets. Given that the LSCC approach typically yields about 1–1.5 mL of highly concentrated platelet preparation, a second centrifugation was decided upon. This allowed for optimal recovery of a higher quantity of platelets and optimization of the process with just a single blood draw from the patient.
In the clinical phase, volunteers had blood drawn from the peripheral vein in a single collection procedure, successively into two tubes, i.e., a 10 mL tube containing 1 mL of anticoagulant (sodium citrate) (T-LAB, Bursa, Turkey) and an empty 10 mL polycarbonate tube (T-LAB, Bursa, Turkey). The material was centrifuged in a fixed-angle centrifuge (Neuation iFuge D06, eqlab, Poland).
3.2. Laboratory Stage
During the laboratory stage, work was conducted in three areas. Area I: to determine the best centrifugation parameters that allow for maximum platelet (PLT) recovery from a single centrifugation of whole blood with and without anticoagulant. The studies were conducted using the following centrifugation parameters: 190 G/7 minutes, 210 G/6 minutes, 250 G/4 minutes, 250 G/5 minutes, 250 G/6 minutes, and 288 G/5 minutes.
Area II: to determine the best centrifugation parameters to produce the highest PLT concentration from a single centrifugation of whole blood with and without anticoagulant. The studies were conducted using the following centrifugation parameters: 55 G/2 minutes, 55 G/3 minutes, 55 G/4 minutes, 72 G/3 minutes, and 288 G/5 minutes.
Area III: to develop a protocol for the combined, double centrifugation of PRP and PRF fractions based on the previous results.
3.3. Centrifugation Protocol
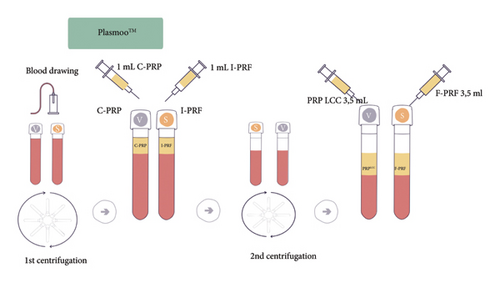
Based on the analyses from the first two stages, a procedure of simultaneous, double centrifugation of PRP (with sodium citrate) and PRF (no sodium citrate) was developed to obtain different autologous fractions. Fraction I (from the first centrifugation of whole blood) exhibited the highest PLT concentration. The procedure applies to samples with and without anticoagulant.
C-PRP (with anticoagulant) and I-PRF (without anticoagulant) were obtained from fraction I.
Fraction II (from the second centrifugation, postextraction of C-PRP or I-PRF) showed the highest quality PLT recovery. The procedure applies samples with and without anticoagulant. PRP LCC plasma (with anticoagulant) and F-PRF fibrin (without anticoagulant) were obtained from fraction II.
A protocol for separate centrifugation of fractions I and II was devised, to optimize blood usage within one procedure for one patient, due to mutually exclusive nature of centrifugation parameters for each fraction.
The separate centrifugation procedures allowed obtaining the following four types of plasma fractions: platelet-rich plasma (C-PRP and PRP LCC) and platelet-rich fibrin (I-PRF and F-PRF), with two different concentrations of platelets each (Figure 1).
3.4. Commercial Application
Based on these studies, certified sets with the commercial name PLASMOO (Innmedis, Poland) were designed for use in the designed clinical protocol for combined PRP + PRF therapy. Each PLASMOO kit consists of two tubes for PRP and PRF extraction, as well as blood collection and injection preparation elements.
3.5. Clinical Stage
In the clinical stage of the study, blood drawn from each participant of the study was collected into two tubes (with and without coagulant). The processed preparations were then injected in the treatment area, previously disinfected (Skinsept, Ecolab) and anesthetized (Anesderm, Pierre Fabre Medicament), using the mesotherapy technique with 30 G/4 mm needles.
