Curcumin as a Supportive Therapy in Type 2 Diabetic Patients
Abstract
Type 2 diabetes mellitus (T2DM) is a growing public health concern. The management of diabetes is a lifelong process that requires diabetic education, attempts to achieve near optimal glycemic control, minimizing cardiovascular and other long-term risk factors, and avoidance of drugs that can exacerbate abnormalities of insulin and lipid metabolism. In the treatment of T2DM, curcumin has demonstrated much potential as an adjunct therapy due to its anti-inflammatory properties and its ability to reduce oxidative stresses within the body. However, the low bioavailability poses limitations to its therapeutic potential. In this paper, we discuss the benefits of curcumin in a healthy diet, its potential and limitations as a supportive therapy. The Ayurvedic perspective of curcumin in diabetes is also discussed. Mechanisms for increasing the bioavailability of the supplement is discussed. Also, epigenetic regulation of type 2 diabetes is discussed, especially the role of DNA methylation.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a rapidly growing public and global health concern. T2DM results when the body cannot properly utilize the glucose from food for cellular function [1]. The consequences of a buildup of glucose within the bloodstream often indicate insulin resistance and occur due to physical inactivity, obesity, or genetics. Recently, epigenetic mechanisms, mainly through aberrant DNA methylation and imbalance of histone modifications, are clear in pathogenesis of diabetes [2]. In most cases, T2DM is often preventable or potentially reversible in the early stages with proper diet and lifestyle modifications. Curcumin is a polyphenol that has been demonstrated to alleviate many common T2DM symptoms, such as inflammation and reactive oxidative stresses within the body [1]. The Ayurvedic perspective of curcumin in diabetes is also discussed. However, the compound’s low bioavailability and absorption pose clinical limitations to its treatment potential. Here, we review the effects of curcumin on T2DM as a potential supplement alone or in combination with other supplements and pharmacological agents alongside healthy lifestyle modifications.
2. Search
Strategy: initially, PubMed and Google Scholar databases were searched. The following keywords were used: “Type 2 diabetes mellitus,” “T2DM,” “Curcumin,” “Turmeric,” “Bioavailability,” and “Clinical trials of curcumin and diabetes.” The existing studies on the bioavailability of curcumin and studies relating to the reduction of common symptoms of T2DM were evaluated. We also searched articles published in journals such as Nature Medicine, Biomedicines, Phytotherapy Research, Diabetes Care, Scientific Reports, Nutrients, Frontiers in Medicine, etc. We revised the information in the searched articles by checking similar articles in other journals indexed in PubMed and Google Scholar. If the accuracy of information was not verifiable, then such articles were excluded from further consideration.
3. Inflammation, Oxidative Stress, and Type II Diabetes
T2DM is recognized as being one of the leading public health concerns. In the United States, 85.2% of the population with T2DM are overweight and obese [3]. Based on the National Diabetes Statistics Report, age-adjusted T2DM incidence aged 18 years or older was 6.6 per 1000, which dropped to 5.9 per 1000 (95% CI: 5.0–6.9) in 2018–2019 or 1.4 million new cases of diabetes. T2DM is defined as having chronically elevated blood glucose levels that augment the inflammatory and oxidative states and cause dyslipidemia. If not addressed correctly in the early stages of the disease, progressions can eventually result in other complications like diabetic retinopathy or diabetic neuropathy [1]. Studies have shown a correlation between poorly controlled T2DM and heart disease [1]. In addition, studies have also shown an association between inflammatory biomarkers and the occurrences of this disease and its complications. Pro-inflammatory cytokines, like TNF-α, can activate several intracellular signaling molecules, such as a JNK and IKK-β, that are critical components of the inflammatory signaling system, leading to impaired insulin action [4]. Oxidative stress has been known to play a pivotal role in the development of diabetes complications both at the microvascular and cardiovascular levels. Free radicals are formed disproportionately in diabetes by glucose oxidation, nonenzymatic glycation of proteins, and the subsequent oxidative degradation of glycated proteins [5]. Recent evidence suggests that the cellular reduction–oxidation imbalance notable in diabetic patients leads to oxidative stress and subsequent occurrence and development of diabetes and related complications by regulating specific signaling pathways involved in β-cell dysfunction and insulin resistance. Reactive oxygen species (ROS) can also directly oxidize specific proteins (defined as redox modification) involved in the diabetes process [6]. Increasing exercise and incorporating a low carbohydrate diethave been proven beneficial in alleviating many of the symptoms, including inflammation [7]. Currently, there are many treatments available for treating T2DM in individuals who are genetically and environmentally predisposed to this disease [7].
