Plant Growth Promoting Rhizobacteria (PGPR): Reports on Their Colonization, Beneficial Activities, and Use as Bioinoculant
Abstract
Recurring use of chemical fertilizers (CFs) in agriculture has resulted the remarkable improvement in crop productivity but their ruinous effects on environment have made a serious issue. Biological entities (e.g., several microorganisms) showing fertilizer-like activities have gained attention in this regard. Several soil resident microorganisms interact strongly with neighboring plants and promote the growth and development of those plants through various means. In exchange of this, microbes utilize different compounds released from plant roots for their own nutrition. This mutualistic mode of interrelation predominantly relies on the transmission of signals from microbes to plants and vice versa. However, climatic factors (e.g., CO2 level, temperature, and water availability) are also important for this association. These bacterial strains are literally known as plant growth promoting rhizobacteria (PGPR) which facilitate plant growth through nitrogen fixation, mineral solubilization, phytostimulation, stress resistance, etc. Responding to the external environmental stimuli, they often modulate the expression of genes responsible for the transport of nutrients. Reduction of the use of CFs through the application of PGPR strains in the cultivation of some economically important plants has been reported by several authors. Significant yield improvement compared to the control groups was found in all experimental studies. Commercial development of the PGPR inoculants with remarkable biostimulating activities and their successive application should be expanded through collaborative association with different sectors after the removal of existing lacunae. Reading more than 100 articles on various aspects of rhizobacteria, the plan of writing this article has been executed. In this review, we have discussed about the colonization and potency of PGPR strains and how their well-planned application in agriculture could evidently reinforce the global economy. The main structure of this text is designed as an outline from the development of interrelation between plants and PGPR to the commercialization of PGPR based on their potential role in the field of agriculture.
1. Introduction
Ever since the dawn of civilization of human being, farmers have been trying to produce different types of crops. The green revolution occurred with the introduction of high-yield crop varieties by N. Borlaug, United States, G. Khush, IRRI, and M.S. Swaminathan, India. Those require high input of chemical fertilizers (CFs), pesticides, and water which ultimately deteriorate soil health. Eco-friendly organic revolution has become a popular choice where restoration of soil condition is possible by introducing bioorganic inputs without compromising crop yield. Plant growth promoting rhizobacteria (PGPR) plays an important role, here. The ecological community within which plants grow consists of different groups of organisms including the rhizomicrobiome. One of the major components of rhizomicrobiome is the PGPR which functions in maintaining plant health during normal and stressed conditions. These are the microbial population associated with plant root surface and the surrounding rhizospheric soil layer, which exhibit a close relationship with plants. These microbes have beneficial effects on plants in exchange of absorbing several nutrients for survival. They promote plant growth by facilitating mineral acquisition, synthesizing phytohormones, modulating the effects of some deleterious abiotic stresses, showing antagonism to some pathogenic microorganisms, development of induced systemic resistance (ISR) in plants, etc. [1]. Rhizosphere includes the soil attached with the plant root surface besides a little extended area of the surroundings. It is an important zone for plant–microbe interaction as the plant roots release a lot of compounds attracting the heterogeneous groups of microorganisms [2]. Autotrophic plants provide carbon compounds and energy sources to the heterotrophic soil microbiota which facilitate the acquisition of nutrients by the host in return [3]. Timing of root exudation and balancing among the released compounds determine the physiological, biochemical, and ecological characteristics of the rhizosphere. Interaction between plant and rhizospheric microorganisms plays a vital role in determining plant health, productivity, and soil fertility as well as transformation, mobilization, and solubilization of nutrients from the mineral pool and subsequent nutrient uptake of plants [4]. Micronutrients and fatty acid content enrichment in seed after PGPR inoculation were described in several reports [5, 6]. This ultimately leads to the positive effects on plant growth as a biostimulant with less hazardous impact on the environment. Multifunctionality of the PGPR has made it a popular additive besides CF for the improvement in crop production in recent years [2]. Many laboratories have reported the bioactivities of PGPR during crop development. However, complete information on the mechanisms behind these activities are lacking. More detailed investigation of the diverse plant–PGPR interactions is needed to be explored. In this review, we have focused on the successful plant–PGPR interaction, potentialities of PGPR for promoting plant growth and stress management, their promising role in reducing the use of CF, and a few other aspects such as influence of PGPR in plant gene expression, commercialization of PGPR strains, difficulties in PGPR application with their possible solutions, and future prospect for studying those organisms broadly.