The preparations were centrifuged in a fixed-angle centrifuge (Neuation iFuge D06, eqlab, Poland) at 55 G for 4 minutes. This setting produced the highest concentration of PLT. The separated plasma fractions were drawn into syringes—1 mL of I-PRF from the tube without anticoagulant and 1 mL of C-PRP from the tube with anticoagulant. Both tubes were then centrifuged at 288 G for another 5 minutes, which allowed for maximum PLT recovery. During fraction II centrifugation, I-PRF and C-PRP from fraction I were injected into the lower eyelid (1 mL of I-PRF) and the forehead (1 mL of C-PRP) areas, respectively. Following these injections, fraction II preparations, PRP LLC and F-PRF, were administered via injections into the cheeks (3.5 mL of PRP LCC and F- PRF on each side). In total, each participant underwent three treatments with 4–6 weeks in between. Special care was taken, during the centrifugation protocol, to prevent any contamination of the biological material collected for injection. Decontamination of the centrifuge rotor and chamber was carried out after each use via appropriate biocidal products (Incidin Liquid Spray, Ecolab).
3.6. Outcome Measures
Results were recorded using high-frequency ultrasonography (device: DUB SkinScanner with a 22 MHz probe) to capture any skin tissue changes over time following treatment. This method allows for high resolution imaging (56 µm) of the epidermis, dermis, and subcutaneous tissues up to a depth of 16 mm. To observe and image any changes of interest along the three layers, we used a focus of 8 mm.
The following areas were evaluated during the ultrasound study: lateral frontal region, lower eyelid, and cheeks. The measurement was performed manually on the ultrasonographic image as an average from a rectangle of 2 mm2 area.
A series of acoustic density values was obtained and normalized to a color scale, which allowed for a comparative analysis of collagen and elastin fibers content in the dermis. We have also measured skin thickness (µm) as the sum of the thicknesses of the epidermis and dermis. The measurement was performed manually on the ultrasonographic image by calculating an average of triplicates in a given area. Measurements were collected before the first treatment and consecutively one month after each treatment.
Patients were identified by sequential numbers and randomly selected for examination during each treatment session. The aesthetic improvement of the treated area was assessed by the subjects one month after completing the full treatment regimen using the Global Aesthetic Improvement Scale (GAIS). GAIS is a scale of 0 = worse, 1 = no change, 2 = improved, 3 = much improved, and 4 = very much improved. The subjects were also asked to report their level of personal satisfaction with the treatment (very satisfied, satisfied, and not satisfied) after the treatment and whether they would recommend the treatment to their friends (yes or no).
3.7. Statistical Analysis
Statistical analysis was performed using the STATISTICA 13.5 PL package. Descriptive statistics (mean, standard deviation, minimum, and maximum) were calculated for the collected data at each time point. Changes over time were analyzed using repeated measures analysis of variance (ANOVA). In cases of significant effects, detailed comparisons were made using the Tukey’s post hoc test. Significant effects were also illustrated with box-and-whisker plots, and the power and effect size were calculated. The paired t-test was used to compare two related measurements. The assumptions of normality and sphericity were checked using the Shapiro–Wilk test and Mauchly’s test of sphericity. In all analyses, effects were considered significant if the probability level was less than the accepted significance level of p < 0.05.
4. Results
Data from all 20 enrolled participants (aged 30–60) were analyzed. Reported adverse events, not associated with the study agent, included redness (n = 20), swelling (n = 15), and bruising (n = 11) following injections. No participants reported any adverse events at 4 months posttreatment.
4.1. Centrifugation Protocol
Centrifugation parameters that produced the highest PLT concentration in the single centrifugation of whole blood were determined by testing the following parameters: 55 G/2 minutes, 55 G/3 minutes, 55 G/4 minutes, 72 G/3 minutes, and 288 G/5 minutes. The best results were obtained at 55 G/4 minutes. The average PLT concentration in the study group was 197%/µl (with the highest result of 208%/µl).
