The Impact of Growth Factors in Platelet-Rich Plasma Combination Therapy for Androgenic Alopecia
Abstract
The efficacy of platelet-rich plasma (PRP) therapy for androgenic alopecia (AGA) varies among diverse populations. While prior research has emphasized the pivotal role of growth factors as active components in PRP, the specific relationship between growth factors and treatment outcomes of AGA remains unclear. This study aims to explore how the efficacy of PRP therapy for AGA is influenced by the types and concentrations of growth factors. The analysis of PRP samples from 46 AGA patients involved assessing seven growth factors using an enzyme-linked immunosorbent assay. Patients received a course of three PRP treatments along with traditional medicines. The assessment of treatment outcomes involved conducting trichoscopy tests before and after the treatment, measuring both hair density (HD) and hair caliber (HC). The findings revealed that HD increased in 36 patients, positively correlating with glial cell-derived neurotrophic factor (GDNF) concentration (p = 0.005). In addition, HC increased in 35 patients, demonstrating a positive correlation with platelet-derived growth factor-BB (PDGF-BB) concentration (p < 0.05). Notably, a gender-based analysis identified a statistically significant difference in HC increase post-PRP therapy (p = 0.005). In addition, no correlations were observed between demographic factors and changes in HD/HC (p > 0.05). The study confirms the beneficial influence of certain growth factors in PRP on AGA treatment outcomes. Future research should further clarify their mechanisms in promoting hair growth, paving the way for the development of novel therapeutic agents.
1. Introduction
Androgenetic alopecia (AGA), also known as male-pattern hair loss (MPHL) and female-pattern hair loss (FPHL), stands as the most prevalent form of alopecia. Its primary hallmark is the nonscarring, progressive miniaturization of hair follicles [1]. The common therapeutic modalities for AGA encompass medications, low-level laser, and hair transplantation, etc. Despite the diversity of treatment options, a universally effective approach remains inexistent. Oral finasteride operates by inhibiting 5α-reductase to reduce dihydrotestosterone production, but its efficacy is limited in FPHL, and concerns about sexual dysfunction persist in male patients [2]. Topical minoxidil operates by enhancing microcirculation and prolonging the anagen phase [3]. Nonetheless, the potential side effects such as scalp itching, increased dandruff, facial hair growth, and headaches may hinder patient adherence [4]. Low-level laser therapy has demonstrated efficacy in treating AGA; however, a consensus on optimal parameters such as wavelength and energy remains elusive [5]. Hair transplantation offers a rapid improvement in aesthetics but is accompanied by a high cost and invasiveness, making it not widely applicable [6].
Platelet-rich plasma (PRP) is derived from autologous venous blood, contains a concentrated amount of platelets, growth factors, and other bioactive proteins, which play a crucial role in tissue healing and regeneration [7–9]. In 2006, Uebel et al. first observed that hair follicle unit yield increased (18.7 vs. 16.4 units/cm2, p < 0.001) when combined with PRP in hair transplantation in contrast to control areas [10]. In recent years, multiple clinical studies have substantiated the efficacy of PRP alone or in combination for AGA therapy. Pietro et al. conducted a randomized, placebo-controlled, half-scalp clinical study, showing that after three PRP treatments, there was an increase in hair quantity and hair density on the treated side (p < 0.001) [11]. Danielle et al. administered PRP treatments to 15 FPHL patients, and the results demonstrated significant improvement in mean density and caliber in the PRP group compared to the placebo group at both week 8 and week 24 (p < 0.01) [12].
PRP is rich in various growth factors stored in the alpha granules and dense granules of platelets, including epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), glial cell-derived neurotrophic factor (GDNF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF), and transforming growth factor-beta (TGF-β) [13, 14]. The precise mechanisms by which these growth factors interact to promote hair growth are not yet fully understood. Possible reasons include the activation of ERK, enhancement of β-catenin expression, release of Bcl-2, and activation of Akt, all of which influence hair growth [15]. Although growth factors play a crucial role in PRP therapy for alopecia, research on the correlation among growth factor types, concentrations, and clinical treatment outcomes remains restricted.