2. Methodology
Internationally popular search engines like PubMed, SpringerLink, Scopus, Google Scholar, and ScienceDirect were used for retrieving necessary data from the original articles and review articles. Searching different types of articles using the keywords like “rhizospheric microorganisms and plant root,” “PGPR and nitrogen fixation,” “PGPR and soil mineral solubilization,” “PGPR as biostimulant,” “antagonistic activity of PGPR against other microorganisms,” “PGPR and plant gene expression,” “PGPR as biofertilizer,” “commercialization of PGPR,” etc., have displayed about 150 articles from which principal ideas were taken for writing the whole and 102 literatures (years of 2000–2022) were cited as per the requirement for respective statements. Some books and book chapters were consulted also for obtaining relevant information. References of publications were cited using the software Mendeley following the journal style.
3. Colonization
Exchange of the signal between plant and microbes is a critical factor in rhizospheric colonization of bacteria. Composition and abundance of root-associated microorganisms depend on timing, amount, and constituents of root exudation. The secretion is greatly influenced by some factors such as temperature, light, plant age, and soil features. Sugars, amino acids, organic acids, fatty acids, and secondary metabolites like flavonoids, terpenes, phenolics, etc., were commonly found in plant root exudates [7]. These compounds attract microbes and therefore can be considered as the initial step for colonization of the rhizobacteria. Flavonoids secreted from legume root were reported to be involved in the establishment of root–rhizobia interrelation through the alteration in rhizobial gene expression. Genetic regulation of root exudation determines distinct rhizobacterial communities for different plants [7, 8]. In associative mutualism, signaling molecules function substantially in rhizospheric environment. A signaling compound acyl-homoserine lactone has a pivotal role in cell-to-cell communication of bacteria as well as it also interacts with plant. Microbial distribution near the plant root generally develops strong interaction after a long-term association. However, sudden replacement of host plant delivers selective pressure on the rhizospheric microbiome and leads to the rearrangement and ultimately the gradual alteration in microbial pool [9]. Regulation of this shifting principally depends on quorum sensing among the members of the microbial population [10]. Plants also respond to the signals coming from microbial origin. A signaling molecule 2,4-diacetylphloroglucinol secreted from Pseudomonus fluorescens and triggered ISR in Arabidopsis thaliana [11]. A. thaliana plant inoculated with Bacillus megaterium has shown altered root architecture and increase in biomass, photosynthesis rate, and drought tolerance according to the report of Zhou et al. [12]. Secretion of spermidine (a precursor of polyamine biosynthesis) from the bacterial strain appears to mediate all the events reported. Lumichrome and riboflavin are the two rhizobacterial signal molecules, elicit changes like rapid leaf development, leaf expansion, increase in plant height, and improvement in biomass production [13]. Thus, plant-microbe signaling is potentially important for the successful colonization of rhizospheric microorganisms in a particular zone and that is also significant in the agricultural system. Rhizospheric colonization of bacteria is diagrammatically presented in Figure 1.
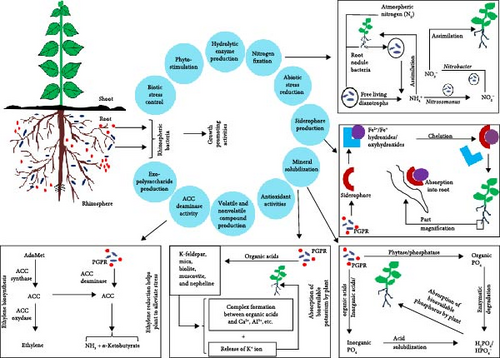
4. Beneficial Activities of PGPR
In 1970, Joseph W. Kloepper used the term PGPR for the first time which later became a common term in scientific literatures. Several species of soil bacteria grow around the plant tissue and stimulate plant growth through different mechanisms [14]. According to their beneficial effects, some of the important activities are discussed below.