The corresponding fractions of PRP and PRF were assigned the following commercial names: tubes with anticoagulant (C-PRP) and without anticoagulant (I-PRF). To determine the optimal centrifugation parameters for maximum PLT recovery in the single centrifugation of whole blood, a series of measurements was carried out using the following parameters: 190 G/7 minutes, 210 G/6 minutes, 250 G/4 minutes, 250 G/5 minutes, 250 G/6 minutes, and 288 G/5 minutes. The optimal results were obtained at 288 G/5 minutes, producing an average PLT recovery of 44%. The corresponding fractions of PRP and PRF were assigned the following commercial names: tubes with anticoagulant (PRP LCC) and without anticoagulant (F-PRF). The double centrifugation procedure allowed obtaining the following four types of plasma fractions with different parameters: platelet-rich plasma (C-PRP and PRP LCC) and platelet-rich fibrin (I-PRF and F-PRF), each with two different platelet concentrations. The total PLT recovery from the procedure was 76%, with an average of 32% in the first centrifugation and that of 44% in the second one. The platelet concentrations were 202% in the first centrifugation and 148% in the second centrifugation.
4.2. Skin Density Results
In the ultrasound skin examination, a series of acoustic density values, directly proportional to skin density, was obtained. Skin thickness was also recorded (µm) as the sum of the epidermis and the dermis thickness. Measurements were collected in the lateral forehead, lower eyelids, and cheek regions.
Changes over time in the forehead area were measured using ANOVA (between treatment groups). The F-test result produced was significant (F(3, 24) = 9.2327 with p = 0.000305), which supports the observation of substantial changes in skin density over time during the treatment. The chart of averages is reported in the following graph (Table 1).
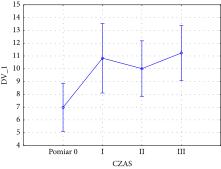 |
Furthermore, the analysis of power and effect size for the forehead area (partial Eta-squared: 0.54) reports the high impact of the therapy. Measurements I, II, and III (Table 1) significantly depart from the baseline, which indicates a gradual increase in skin density of the forehead area and thus demonstrates the effectiveness of C-PRP injections. Results in the lower eyelid area were recorded via acoustic density measurements and ANOVA (between treatment groups). The F-test result produced was not significant (F (3, 24) = 1.1549 with p = 0.3474), which indicates that I-PRF injection treatment of the lower eyelid skin does not result in substantial improvements between treatments. However, when a paired t-test analysis was used to analyze compare the baseline and the final treatment groups, a statistically significant result was produced (p = 0.0461). This result, therefore, supports a substantial change in lower eyelid skin density posttreatment completion, which is illustrated by the following graph (Table 2).
 |
Over time changes in the cheek area were recorded using acoustic density and ANOVA (between treatment groups). The F-test result produced was significant (F (3, 24) = 4.5170 with p = 0.011971), which supports the observation of substantial skin density increase during treatment regimen. The chart of averages is reported in the following graph (Table 3).
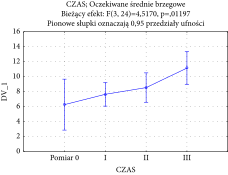 |
Substantial changes over time were analyzed using the Tukey’s post hoc test and revealed significant differences between individual measurements, which indicated systematic improvements in cheek skin density following PRP LCC and F-PRF treatments. Analysis of power and effect size (partial Eta-squared equals 0.36, and the power of analysis is 0.82) reports the high impact of such therapy. These results therefore confirm the effectiveness of PRP and PRF therapy in skin density increase, when applied in the regiment designed for this study.
4.3. Skin Thickness Results
ANOVA (between treatment groups) was used to analyze the forehead skin thickness results over time.
The F-test result produced was not significant (F (3, 6) = 2.383248 with p = 0.168172), which indicates no substantial changes in skin thickness over time. Furthermore, the paired t-test used to compare the baseline and the final treatment groups also showed lack of statistical significance (p = 0.0928).
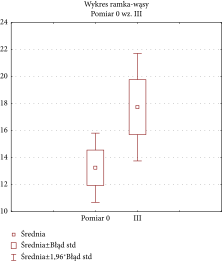
Similarly, no significant changes were observed in cheek skin thickness, as the F-test for changes over time was not significant (F (3, 6) = 1.438771 with p = 0.321616), which indicates no substantial changes in skin thickness over time. The paired t-test also showed a nonsignificant difference between group means (p = 0.08984) when comparing baseline and final treatment data. However, a statistically significant result was produced for the F-value for the lower eyelid skin thickness changes over time (F (3, 24) = 3.2653 with p = 0.038881), which supports the observation of substantial skin thickness increase during treatment regimen. The chart of averages is reported in the following graph (Table 4).