To assess the efficacy of PRP in conjunction with traditional medicines for AGA and explore possible influencing factors, the research group analyzed data from 46 AGA patients who underwent hair loss treatments at our center between 2021 and 2022. The study aimed to conduct a comprehensive analysis of the relationships between treatment outcomes and various factors, including age, gender, disease duration, weight, height, blood cell parameters, and plasma growth factors.
2. Material and Methods
2.1. Patient Population
This study was conducted at Beijing Tsinghua Changgung Hospital and included 46 patients with AGA who were scheduled to receive treatment at the Hair Disorders Clinic from July 2021 to April 2022. The patient group comprised 28 males and 18 females, with MPHL patients graded based on the Hamilton–Norwood criteria and FPHL patients classified using the Ludwig scale. Prior to their initial treatment, all patients had not undergone any hair loss-related treatments, including medications, PRP, lasers, and hair transplantation. They were also screened for malignancies, hematological disorders, acute infections, severe hepatic or renal disease, severe cardiovascular diseases, and autoimmune diseases. Informed consent was obtained from all participants, and the study received ethical approval from the Ethics Committee of Beijing Tsinghua Changgung Hospital.
2.2. Platelet-Rich Plasma Preparation
For each treatment, 18 mL of whole blood was drawn from the antecubital vein of each patient using two 9 mL anticoagulant tubes containing heparin sodium. The blood was then subjected to centrifugation with speeds of 2700, 2400, 2700, and 3000 rpm for 12 minutes in a variable speed centrifuge (Medifuge, Silfradent, Italy). A final volume of 5 mL of PRP, containing growth factors located above the interface of the red blood cells at the bottom layer, was collected. In this study, PRP preparation utilized variable speed centrifugation technology without the addition of any activators.
2.3. Growth-Factor Testing and Complete Blood Count
The PRP samples were collected for the measurement of EGF, FGF2, platelet-derived growth factor subunit B (PDGF-BB), VEGF, GDNF, HGF, and transforming growth factor beta-1 (TGF-β1). Concentrations of these factors in the PRP samples were determined using ELISA kits following the manufacturer’s instructions (ImmunoWay Biotechnology, USA). In addition, peripheral venous blood was drawn from 44 of the 46 patients for a complete blood count (CBC), which provided data on white blood cell (WBC), red blood cell (RBC), hemoglobin (HGB), platelet (PLT), platelet crit (PCT), platelet distribution width (PDW), mean platelet volume (MPV), platelet large cell ratio (P-LCR), and other parameters.
2.4. Interventions
All patients underwent a combined treatment regimen consisting of conventional pharmacological and PRP treatment, with both therapies initiated concurrently.
Conventional pharmacological treatment: all male patients were treated with oral finasteride tablets (1 mg, once daily) and topical 5% minoxidil liniment (6 sprays, twice daily, applied to the affected area of the scalp). All female patients were treated with oral spironolactone and topical 5% minoxidil liniment (6 sprays, once daily, applied to the affected area of the scalp).
PRP treatment: after disinfection of the scalp in the area of hair loss using 70% alcohol, 5 mL of PRP was injected with a 30G syringe needle. No anesthesia was administered. The injections were made into the scalp at a depth of 2–4 mm, at an angle of approximately 45 degrees, at a quantity of 0.1–0.2 mL per cm2, and spaced 0.5–1 cm apart. Each patient received three treatment sessions at one-month intervals, with a final follow-up visit one month after the last treatment.
2.5. Main Outcome Measures
Trichoscope (Dermatoscope-2K, HongXin Medical Equipment Co. Ltd, China) was utilized to evaluate AGA patients before the combination therapy. The centroparietal scalp area, located 15–20 cm superior to the glabella, was chosen for trichoscopy test in each patient. The provided hair image analysis software was used to calculate hair density (HD) and hair caliber (HC) for each patient before and after treatment.
2.6. Statistical Analysis
Statistical analysis was conducted using IBM SPSS® Statistics software (Version 23). Descriptive statistical data were provided for patient demographics, including gender, age, disease duration, height, weight, AGA types, and blood cell parameters. Spearman correlation analysis was used to examine the relationships between growth factor concentrations and changes in HD and HC. Similarly, the same methods were performed to investigate the associations between age, disease duration, height, weight, AGA types, blood cell parameters, and changes in HD and HC. The paired t-test was used to analyze the changes in HD and HC before and after treatment. Nonparametric tests were applied to assess the relationship between gender and changes in HD and HC.