4.1. Biological Nitrogen Fixation
Molecular dinitrogen (N2) of the atmosphere is almost metabolically useless except for a few microorganisms. Free-living diazotrophs and several symbiotic diazotrophs (root nodule bacteria) convert N2 into ammonia using their own nitrogenase enzyme. Plants easily assimilate ammonia by involving glutamine synthetase and glutamate synthase enzymes. Plants accommodate those bacteria and gain nitrogen by providing their own metabolites to those microbes [15]. Free-living diazotrophs like Azotobacter, Clostridium, Azospirillum, Chromatium, Beijerinckia, Derxia, Methanococcus, Rhodospirillum, etc., cyanobacteria like Anabaena, Nostoc, Calothrix, etc., and symbiotic legume-root nodule bacteria like Rhizobium, Azorhizobium, Bradyrhizobium, Sinorhizobium, Photorhizobium, etc., are the important nitrogen fixing agents. Root nodule forming bacteria like Frankia, Acetobacter noticed in nonleguminous plants, can also fix atmospheric nitrogen [16]. Methylobacterium nodulans, a facultative legume-root nodule forming bacteria, fixes nitrogen [17]. On the other hand, several Nitosomonus bacteria like Nitosomonus europea, Nitosomonus oligotropha, Nitosomonus marina, Nitosomonus communis, Nitosomonus nitrosa, Nitosomonus cryotolerance, etc., oxidize ammonia and convert into nitrite using two different enzymes, ammonia monooxygenase (which catalyzes oxidation of ammonia to ammonium hydroxide) and hydroxylamine oxidoreductase (catalyzes oxidation of ammonium hydroxide to nitric oxide that is partitioned into nitrous oxide and nitrite, later) [18]. Another group of soil bacteria called Nitrobacter, further oxidize nitrite to nitrate with the help of nitrite oxidoreductase. Those are namely, Nitrobacter alkalicus, Nitrobacter hamburgensis, Nitrobacter winogradskyi, etc. [19]. Plants assimilate nitrate through photosynthetic green shoots as well as using nonphotosynthetic root parts. Biological nitrogen fixation represents the entry of atmospheric nitrogen into the plant tissue and contributes to the crop cultivation. This mechanism is schematically represented in Figure 1.
4.2. Mineral Solubilization
In most of the regions, soil phosphorus level is less available to the plants due to low diffusion rate and metal chelation. A diverse group of rhizospheric bacteria secret organic/inorganic acids, phosphatase, phytase which produce H2PO4/HPO4− from inorganic and organic phosphorus compounds through acid solubilization and enzymatic degradation through which increase their accessibility to the plants [20]. Significant phosphate solubilizing activities were found in some species of Pseudomonus, Pantoea, Gluconacetobacter, Burkholderia, etc. [21]. Acinetobacter rhizosphaerae solubilized tricalcium phosphate by secreting gluconic, oxalic, lactic, malic, and formic acids [22]. Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans both are capable of solubilizing significant amount of phosphate through the process of bioleaching and are considered as the potential candidate for phosphorus supplementation of soil [23]. A vast amount of insoluble crystalline soil potassium is unavailable to the plants. Rhizospheric bacteria like Agrobacterium, Rhizobium, Flavobacterium, and Bacillus sp. solubilize that form of potassium (e.g., K-feldspar, mica, muscovite, biolite, nepheline, etc.) through diversified mechanisms including acidolysis, complexolysis, chelation, exchange reaction, etc., and therefore turns into easily available ones [24]. Some strains of Burkholderia, Rhizobium larrymoorei can also solubilize potassium [25]. Through mineral solubilization, PGPR facilitates the complex, dynamic nutrient uptake process of plant and accordingly reduces the requirement for synthetic agrochemical supplementation. Mineral solubilization principles are outlined in Figure 1.
4.3. Siderophore Production
Iron (Fe), an essential micronutrient of plant, involved in chlorophyl synthesis and metabolic processes like DNA synthesis, respiration, amino acid biosynthesis, etc., mainly exists in soil as Fe2+ or Fe3+ forms with insoluble hydroxides or oxyhydroxides which cannot be easily assimilated by plants. Plants mainly acquire Fe from soil through Fe reduction and Fe chelation. Some of the rhizobacterial species of Pseudomonus, Enterobacter, Azotobacter, Rhizobium, Serratia, and Bacillus help in Fe absorption of plants by producing siderophore. Siderophore is a chelating substance having Fe-binding affinity. From minerals and several organic compounds, siderophore releases Fe and forms Fe–siderophore complex, which is directly taken by plants as per their requirement [26]. It is schematically presented in Figure 1.