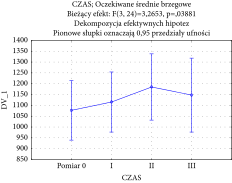 |
A paired t-test was also carried out on the baseline and final treatment groups and produced a statistically significant result (p = 0.048317). This result indicates a positive impact of I-PRF injections on skin thickness in the lower eyelid area.
4.4. Ultrasound Results
The ultrasound measurements of skin subjected to injections showed significant improvement of skin density in all treated areas, indicating the effectiveness of I-PRF, C-PRP, F-PRF, and PRP LCC preparations for this purpose. However, only I-PRF injections in the lower eyelid area showed a substantial improvement of skin thickness (Figure 2).
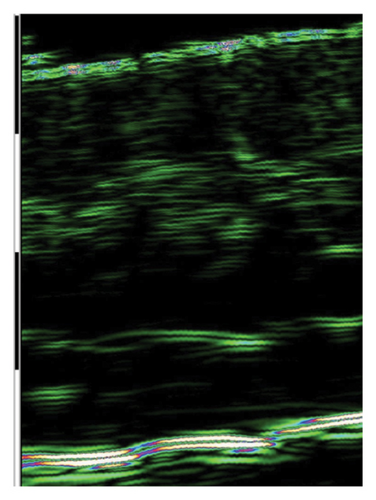
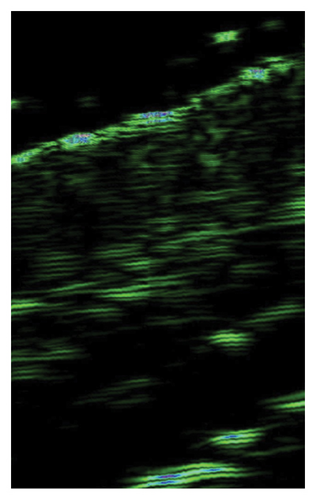
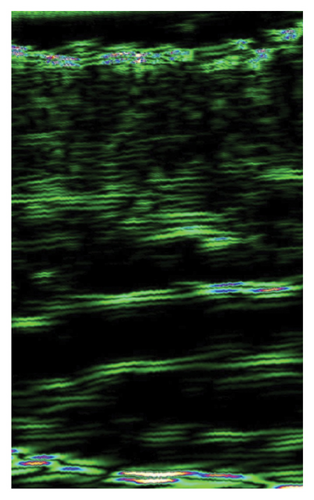
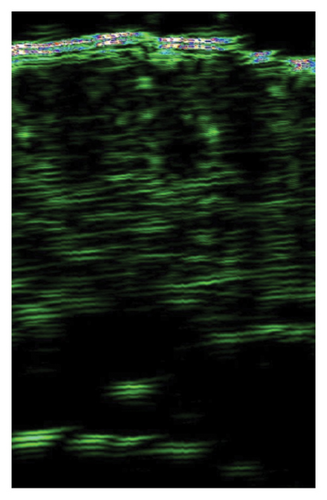
4.5. Survey Results
The analysis of survey results concerning the aesthetic effects of treatment and patient satisfaction indicates a high potential of the applied therapy. Patient satisfaction clearly improved (12 (60%) were “satisfied” and 8 (40%) were “very satisfied” with the effects) posttreatment, which was also confirmed by the “much improved” aesthetic result in the GAIS scale (average result of 2.75). All patients declared that they would recommend the treatment to their friends. Digital photographs of before/after show improvement of skin condition (Figures 3(a) and 3(b)), most significant in the lower eyelid area (Figure 4).