3. Results
3.1. Demographics
Out of the 46 patients, males accounted for 60.9%, while females comprised 39.1% of the total. The age range spanned from 24 to 51 years, with disease duration ranging from 0.3 to 15 years. Heights varied from 155 to 185 cm, and weights varied from 45 to 110 kg. Within the MPHL patients, a significant proportion presented with Hamilton–Norwood type III-IV, while the majority of FPHL patients were classified as Ludwig grades I-II (Table 1).
| Characteristic | Patients (n = 46) |
|---|---|
| Sex, no. (%) | |
| Men | 28 (60.9) |
| Women | 18 (39.1) |
| Age, mean ± SD, (y) | 34.48 ± 7.10 (range 24–51) |
| Disease duration, mean ± SD, (y) | 5.87 ± 4.05 (range 0.3–15) |
| Height, mean ± SD, (m) | 169.37 ± 8.38 (range 155–185) |
| Weight, mean ± SD, (kg) | 68.79 ± 13.38 (range 45–110) |
| AGA type, no. (%) | |
| Hamilton–Norwood classification | |
| Type I | 4 (14.2) |
| Type II | 2 (7.1) |
| Type III | 11 (39.2) |
| Type IV | 9 (32.1) |
| Type V | 1 (3.5) |
| Type VI | 1 (3.5) |
| Ludwig classification | |
| Grade I | 10 (55.5) |
| Grade II | 8 (44.4) |
- SD: standard deviation; y: years; m: meters; kg: kilograms; AGA: androgenetic alopecia.
3.2. Results of CBC
The platelet count in the blood of the 44 patients was 259.25 ± 63.05 × 10 ^ 9/L. Detailed data for other parameters such as WBC, RBC, HGB, PCT, PDW, MPV, and P-LCR can be found in Supplementary File 1.
3.3. Growth Factor Concentrations
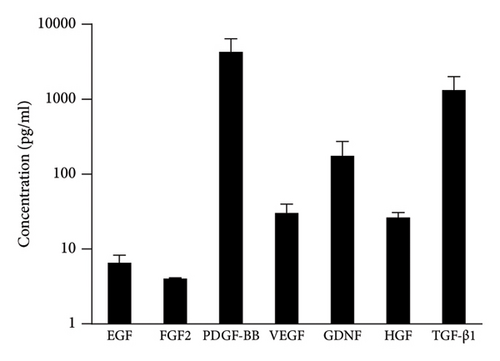
The levels of seven growth factors, including EGF, FGF2, PDGF-BB, VEGF, GDNF, HGF, and TGF-β1, in the PRP samples of all patients are depicted in Figure 1. Among these growth factors, PDGF-BB exhibited the highest concentration. Conversely, FGF2 had the lowest concentration. Detailed data can be found in Supplementary File 2.
3.4. Changes in HD
The pretreatment HD value was 118.28 ± 26.10 hairs per cm2, while the HD value at the final follow-up increased to 133.30 ± 26.56 hairs per cm2 (p < 0.001). An increase in HD was observed in 36 out of 46 patients (78.3%). The concentration of GDNF exhibited a positive correlation with the increase in HD (p = 0.005), whereas the concentrations of the other six growth factors showed no significant correlation with changes in HD (p > 0.05) (fitted regression curves were shown in Figure 2).