4.4. Phytostimulation
Phytostimulation naturally occurs through the active involvement of phtohormones or plant growth regulators. Those are basically the signal molecules which are produced within plant in very low concentration, stimulate plant growth by controlling all aspects growth and development, such as embryogenesis, organ size regulation, reproductive development, biotic and abiotic stress defense, etc. [27]. Auxin, cytokinin, and gibberellin are the most important plant growth regulators for maintaining development-related events through their respective pathways. Auxin is essentially required for cell growth specifically apical growth which contributes to the size of plant, organ direction, tropism (i.e., turning movement in response to external stimulation), etc. [28]. Gibberellin regulates different plant developmental processes such as flowering, stem elongation, seed germination, etc. [29]. Cytokinin promotes cell division, cell differentiation, lateral growth, etc. [30]. Several PGPR strains produce plant growth regulators through which they enhance plant growth and manipulate stress responses. Bacillus altitudinis triggers the expression of auxin-responsive genes and enhances indole-3-acetic acid (IAA) one of the bioavailable from of auxin level in rice [31]. Klebsiella oxytoca and Arthrobacter nitroguajacolius produced a high amount of IAA in L-tryptophan (precursor of auxin biosynthesis) medium [32]. Bacillus cereus, Bacillus pumilus, and Bacillus macroides were found capable of inducing endogenous gibberellin in plant [33]. PGPR strains like Acinetobacter calcoaceticus, Pseudomonas koreensis, Leifsonia soli, and Serratia nematodiphila isolated from soil produce bioactive gibberellin which increases plant growth [34]. Some species of Bacillus, Pseudomonus, Azospirillum, Arthrobacter, Agrobacterium, and Xanthomonus were found to produce cytokinin [35]. Reports have shown that Bacillus subtilis and Bacillus toyonensis produced cytokinin, possessed the ability to trigger plant growth, and mitigated metal-induced stress [36]. B. subtilis also enhanced auxin and gibberellin content in tomato plant [37]. There are many other reports available stating the involvement of PGPR in phytohormone production.
4.5. Biotic Stress Control
Rhizobacteria exhibit different types of antagonistic activities against plant pathogens. P. fluorescens was reported to have the ability to produce antifungal agents, like pyoleuteorin and 2,4-diacetylphloroglucin [38]. Some species of bacterial genera revealed antimicrobial activities by producing hydrogen cyanide (HCN), visconamide, tensin, phenazines, and pyrrolnitrin [39]. Bacillus amyloliquefaciens inhibited the growth of Rhizoctonia solani in rice and enhanced host defense against this pathogen through the production of secondary metabolite suppression and reactive oxygen species (ROS) modulation [40]. Gossypol and jasmonic acid production were escalated by Bacillus sp. in cotton, causing a reduced larval attack of Spodoptera exigua [41]. Inoculation of Paenibacillus lentimorbus decreased the virulence of cucumber mosaic virus in infected Nicotiana tabaccum (tobacco) plant and increased the tolerance against southern blight disease of tomato caused by Scelerotium rolfsi. Induction of systemic resistance in the host plant was observed for both cases [42]. Rhizobacterial strain of Enterobacter asburasburiae enhanced plant resistance for tomato leaf curl virus through the expression of pathogenesis-related gene, phenylalanine ammonia lyase, catalase, peroxidase, and superoxide dismutase [43]. Serratia liquefacience and Pseudomonas putida were found to have a significant role in eliciting ISR in tomato plant for the pathogen Alternaria alternate [44]. P. fluorescens helps in the accumulation of salicylic acid and develop ISR in chickpea against Fusarium oxysporum f. sp. [45]. A number of PGPR strains produce HCN through which they inhibit the growth of some harmful rhizospheric microbes [46]. Several biotic stress responses of PGPR strains are mentioned in Table 1.