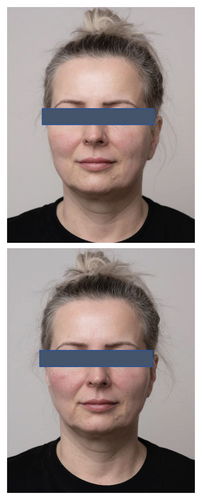
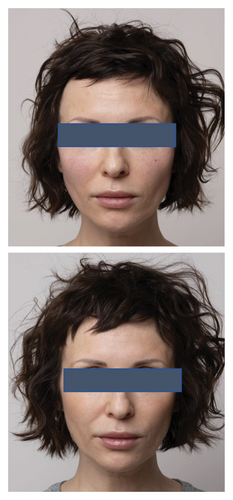
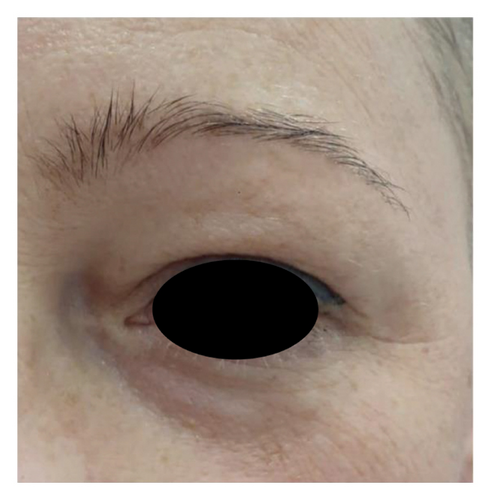
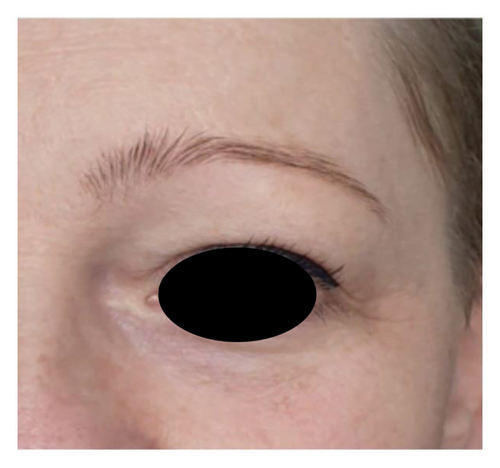
5. Discussion
Comparisons between therapeutic efficacy of different plasma or platelet-rich fibrin variants are impossible due to biological activity differences of preparations obtained in distinct preparation protocols [12]. Over the years, several classifications have been proposed to structure this area of investigation [13–15].
In 2016, Magalon [16] introduced the DEPA system, criticizing predecessors for lack of consideration of final volume of the product obtained, platelet recovery, and erythrocyte contamination in the preparation. The DEPA system is based on an assessment of platelet quantity, their recovery, and the number of erythrocytes in relation to the quantity of platelets and leukocytes. The authors of this publication fully agree with the assumptions of the DEPA system.
According to DEPA, platelet recovery in tested systems ranges from 13.1% to 79.3%, none of which achieved high efficiency, i.e., above 90%, which indicates that PLASMOO procedure results in a high-quality preparation. In this work, double centrifugation of the preparations produces a total volume of approximately 9-10 mL of final product. The total PLT recovery in the procedure is 76% (an average of 32% in the first centrifugation, and that of 44% in the second one). Platelet concentrations of individual PLT fractions vs whole blood were reordered at 202% in the first centrifugation, and at 148% in the second centrifugation.
Over the span of several decades, many works [17–19] based on different protocols for preparing blood-derived products have been published, without reaching a clear conclusion about the optimal method. This is due not only to individual differences in blood parameters among different individuals (including-the amount of erythrocytes, platelets, lymphocytes, hematocrit level) but also to the use of different systems - with or without an anticoagulant, with or without an activator, with centrifugation at a reduced temperature, using single or double centrifugation, as well as the volume of blood processed, speed, duration of centrifugation, and the number of centrifugations.
From the above analysis, it follows that different preparation protocols are used to obtain various fractions of platelet-rich plasma. Many authors [20, 21] point out the higher quality of PRP preparations prepared using the double centrifugation method, including the American Association of Blood Banks Technical Manual. Tamimi et al. and Harrison et al. [19, 22] are also of the same opinion, indicating that double centrifugation allows for higher platelet concentration. Mazzucco et al., [21] previously cited, after examining four commonly used methods of preparing PRP, demonstrated that single centrifugation results in significantly lower concentrations of growth factors and platelets in the preparation than double centrifugation. In 2021 the Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) issued guidelines [20] based on the analysis of 45 articles regarding the preparation of PRP preparations, also recommending the double centrifugation method.