Many pharmaceutical medications have been introduced to treat T2DM, such as SGLT2 (sodium–glucose cotransporter-2) inhibitors, biguanides (e.g., metformin), and sulfonylurea. Despite the availability of these antidiabetics, most subjects do not achieve optimal levels of glucose control. Additionally, blood glucose control per se is not always sufficient to prevent the long-term complications of diabetes. Studies have shown that, except for metformin, most antidiabetics do not delay the progression of diabetes [8]. This research highlights the importance of using supplements in conjunction with antidiabetics to improve outcomes. A growing body of literature has shown the effect of curcumin on different features of metabolic syndrome [9]. Due to their anti-inflammatory and antioxidant properties, turmeric and its bioactive ingredient, curcumin, exhibit antidiabetic effects by increasing insulin release [9]. Turmeric has been shown to decrease the phosphorylation level of insulin and inhibit the activation of the NF-κB [9]. Hence, curcumin might possess therapeutic potential in delaying the progression or reducing the oxidative stress that happens in T2DM [10]. One could hypothesize that curcumin could exhibit a synergistic effect in delaying the disease progression in conjunction with antidiabetic medications currently available.
4. Curcumin: What Is It?
The spice turmeric, derived from the root of the Curcuma longa, has been described as a treatment for diabetes in Ayurvedic and traditional Chinese medicine for thousands of years. The turmeric root is related to the ginger family. It has been used to treat various conditions and disease states, from upper respiratory illnesses, arthritis, and diabetes to various skin conditions [7]. Studies have shown that it reduces inflammation by regulating the secretion of inflammatory cytokines through protein and enzyme signaling [11], as well as preventing the formation of ROS by utilizing its antioxidant properties, which may be effective in regulating diabetes. Studies in rodent models have shown that curcumin effectively reduces hyperglycemia and hyperlipidemia [12–14]. Although there are turmeric supplements available that consist of a mixture of curcuminoids which possess many beneficial biological activities, the low bioavailability, adsorption and metabolism of the compound, along with its relatively low stability, prevents it from being a singular reliable treatment despite its potential [15].
5. How Is Curcumin Metabolized?
Some research has also been conducted on maximizing curcumin’s absorption by pairing it with other common ingredients. For example, pairing the spice with a source of fat like avocados and oils like Ghee (clarified butter) and other spices like black pepper would also assist in augmenting absorption [16, 17]. Some of these polyphenols can bypass liver metabolism by pairing it with fats. A few published studies have demonstrated that curcumin’s absorption increased manifold after giving curcumin with black pepper in humans [16, 17]. In that same study conducted with rats, curcumin absorption increased by 154% after about 1–2 h with the addition of piperine [16, 17]. Studies have shown that curcumin is lipophilic and attaches to fats and oils very quickly, which may also help slow the degradation of the molecule once it is in the bloodstream. Although some research has been conducted regarding the other methods of drug transport, such as intravenous, subcutaneous, or nasal, to increase bioavailability in tissues, these studies have been conducted minimalistically in laboratory animals [16, 17].
Curcumin is typically metabolized in the liver, where enterocytes and hepatocytes break it down via enzyme reductase. From there, it is broken down further into dihydrocurcumin, tetrahydrocurcumin, hexa-hydrocurcumin, and octahydrocurcumin (Figure 1) [18]. Curcumin can help reduce inflammation, and a dry culture of lactic acid bacteria, commonly found in probiotics, can help metabolism. Together, these two things, along with a change in diet and exercise, may help prevent T2DM [19].
6. Supporting Role of Curcumin in T2DM
A clinical study was done in Mumbai, India, with 50 people who had advanced type 2 diabetes and whose condition was not getting better with standard medical treatment. The study looked at whether a herbal supplement with curcumin, grape seed, Indian gooseberry and fenugreek would help with diabetes management along with standard treatment (metformin and sulfonylurea). This study’s results were based on glycated hemoglobin (HbA1C) and blood sugar levels. It was determined that this herbal supplement, paired with prescribed diabetes medication such as metformin and sulfonylurea, reduced from 8.8%/73% to 7.5%/58% mmol/ml (p < 0.001). This resulted in an average glycated hemoglobin value. The postprandial blood sugar values also decreased from average glycated hemoglobin value 14.6–10.4 mmol/l of blood [20]. The role of curcumin as a supportive therapy in T2DM is presented in Figure 1.