| Name of PGPR strains | Plant | Biotic/abiotic stress | Function | References |
|---|---|---|---|---|
| Serratialiquefacience, P. putida | Tomato | Alternaria alternate infection | Eliciting-induced systemic resistance | [44] |
| B. amyloliquefaciens | Rice | Rhizoctonia solani infection | Secondary metabolite production and ROS modulation | [40] |
| P. lentimorbus | Tobacco | Cucumber mosaic virus infection | Induction of systemic resistance | [42] |
| Paenibacillu slentimorbus | Tomato | Scelerotium rolfsi infection | Induction of systemic resistance | [47] |
| Enterobacter asburiae | Tomato | Tomato leaf curl virus infection | Expression of pathogenesis-related gene, phenylalanine ammonia lyase, catalase, peroxidase, and superoxide dismutase | [43] |
| Bacillus sp. | Cotton | larval attack of S. exigua | Gossypol and jasmonic acid production | [41] |
| Pseudomonus fluorescens, Bacillus sp. | Many plants | Microbial infection | Production of pyoleuteorin, 2,4-diacetylphloroglucinol, HCN, visconamide, tensin, phenazines, and pyrrolnitrin | [38, 39, 45] |
| P. putida | Chick pea | Drought | Membrane integrity modulation, osmolyte accumulation, and ROS scavenging | [48] |
| Pseudomonas fluorescens | Rice | Flood | ACC deaminase production, root elongation | [49] |
| Variovorax paradoxus | Pea | Salinity | Increasing photosynthetic rate, electron transport, K+ movement toward the shoot and Na+ deposition in root, and decreasing stomatal resistance | [50] |
| B. amyloliquefaciens | Maize | Salinity | Upregulation of HKT1, H+PPase, NHX1, NHX2, NHX3, RBCS, RBCL, and downregulation of NCED gene expression | [51] |
| Dietzia natronolimnia | Wheat | Salinity | Upregulating the genes involved in ABA-signaling cascade, SOS pathway, ion transport, and enzymatic activities | [52] |
| Serratia nematodiphila, P. putida | Capsicum | Cold | Increasing endogenous ABA-level and reducing salicylic acid and jasmonic acid content | [53] |
| B. pumilus | Rice | Salinity and boron stress | Alteration in root system architecture | [54] |
| Burkholderia phytofirmans | Grapevine | Cold | Modulation of carbohydrate metabolism | [55] |
| P. vancouverensis, P. frederiksbergensis | Tomato | Cold | Enhancement of cold acclimation genes expression and antioxidant activity in leaf tissue | [56] |
| Kochuria rhizophila | Maize | Salinity | Exclusion of Na+ ion from cells | [57] |
| Sphingobacterim sp. | Tomato | Salinity | Expression of enolase, ATP synthase, thiamine biosynthesis protein, EF-α, and catalase | [58] |
- Abbreviations: ABA, abscisic acid; ACC, 1-aminocyclopropane-1-carboxylic acid; ATP, adenosine triphosphate; EF, elongation factor; NCED, 9-cis-epoxycarotenoid dioxygenase; NHX1, Na+/H+ exchanger 1; NHX2, Na+/H+ exchanger 2; NHX3, Na+/H+ exchanger 3; PGPR, plant growth promoting rhizobacteria; RBCL, ribulose bisphosphate carboxylase/oxygenase large subunit; RBCS, ribulose bisphosphate carboxylase/oxygenase small subunit; ROS, reactive oxygen species; SOS, salt overly sensitive.
4.6. Reduction of the Abiotic Stress Response
Several research groups reported the involvement of PGPR in the reduction of abiotic stress in plants. P. putida decreased the effect of drought in chickpea plant through membrane integrity modulation, osmolyte accumulation, and ROS scavenging which result the change in expression of MYC2, ACS, ACO, LEA, DHN, NAC1, and DREB1A genes [48]. Pseudomonas fluorescens produced 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase which thereby protects the rice plant from flooding condition by promoting root elongation [49]. Variovorax paradoxus reduced salt stress effect in pea plant with increasing photosynthetic rate, electron transport, increasing K+ movement toward the shoot, and Na+ deposition in root with decreasing stomatal resistance [50]. B. amyloliquefaciens enhanced salt tolerance in maize with upregulating HKT1, H+PPase, Na+/H+ exchanger 1 (NHX1), Na+/H+ exchanger 2 (NHX2), Na+/H+ exchanger 3 (NHX3), ribulose bisphosphate carboxylase/oxygenase small subunit (RBCS), ribulose bisphosphate carboxylase/oxygenase large subunit (RBCL), and downregulating 9-cis-epoxycarotenoid dioxygenase (NCED) gene expression [51]. Inoculation of halotolerant bacteria Dietzia natronolimnia into wheat plant has shown salt stress tolerance by upregulating the genes involved in abscisic acid (ABA)-signaling cascade, salt overly sensitive pathway, ion transport, and enzymatic activities [52]. Capsicum annum plant inoculated with Serratia nematodiphila have increased endogenous ABA level, reduced salicylic acid and jasmonic acid content through which represented well growth in low-temperature stress condition in comparison with noninoculated plants [53]. Modulation of carbohydrate metabolism in grapevine by the inoculation of Burkholderia phytofirmans has reduced chilling damage of the plantlets [55]. Enhanced expression of cold acclimation genes and elevated antioxidant activity in leaf tissue were observed in low-temperature grown tomato plant when inoculated with Pseudomonas vancouverensis and Pseudomonas frederiksbergensis [56]. Kochuria rhizophila further improved salt tolerance in maize with Na+ ion exclusion from cells [57]. Sphingobacterium sp. reduced salt stress in tomato plant through the expression of enolase, adenosine triphosphate (ATP) synthase, thiamine biosynthesis protein, and catalase [58]. These reports of PGPR would obviously help to develop abiotic stress tolerance in plants after rigorous study. The abovementioned activities of PGPR are summarized in Table 1.