Numerous in vitro studies [23–27] confirm the impact of PRP and PRF on fibroblasts. demonstrating that high concentrations of PRP induce an increase in the expression of G1 cell cycle regulators, an increase in type I collagen expression, and MMP-1 metalloproteinases, thus accelerating the wound healing process. The use of PRP is associated with the release of large amounts of PDGF-AB and TGF-beta1 and the addition of activated platelet-rich or platelet-poor plasma significantly favors the proliferation of human stem cells derived from adipose tissue and human skin fibroblasts. These results confirm the possibility of clinical application of platelet-rich plasma in cell engineering based on soft tissues and wound healing.
Despite the extensive literature on the cellular-level effects of PRP, its molecular mechanism of action is not fully understood. In a 2023 study, Manole [28] emphasizes the potential role of telocytes in the mechanism of wound healing and skin regeneration through the stimulation of neoangiogenesis following PRP injection, thus indicating a new area for research on the mechanism of PRP action.
Wang et al. [29] compared the in vitro effects of PRP and liquid PRF, demonstrating the superiority of PRF. Although both PRP and liquid PRF significantly induced the level of cellular PDGF mRNA, it was observed that mRNA levels of TGF-beta, collagen 1, and fibronectin were significantly highest in the liquid PRF group. Liquid PRF showed a significantly greater ability to induce collagen matrix synthesis compared to PRP, which is consistent with the findings of the present study, as in area of I-PRF application, an increase in both the density and thickness of the skin was demonstrated.
Buzalaf and Levy [30], based on the analysis of 100 available literature items, indicate that second and third generation products, platelet-rich fibrin (PRF) and injectable platelet-rich fibrin (i-PRF), seem to induce greater collagen production than PRP, especially under lower relative centrifugation forces. However, they also emphasize that when planning studies using PRP, one should consider patients’ characteristics, the optimal number of sessions, and the development of more effective instruments to evaluate skin aging. Phoebe et al. [31] also recommend the appropriate selection of patients for PRP treatments based on physical and biometric data. In their study, PRP treatment led to improvements in skin pore size, texture, wrinkle reduction, pigmented spots, and collagen density after one to three PRP sessions. Additionally, the authors concluded that combining PRP with hyaluronic acid enhanced skin elasticity in patients with lower BMI and skin firmness in individuals in their 50 s and 60 s.
In another effective application of PRF, it is used in periodontology and dental surgery. In a systematic review [32] published in 2021, Fernandes et al. highlight that second-generation PRF (membranes) can be a substitute biomaterial for treating gingival recessions due to their ease of use, low cost, good mechanical properties, and stimulation of tissue healing, especially in the early postoperative period. They note that the three-dimensional scaffold created by PRF serves as a structure that allows for the continuous release of growth factors and cytokines for up to 10 days. Additionally, PRF influences the release of alkaline phosphatase. In another study within the field of dentistry [33], the authors emphasize the potential impact of PRF as a bioactive material on osseointegration, which can shorten the postoperative rehabilitation period.
In the area of aesthetic medicine there are only few publications showing the clinical effects of PRP/PRF products, taking into account objective and instrumental evaluation parameters [34]. One such study is the publication by Alam et al. [35] where the authors attempted to answer whether PRP injections are effective in improving the quality of photo-damaged skin. It was a randomized, blinded clinical trial. Primarily, the effect of PRP on signs of photoaging of the skin (fine wrinkles, pigmentation, roughness, and skin color) was assessed. Secondary outcomes included participant self-assessment of skin quality improvement and overall participant satisfaction. The results of this study suggest that PRP may temporarily improve the appearance of photoaged skin. The improvement in skin condition is subtle and difficult to detect for external evaluators based on photographs. Participants noticed the differences more clearly because they knew their faces well and had time for detailed observation of changes. Interestingly, in studies assessing the effectiveness of PRP in skin bioregeneration, patient observations are much more positive than those made by researchers [36].