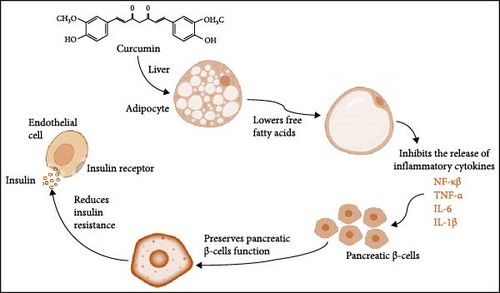
In a double-blind, randomized clinical trial, 44 patients were given 1500 mg of curcumin or a placebo group to take daily for 10 weeks. Triglycerides (TGs), cholesterol levels, C-reactive protein (a significant inflammatory marker for T2DM), and adiponectin serum levels were measured at the beginning and end of the study after a 12-h fasting. It was determined that the TG levels (mg/dl) in the curcumin group decreased from 124 ± 36 to 109 ± 36 (p < 0.03) while the decrease in the control group was not statistically significant (126 ± 52 to 121 ± 44, p = 0.731). Delta changes between the two groups were not statistically significant (p = 0.40).
The mean serum concentration of adiponectin decreased in the curcumin group with a baseline of 64 ± 3 to 63 ± 4 (p < 0.05) [11]. The study results indicate that curcumin consumption may reduce diabetic symptoms and complications by reducing TG/fat levels and inflammation [11]. Although current medications are available to treat diabetes, curcumin alone is insufficient as a stand-alone treatment. Regular consumption of this herb could provide many long-term benefits to individuals at risk for developing T2DM due to its anti-inflammatory and ROS-minimizing effects on the body. Such a formulation must be tested in a properly designed clinical study with or without different T2DM drugs. However, it is essential to note that consuming about 30–90 mg/kg of body-weight curcumin per day for about a month may potentially induce a synergistic hypoglycemic effect if taken with other blood sugar medications. Thus, it is crucial to monitor turmeric consumption if taking blood sugar medications like metformin [21]. In a study conducted with diabetic mice, it was determined that both curcumin and metformin separately were able to decrease glucose, TG, and cholesterol levels while simultaneously increasing PON 1 (Paraoxinase 1) levels, as well as indicating that these two substances could work synergistically to combat the cardiovascular events that result from diabetes [21]. PON 1 levels are essential because they provide antioxidant properties in and helps to minimize inflammation [22].
A randomized, double-blind placebo-control add-on clinical trial (N = 80) by Rahimi et al. [23] also showed that curcumin, administered orally (80 mg/day in nanomicelles) for 3 months, improved the lipid profile and decreased fasting blood glucose, glycosylated hemoglobin, and body mass index (BMI). The authors of this study pointed out that “all other necessary medications were given to subjects” (add-on therapy), without specifying the kind of drug they used; therefore, it is not possible to know the influence of that medication on curcumin pharmacokinetics or on the observed effects. In another study, at a dose of 500 mg/day for 15 or 30 days, curcumin did not produce glucose-lowering effects nor changes in lipid profile in patients that were also under treatment with one or more of the following drugs: insulin, metformin, rosiglitazone, glibenclamide, gliclazide, acarbose, or aspirin, except for the diminished concentration of LDL cholesterol. This study had 59 patients on curcumin and 58 patients on placebo. The possibilities in this last case could be (a) effects on lipid profile were not so evident because the dose in this study was smaller than that used in other studies where improvement of lipid profile was observed; for instance, the one by Panahi et al. [24]; (b) curcumin was not protective against degradation as in the study by Rahimi et al. [23]; or (c) curcumin effects are negligible compared with the antidiabetic drugs the patients were taking.