Different experimental studies also reported many other aspects of PGPR like antioxidant production (e.g., Bacillus, Pseudomonus, Sphingobacterium, Serratia, etc.), ACC deaminase activity (e.g., Achromobacter, Variovorax, Acidovorax, Entrobacter, etc.) which reduces ethylene biosynthesis followed by several stress responses [47] (Figure 1), production of volatile organic compounds (Xanthomonus, Paenibacillus, Agrobacterium, Bacillus, Pseudomonus, Burkholderia, etc.), hydrolytic enzyme production (several species of Bacillus and Pseudomonus), biocidal activities with nonvolatile compound secretion (some species of Bacillus and Pseudomonus), suppression of pathogenesis with quorum sensing disruption (e.g., Bacillus, Pseudomonus, Arthrobacter, Agrobacterium, etc.), osmo-protection with exopolysaccharide secretion (e.g., B. subtilis sp. inaquosorum, Marinobacter lipolyticus), etc., which facilitate plant growth in many ways [59]. Detailed study of these microorganisms would unveil the potential for their possible commercial usage. Major activities of PGPR are schematically shown in Figure 1.
4.7. Influence of PGPR on the Expression of Host Nutrient Transport Gene
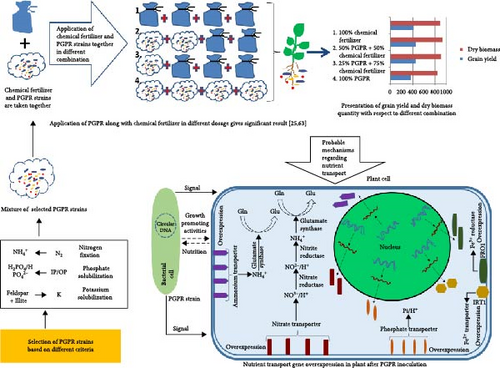
Some PGPR strains have an important role in the transport of nutrient within host. Calvo et al. [60] reported that PGPR can induce the expression of nitrate and ammonium transport-related genes along with improved nitrogen, phosphorus, and potassium (NPK) uptake. A. thaliana seeds soaked with the mixture of different rhizobacteria, namely B. subtilis, B. pumilus, B. safensis, B. amyloquefaciens, Lysinibacillus xylanilyticus, and Paenibacillus peoriae, and revealed varied level of gene expression after 6 weeks of sowing. Increased uptake of NPK was detected in treated plants in comparison with control. Transcripts of nitrate transporters (AtNRT1.1, AtNRT1.2, AtNRT1.5, AtNRT2.3) and ammonium transporters (AtAMT1.3, AtAMT1.5, AtAMT1.2) were increased in plants treated with PGPR mixtures unlike lower expression in untreated ones [60]. PGPR inoculum and arbuscular mycorrhizal fungi (AMF) promoted plant growth and nutrient transport gene expression in Triticum durum, when worked in concert. The admixture of AMF spores of Scutellospora calospora, Acaulospora laevis, Glomus aggregatum, Gigaspora margarita, G. intraradices, G. mosseae, G. fasiculatum, G. etunicatum, and G. deserticola and some PGPR strains, viz., Bacillus brevis, B. circulans, B. coagulans, B. firmus, B. halodenitrificans, B. laterosporus, B. licheniformis, B. megaterium, B. mycoides, B. pasteurii, B. polymyxa, and B. subtilis remarkably upregulated the expression of ammonium transporter (AMT1.2), nitrate transporters (NRT1.1, NRT2, NAR2.2), and phosphate transporters (Pht1, Pht2, PT2-1) in the plant [61]. Combined inoculation of B. subtilis, B. pumilus, and P. fluorescens into Moringa oleifera improved Fe accumulation efficiency in foliage with increased expression of Fe-phytosiderophore oligopeptide transporter gene [62]. A report has stated that treatment with B. subtilis, upregulated the expression of FRO2 and IRT1 genes encoding Fe3+ chelate reductase and Fe2+ transporter, respectively, in Arabidopsis seedling [63]. Further investigation is necessary to analyze the nature of gene expression (upregulation or downregulation) for nutrient transport in different plants after PGPR inoculation and that might help to understand the specific role of PGPR in this regard. Host gene overexpression after PGPR inoculation is schematically shown in Figure 2.