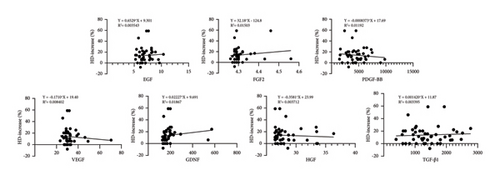
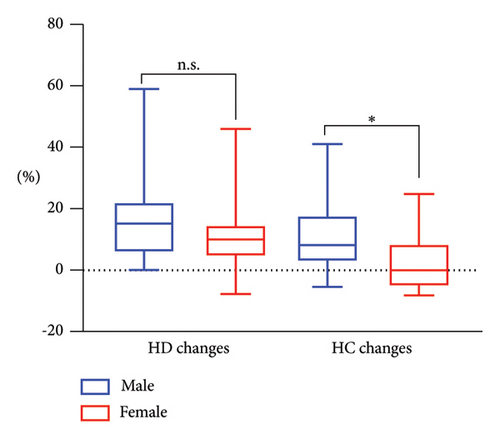
After treatment, MPHL patients experienced a median HD increase of 15.13%, with an interquartile range (IQR) of 5.68%–22.22%. In comparison, FPHL patients had a median HD increase of 9.94%, with an IQR of 4.38%–14.7%. Statistical analysis revealed no significant difference in posttreatment HD change between males and females (p = 0.21) (Figure 3). It was confirmed that variables such as age, disease duration, weight, and height showed no significant correlation with the posttreatment change in HD (p > 0.05). Furthermore, no significant correlations were found between the results of CBC analysis and the posttreatment change in HD (p > 0.05).
3.5. Changes in HC
The pretreatment HC value was 54.37 ± 10.28 μm, while at the final follow-up, the HC value measured 57.99 ± 9.96 μm (p < 0.001). An increase in HC was observed in 35 out of 46 patients (76.1%). The concentration of PDGF-BB exhibited a positive correlation with the increase in HC (p < 0.05), whereas the concentrations of the other six growth factors showed no significant correlation with changes in HC (p > 0.05) (fitted regression curves were shown in Figure 4).
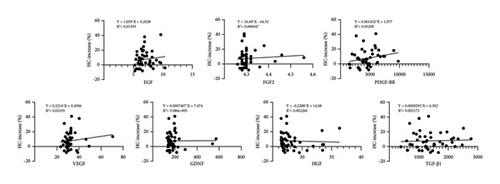
After treatment, MPHL patients demonstrated a median HC increase of 8.15%, with an IQR of 2.73%–17.83%. Conversely, FPHL patients showed no median HC increase posttreatment, with an IQR of −5.35%–8.50%. Nonparametric analysis revealed a statistically significant difference (p = 0.005) in the posttreatment change of HC between males and females (Figure 3). Correlation analysis confirmed that variables such as age, disease duration, weight, and height exhibited no significant correlation with the posttreatment change in HC (p > 0.05). In addition, no CBC parameters were identified to demonstrate a significant correlation with the posttreatment change in HC (p > 0.05).
4. Discussion
Oral finasteride and topical minoxidil are considered the classic therapeutic modalities for AGA. However, these treatments come with an extended duration, typically requiring continuous use for 6–12 months before discernible therapeutic effects manifest [16]. Therefore, the search for more rapidly effective treatment methods has become crucial. PRP is a novel treatment approach for AGA that has emerged in recent years, known for its high safety and minimal adverse reactions [17], mostly limited to transient pain during the treatment process [18]. This has contributed to a favorable acceptance of PRP among patients. In this study, we combined PRP with traditional medicines for AGA patients and observed significant therapeutic effects under trichoscopy evaluation after a 3-month treatment period. The majority of patients experienced increased HD and HC, suggesting that this combination therapy produces therapeutic results more rapidly than conventional treatment approaches used alone, similar to the findings reported by Soorumsetty [19].
The effectiveness of PRP therapy for AGA is influenced by various factors, including individual patient characteristics, the progression and grading of hair loss, PRP preparation methods [20, 21], single treatment dosages, treatment frequency [22], and key components. In this study, no significant correlations were found between age, body weight, height, disease duration, hair loss grade, and the efficacy of PRP when combined with conventional medications. The uniformity of PRP preparation methods, treatment dosages, and frequencies among all participants highlights the significant influence of PRP components on therapeutic outcomes. Previous research has highlighted the crucial role of growth factors as the primary active components in PRP [23]. These growth factors contribute to enhancing the follicular microenvironment, stimulating the differentiation of follicular stem cells, regulating the follicular cycle, and promoting follicular formation [24]. Analyzing variations in growth factor content within PRP across different patients can help discern the impact of growth factor types and concentrations on treatment efficacy.