Na et al. [25] administered curcumin at a dose of 300 mg/day to overweight/obese type 2 diabetic mellitus patients, and they found that the treatment reduced BMI, fasting blood glucose, glycosylated hemoglobin, insulin resistance index (HOMA-IR), and free fatty acids [25]. It is worth mentioning that the decrease in glucose was 18% and that of glycosylated hemoglobin was 11% compared to baseline within the group. The same group later found that the reduction in free fatty acid levels could be due to curcumin diminishes adipocyte-fatty acid binding protein (A-FABP), an adipokine secreted from adipocyte [26] and other tissues that coordinate lipid-mediated processes [27]. Recent advancements and clinical trials of curcumin as a supportive therapy for T2DM are presented in Table 1.
| S. no. | Study | Outcome | Reference |
|---|---|---|---|
| 1 | Assessed the efficacy of curcumin in delaying development of T2DM in the prediabetic population | After 9 months of treatment, 16.4% of subjects in the placebo group were diagnosed with T2DM, whereas none were diagnosed with the disease in the curcumin-treated group | [28] |
| 2 | Assessed the possible beneficial effects of curcumin capsules as lipid-lowering effects and as a permeability glycoprotein (P-gp) inhibitor on the pharmacokinetics and pharmacodynamics of glyburide and as a P-gp substrate with glyburide in patients with type-2 diabetes mellitus | Coadministration of curcumin capsules with glyburide may be beneficial to the patients in better glycaemic control. The lipid lowering and antidiabetic properties of the curcumin show as a potential future drug molecule | [16] |
| 3 | Evaluated the effects of curcumin and/or long-chain omega-3 polyunsaturated fatty acids on improving glycosylated hemoglobin as a primary outcome, along with secondary outcomes of glycaemic indices, lipid profile and inflammatory parameters | Determined whether curcumin and/or long-chain omega-3 polyunsaturated fatty acids affect surrogate markers of glycaemic control which is relevant to delaying T2D | [29] |
| 4 | Evaluated the effects of a single dose of curcumin and/or fish oil on postprandial glycaemic parameters in healthy individuals | Curcumin, but not fish oil, reduces postprandial glycaemic response and insulin demand for glucose control | [30] |
| 5 | Investigated the effect of a curcumin–phospholipid lecithin formulation (Meriva) on visual acuity and OCT retinal thickness in patients with chronic diabetic macular edema | Curcumin–phospholipid formulation (Meriva), administered as Norflo tablets, may be feasible in the improvement of visual acuity and reduction of macular edema in patients with diabetic retinopathy | [31] |
| 6 | Studied that curcumin consumption may reduce diabetes complications through decreasing TG level as well as indicators of inflammation | At the end of study, the mean concentration of high-sensitivity C-reactive protein decreased and mean serum concentration of adiponectin increased in the curcumin group compared to the control | [11] |
| 7 | Evaluated the effects of curcumin or long-chain omega-3 polyunsaturated fatty acids (LCn-3PUFA) supplementation on glycaemic control and blood lipid levels in individuals at high risk of developing T2D | Reduction in insulin resistance and triglycerides by curcumin and LCn-3PUFA appears to be attractive strategies for lowering the risk of developing T2D | [32] |
| 8 | Examined the effects of Theracurmin, a highly absorbable curcumin preparation, on glucose tolerance, adipocytokines, and oxidized LDL, we conducted a double-blind placebo-controlled parallel group randomized trial in patients with impaired glucose tolerance or noninsulin-dependent diabetes mellitus | Study suggests that the highly absorbable curcumin could potentially inhibit a rise in oxidized LDL in patients with impaired glucose tolerance or noninsulin-dependent diabetes mellitus | [33] |
| 9 | Investigated the effects of dietary supplementation with curcumin on key peptides implicated in insulin resistance in individuals with high risk of developing T2D | Dietary supplementation with curcumin reduced circulating levels of IAPP and GSK-3β, thus suggesting a novel mechanism through which curcumin could potentially be used for alleviating insulin resistance related markers for reducing the risk of T2DM | [34] |
| 10 | Assessed the effects of curcumin intake on psychological status, markers of inflammation, and oxidative damage in patients with T2DM | Curcumin intake for 12 weeks in patients with T2DM and CHD had beneficial effects on PSQI, TAC, GSH, MDA values, and gene expression of PPAR-γ | [35] |
| 11 | Tested the efficacy of nanocurcumin supplementation on depression, anxiety, and stress in patients with diabetic polyneuropathy | Suggested that nanocurcumin supplementation for 8 weeks was effective in reducing depression and anxiety scores in patients with diabetic polyneuropathy | [36] |
| 12 | Studied the management of prediabetes with curcumin and zinc to prevent type-2 diabetes | Zinc and curcumin supplementation exerted a beneficial effect on several key glycemic parameters | [37] |
| 13 | Evaluated the impact of nanocurcumin on cardiovascular risk factors in type 2 diabetic patients with mild to moderate CAD | Nanocurcumin (80 mg/day) might prevent atherosclerosis progression and, in terms of attenuating hs-CRP levels as an inflammation index, succedent cardiovascular events in diabetic heart patients | [38] |
- Abbreviations: CAD, coronary artery disease; OCT, optical coherence tomography; T2DM, type 2 diabetes mellitus; TG, triglyceride.