In another study, Everts et al. [37] used biometric instrumental evaluations and patient-reported outcomes to support the antiaging effects. A series of 3 PRP injections at 6-month follow-up resulted in significant skin rejuvenation. The study used, among other tools, a chromameter and a cutometer to measure skin thickness, showing a significant decrease in brown spot counts and area after 3 months. Wrinkle count and volume were significantly reduced as was skin firmness.
Different types of instrumental measurements were used in the study by Du and Lei [38]. In a group of 30 patients, 3 PRP injections were performed at 15-day intervals. The effects of PRP injections were evaluated using the VISIA® Complexion Analysis System and skin computed tomography. In addition, a human organotypic skin model was used and treated with PBS or PRP before (UV)-B light irradiation. It was demonstrated that PRP treatment ameliorated skin photoaging through the regulation of metalloproteinase 1, tyrosinase, fibrillin, and tropoelastin expression.
An objective instrumental way to assess skin quality is the use of high-frequency ultrasound and the assessment of skin density and thickness, as well as their changes over time [39]. In the study [40] assessing the impact of PRF on improving skin quality, a high-frequency ultrasound device was used to measure skin density. Skin density analysis was performed before the administration of PRF, one month after the second and one month after the third mesotherapy treatment in the periocular area. The results showed an increase in skin density in all patients after just one treatment. The author concluded that I-PRF was a promising treatment modality for skin rejuvenation in the periorbital area, and PRF products can be used as an antiaging treatment and for improving skin tone and texture. These results correspond with our findings. In another study [41] using autologous plasma gel, the authors showed an improvement in skin density as well as a reduction in the depth of nasolabial folds using ultrasound to measure skin density and the depth of wrinkles.
There are also numerous publications using other than instrumental outcome measures such as the Wrinkle Severity Rating Scale (WSRS) and the Global Aesthetic Improvement Scale (GAIS) to evaluate PRP effect on skin condition and reducing wrinkles’ depth which demonstrate significant posttreatment improvement [42–45]. In a controlled placebo clinical trial by Abuaf et al. [46], the aim was to examine the impact of PRP on skin rejuvenation (and collagen changes) through histological analysis of skin collagen. PRP was injected into the upper part of the right infraauricular area and the entire face. Saline was injected into the left infraauricular area. On the 28th day after the injection of PRP and saline, a punch biopsy was performed in the PRP treated and control areas. The histological study measured the mean optical densities (MODs) of collagen in the control and PRP-treated areas. In the PRP MOD, an improvement of 89.05% was observed compared to before treatment. The mean MOD of collagen fibers was significantly highest on the PRP side (p < 0.001). The ratio of PRP improvement to saline control was 89.05%–46.01%, confirming the thesis that PRP increases the level of collagen in the skin and can be considered an effective (even with a single application) and safe procedure for facial skin rejuvenation. This result corresponds with our work and confirms PRP efficacy in skin density improvement. In our study, ultrasound measurements of the skin areas subjected to injections indicate a significant improvement in skin density in all areas treated with injections, suggesting the effectiveness of I-PRF, CPRP, F-PRF, and PRP LCC in this indication. However, only I-PRF injections in the periorbital skin area showed an impact on improving skin thickness, which corresponds with the findings of the earlier cited publication [39]. Results showing improvement in both density and thickness of the skin in the periorbital area indicate that liquid I-PRF has higher stimulation efficacy than PRP, which is consistent with the previously cited study results [29]. In our study, a statistically significant improvement in the thickness of the lower eyelid skin was observed after repeated I-PRF injections, while the thickness of the skin in other studied areas did not change. The I-PRF fraction was obtained after centrifuging whole blood, without anticoagulant, using parameters of 55G for 4 minutes. This corresponds to the slow centrifugation concept presented by other authors [11]. Miron et al. showed that both PRP and I-PRF stimulate migration and proliferation of fibroblasts, but I-PRF caused a significant increase in mRNA levels for TGF-β on the 7th day after administration, an increase in type I collagen expression after 3 and 7 days, and an increase in PDGF levels after 3 days compared to PRP. The authors demonstrated that PRP was responsible for the rapid release of growth factors after administration, while I-PRF provided long-term (up to 10 days) release of IGF-1, EGF, PDGF-AA, and PDGF-AB, which may explain the impact of this preparation on improving skin thickness, compared to the action of PRP. This is also consistent with the findings of Kobayashi et al. [47], who found that different platelet concentrates have different kinetics and undoubtedly, PRF causes continuous and stable release of growth factors and an increase in collagen production to a greater extent than PRP. The concept presented in this work of optimizing the procedure for obtaining PRP and PRF with only one blood collection from the patient significantly simplifies and shortens the duration of the procedure, while maintaining its effectiveness, as objectively confirmed by the results of skin ultrasound studies.