Our study has extensively covered various common categories of growth factors within PRP, elucidating their associations with follicular growth through cell and animal research [25–32]. Several clinical studies have investigated the influence of specific growth factors on the efficacy of PRP therapy. In PDGF family members, PDGF-AA has been previously confirmed to lack association with hair loss improvement following PRP therapy [33]. Our research findings establish a strong correlation between PDGF-BB concentration and HC increase; this may be related to its critical role in repairing the subcutaneous microcirculatory system, as well as enhancing collagen formation. Another randomized double-blind controlled study demonstrated that the combination of PRP and FGF2 injections resulted in an enhanced hair quantity and a higher terminal hair growth rate compared to sole PRP treatment [34]. However, in our study, we did not observe an association between the increase in FGF2 concentration and the improvement in treatment efficacy. An additional significant growth factor that we discovered is GDNF, which is associated with an increase in HD. This finding aligns with Tee’s research, revealing a positive correlation between GDNF concentration and hair density (p = 0.004) [35].
The main limitation of this study is the relatively small sample size. Another limitation is that we did not employ a fixed tattoo in the process of trichoscopy assessment. However, we ensured that the measurement points on the crown were consistently positioned at the same distance from the brow center for each patient, thereby guaranteeing the reliability of HD and HC calculation.
The common method for preparing PRP uses the patient’s own plasma, known as autologous PRP. This approach minimizes the risks of immune rejection and disease transmission. However, the preparation process is complex and requires specialized equipment. In addition, the effectiveness of autologous PRP can be influenced by the patient’s health and age. In contrast, heterologous PRP, sourced from donor blood, offers a more convenient option, allowing for standardized and large-scale production, which ensures consistency in treatment quality. Heterologous PRP has shown promise in wound healing applications [36]. Immunological and serological testing in some experiments has demonstrated that heterologous PRP is safe from adverse immune reactions [37]. However, ethical concerns and limitations related to donor resources must also be considered.
Purified growth factors, which can be considered a derivative of heterologous PRP, are expected to have a better safety profile. In our study, a positive correlation between the concentrations of PDGF-BB and GDNF and the posttreatment effect was observed. This suggests the need for further research to explore the precise roles of PDGF-BB and GDNF in the hair follicle cycle and their impacts on cellular growth and structural changes. In addition, both basic and clinical studies are necessary to investigate the efficacy of utilizing growth factor preparations alone rather than PRP for AGA treatment, paving the way for the development of more effective treatment techniques in AGA management.
5. Conclusion
Our findings highlight the positive impact of specific growth factors in PRP on the outcomes of AGA treatment. Future studies should further investigate their mechanisms in stimulating hair growth.
Abbreviations
-
- AGA:
-
- Androgenic alopecia
-
- PRP:
-
- Platelet-rich plasma
-
- MPHL:
-
- Male-pattern hair loss
-
- FPHL:
-
- Female-pattern hair loss
-
- DPCs:
-
- Dermal papilla cells
-
- HD:
-
- Hair density
-
- HC:
-
- Hair caliber
-
- EGF:
-
- Epidermal growth factor
-
- FGF:
-
- Fibroblast growth factor
-
- PDGF:
-
- Platelet-derived growth factor
-
- VEGF:
-
- Vascular endothelial growth factor
-
- GDNF:
-
- Glial cell-derived neurotrophic factor
-
- HGF:
-
- Hepatocyte growth factor
-
- TGF:
-
- Transforming growth factor.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Authors’ Contributions
Jie Ren, Yi Zhao, and Hsiaohan Tuan designed the research study. Jie Ren, Jingxuan Sun, and Zhenghui Li performed the research. Jie Ren and Jingxuan Sun collated and analyzed the data. Jie Ren drafted and revised the manuscript with the help from Yi Zhao and Hsiaohan Tuan. Yi Zhao and Hsiaohan Tuan contributed equally to the work. All the authors have read and approved the final manuscript.
Acknowledgments
We thank all other members of our departments for vital discussions and critical suggestions. We thank the subjects for participating in this study. This work was supported by the Beijing Hospitals Authority’s Ascent Plan (no. DFL20240901).
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.