7. Ayurveda Perspective
Prameha is included among Ashta maha gada (eight major diseases) in Brihattrayi (three major books of Ayurveda). Madhumeha (diabetes mellitus) is the advanced stage of Prameha [39]. Hence, diabetes has been a disease since the dawn of civilization, that is, more than 3000 years ago. Various medicinal plants were used to treat and control T2DM. All three major Ayurveda books, viz., Charak Samhita, Sushrut Samhita, and Ashtanga Hridayam have used Harida (Turmeric) and Amalaki (Indian gooseberry) for diabetes [40–42]. The preferred treatment for T2DM in Ayurdvedic medicine is to use turmeric powder mixed with equal quantity of Amalaki powder. Turmeric can also be used by mixing it with Vijaysara (Pterocarpus marsupium) powder. Turmeric, when used alone, can cause hyperacidity or gastric ulcers in some patients due to its ushna guna (hot property) and ushna veerya (hot potency). At the same time, Amalaki (Indian gooseberry) is sheeta Veerya (cold potency). So, the combination of turmeric and Indian gooseberry powders in equal quantities can be absorbed by the body and give the desired results. Since haridra (turmeric) is of Ruksha Guna (dry), it is advised to use it with some fat medium like Ghrita (clarified butter). It is interesting and worth mentioning here that almost all the classical texts of Ayurveda have recommended haridra in Prameha and Madhumeha (T2DM) and Tvak roga (Skin diseases). Charaka Samhita and Ashtanga Hridaya have mentioned haridra (turmeric) as Rasayana (Rejuvenating and immunity modulating) while administering with Madhu (Honey) and Ghrita (Clarified butter).
- a.
Vijaysara (P. marsupium or Malabar kino) wood glass soaked in water overnight,
- b.
water kept in copper glass overnight,
- c.
mixed with Amalaki juice (Indian gooseberry),
- d.
mixed with giloy juice (Tinospora cordifolia),
- e.
mixed with Triphala kwath or juice (decoction or juice of three myrobalans), and
- f.
mixed with lukewarm cow milk or goat milk, etc.
Therefore, haridra with specific Anupana must be considered as a therapeutic or supplemental agent for better assimilation and lowereing blood sugar levels in T2DM patients.
8. Adverse Effects of T2DM Disease State
To better understand curcumin’s potential alleviation or preventative effect, it is essential to examine the adverse effects of T2DM. In general, T2DM decreases the secretion of adiponectin, a fat cell-derived protein that regulates insulin resistance via the glucose transporter 4 (GLUT4) translocation and transport [1]. Adiponectin is essential because it helps to reduce inflammation and fatty deposits within the arteries. Adiponectin is vital because an increase in fat deposits within the arteries increases the risk of cardiovascular disease and other life-threatening states, including the development of clogged arteries and even organ failure [18]. Although it is most common in middle-aged individuals, there has been a rise in reported cases due to the sedentary lifestyle and high-caloric and sugar-based diets [18]. It is also important to note that other pre-existing conditions can also make someone more susceptible to this disease, including hyperinsulinemia or dyslipidemia. An increase in ROS is frequently the cause of type 2 diabetes complications. These can then start intracellular pathways that make pro-inflammatory cytokines stick to cells and change how proteins are expressed in the body, damaging blood vessels and organs. Damage can cause poor circulation in blood vessels, which can lead to diabetic foot ulcers and sometimes osteomyelitis, which can require a sustained course of intravenous antibiotics and sometimes even amputation of the extremities. T2DM is also known to cause diabetic retinopathy, neuropathy, and sometimes decreased urine output, leading to slowly progressing diabetic nephropathy [18].