Strengths of the study are as follows: use of objective measurement techniques: the study utilized skin ultrasound examinations, which provided objective and quantifiable data on the effects of the PRP/PRF treatments and enhanced the reliability and validity of the results; benchmarking against established research: the study was designed with reference to existing research, specifically the work by Alam et al. [34], which ensures that the study methods and results are comparable to established standards in the field; practical application in aesthetic clinics: the use of fixed-angle centrifuges, common in aesthetic medicine clinics, ensures that the procedures developed can be easily implemented in a wide range of settings, increasing the practical applicability of the study findings; and relatively long observation period: the study maintained a 4-month observation period, which is considerable compared to other studies in the field, providing meaningful insights into the longer-term effects of the treatments.
There is, however, a number of limitations of this study such as small sample size, with only 20 participants, the study’s sample size is limited. A larger sample size would increase the statistical power of the study and the generalizability of the findings. Another limitation is the 4-month observation period, which, while relatively long, is shorter than ideal for fully understanding the long-term effects of PRP/PRF treatments. Longer follow-up periods (years) would provide more comprehensive data. Using fixed-angle centrifuges might be considered another limitation of the study; the exclusive use of fixed-angle centrifuges, while practical for widespread application, may have resulted in a lower concentration of platelets compared to horizontal centrifuges, potentially affecting the efficacy of the PRP/PRF treatments. The study design and duration were influenced by the limitations of the research and development grant, which restricted the ability to extend the study period and increase the number of participants.
6. Conclusion
There is still no consensus regarding the most effective methods for preparing PRP. The results obtained by many authors vary although they indicate an improvement in skin quality as a result of PRP and PRF injections. The proposed preparation protocol confirms the effectiveness of PRP and PRF in improving skin density and thickness in specific facial areas. Ultrasound measurements and statistical analysis revealed a significant increase in skin density of the cheek and forehead areas, as well as in density and thickness of the lower eyelid skin, indicating the effectiveness of these treatments in enhancing skin quality. A significant aspect of the study is the use of ready-to-use PLASMOO kits (Innmedis, Poland), which greatly simplify the treatment process. The high levels of patient satisfaction, as reflected in the Global Aesthetic Improvement Scale and the willingness of participants to recommend the treatment, further affirm the positive outcomes. This approach offers a promising direction for future treatments in aesthetic medicine, enhancing both the effectiveness and the convenience of such therapies.
Ethical Approval
The authors declare that all procedures were performed in adherence to the Declaration of Helsinki, in accordance with regional laws and good clinical practice for studies in human subjects. Study design was reviewed and accepted by the Bioethics Committee of the Jagiellonian University in Krakow (approval number 1072.6121.11.2020).
Consent
All patients have signed a consent form for the procedure and for the publication of their image.
Disclosure
The study is listed on the ISRCTN registry with study registration number ISRCTN10538865.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
L.M. and J.K. conceptualized the study; L.M. wrote the original draft; K.D. performed statistical analysis, and L.M. and K.D reviewed and edited the study. All the authors have read and agreed to the published version of the manuscript. All the authors gave their final approval and agree to be accountable for all aspects of the work.
Acknowledgments
This research received external funding from Małopolskie Centrum Przedsiębiorczości, Krakow, Poland. Innovation voucher titled Purchase of R&D work necessary for the launch of innovative aesthetic medicine services for synergistic therapies, application number PRMP.01.02.03-12-0296/19.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.