9. Epigenetic Regulation of T2DM
Epigenetics refers to how external factors such as environment, lifestyle, and diet can contribute to the expression of specific gene activities. Currently, DNA methylation is the most studied regarding epigenetic changes that increase the risk of developing cardiovascular diseases, heart failure, and T2DM disease progression [43]. It has been noted that DNA methylation can be reverted through simple dietary modifications such as adding folic acid, vitamin B6 and B12 [44], which is also often recommended for those at risk for developing T2DM. On the other hand, long-term treatment of metformin, and SGLT2i inhibitors that is commonly used to treat T2DM, has been associated with reducing the risk of incidence of heart failure and delaying the progression of diabetes. Although there have not been any results published, metformin is contraindicated for patients with suspected heart failure. However, recent evidence suggests that the epigenetic interference benefits from metformin and other SGLT2i medications are most prevalent in tissue levels of β-hydroxybutyrate which are associated with the beneficial effects of fasting state [43].
Because most epigenetic changes result from lifestyle and diet, dietary supplements have emerged to assist in adapting these dietary modifications. One of these supplements includes curcumin which has been demonstrated to reduce inflammation and works as an epigenetic modulator in several diseases including T2DM [45]. In a recent study, conducted by Boyanapalli and Kong [45], it was determined that the induction and suppression of several genes related to cell regulation were identified after the administration of curcumin to mice liver and small intestine. In relation to DNA methylation and curcumin, bioactive components in dietary supplements such as curcumin may assist in the treatment of many pathologies due to curcumin’s ability to potentially suppress gene expression and certain transcription factors [45].
10. Discussion
American Diabetes Association Professionals frequently advise patients to switch to a lower caloric/carb/sugar diet and increase their exercise levels in comparison to their prior levels of physical activity as a way to lower their risk of developing T2DM. However, some research has indicated that adding or increasing the amount of turmeric alone or in combination with other phytonutrients in the diet may potentially assist in lowering inflammation levels and induce adiponectin expression as well [46]. Curcumin also has many different antioxidant properties that may also assist in lowering the risks of other T2DM-associated diseases, such as cardiovascular disease.
Overall, as the world is leaning more toward the sedentary lifestyle, high caloric diets, aging population, and genetic dispositions, the risk of developing this disease has quadrupled within the past three decades, and these rates will continue to rise in the foreseeable future [47]. However, despite the rising trend in T2DM diagnosis, the promising potential of curcumin alone or in combination with traditional medications cannot be undermined. A combination that includes Indian Gooseberry, Giloy juice, Triphala kwath, fenugreek, piperine, and curcumin may provideoptimum results. Such a formulation must be tested in a properly designed clinical study with or without different T2DM drugs.
However, due to the extremely low bioavailability and absorption of curcumin, it would be more effective to be used as a part of a healthy lifestyle that includes a balanced diet with moderate exercise plus other ingredients like piperine, fenugreek, Indian gooseberry, and grape seeds. Curcumin is known for its myriad alleviative properties, from anti-inflammatory to anticancer, while remaining nontoxic even when consumed in large quantities, like 6 g/day for several weeks [46]. Most clinicians often recommend a positive lifestyle change over medication, especially if the disease is at its earliest stages. Those with prediabetes should notice an improvement in their overall well-being by increasing their level of exercise and eating healthier food with a diverse diet [48]. Within the clinical trial setting, it was determined that curcumin, even when taken at high doses, provides little to no side effects. Doses up to 12 g/day have been tested in clinical trials and have been demonstrated to be beneficial for alleviating diabetic complications even when taken long-term. In the study conducted by Cas and Ghidoni [7], doses of 2–10 g of curcumin were given to the subjects depending upon the subject’s physiology.
11. Conclusion
Curcumin seems to have shown benefit in T2DM control via its anti-inflammatory properties. The compound’s low bioavailability and low absorption pose clinical limitations for its treatment potential, although the research cited in this review supports ways to improve bioavailability and absorption. This narrative review provides a comprehensive insight into existing research and identifies potential gaps to encourage further research into curcumin’s bioavailability to alleviate some common diabetic symptoms. Dose optimization of curcumin and oral antidiabetics should be an ongoing area for investigation including epigenetic mechanisms in pathogenesis of diabetes. However, there is enough work to support the proposition that curcumin and oral antidiabetics can synergistically improve overall glycemic control along with lifestyle choices.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for the study from any funding agency.
Open Research
Data Availability Statement
The citation data used to support these findings of the study are included within the article under the “References” section.