5. Use as a Bioinoculant
5.1. Preparation of PGPR Inoculant
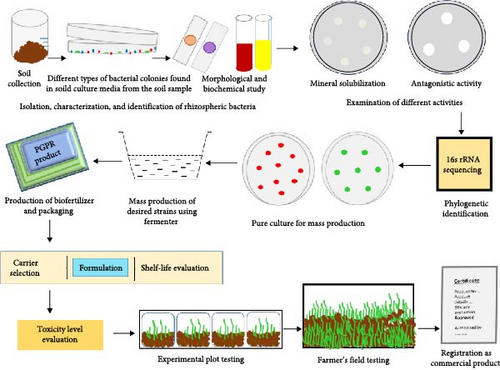
To prepare the PGPR inoculant, bacteria are isolated from the rhizospheric samples (following soil sample collection, suspension in sterile double distilled water, making serial dilution of the suspension, and growing on suitable media), characterized (through several biochemical studies), identified phylogenetically (through 16s rRNA sequencing), and then carefully grown in pure culture for mass production by skilled professionals. In the preparation of a worthwhile PGPR inoculant to enhance plant growth performance, the choice of microbial strains is the crucial factor. Evaluation of their effectiveness (i.e., several growth promoting activities like mineral solubilization, antagonistic activity, etc.) one by one should be based on screening under laboratory conditions in vitro as well as in vivo. To determine whether they are toxic to plant cell or not several tests should be conducted after inoculation. Observation of plant morphology, chromosomal development (e.g., Are there any aberrations?), quantification of defence-related secondary metabolites and photosynthesis monitoring through fluorescence-based assays, etc., can give cues regarding this. Mass production of selected microbial strains with the features of interest are done using industrial fermenter and thereafter formulated through the amalgamation with respective carriers (i.e., solid, liquid, or gel substrate). The raw materials of the carriers include clay, peat, animal manure, earthworm compost, charcoal, lignite, talc, perlite, coir dust, compost, and inorganic soil fraction. Carriers should be absolutely consistent and contamination free. A typical PGPR inoculant should be nontoxic (which shows no detrimental effect on plant development when tested in laboratory condition), biodegradable, have adjustable pH, and sufficient shelf-life during long storage. It should allow for ease of handling for the addition of required nutrients. Biochar made from plant wastes can also be used as a potential alternative due to its porosity and nutrient content. Inoculation of PGPR into the harvested seeds of the mother plant or into the soil before cultivation is the common practice in most cases. Making consortia with a combination of strains showing promising result may give more reliable outcomes rather than using a single strain only. Before using more than one strain, it should be checked that there is no antagonistic activity to each other. Coexistence ensures their compatibility in this regard. Sometimes, signal-based compounds secreted by microorganisms are isolated and purified from liquid culture and also formulated as inoculum. Industrially, the signal compounds are produced often by cultivating PGPR strains along with the specific additives that trigger the secretion of the respective molecules [64]. For example, some compounds like N-(tetrahydro-2-oxo-3-furanyl)-octanamide secreted from Sinorhizobium meliloti influence on nodulation efficiency; ComX pheromone secreted by Bacillus licheniformis inhibit the growth of a pathogenic fungus R. solani, etc., and can be formulated as inoculants [65, 66]. Testing of formulated inoculum in cultivation field is a necessary part. After completion of the experimental plot testing and farmer’s field testing of the inoculant, its registration as a commercial product can be done. According to the intellectual property right, novel technology for the formulation with freedom to operate and sufficient supporting data can be considered for patent [67, 68]. Survival and efficacy of the PGPR inoculant depend on the selection of the carrier and the method of formulation. To get sufficient cells in seeding time, the number of microbial strains should be increased during the processing of inoculant [69]. Authenticity and effectiveness of the PGPR products mainly depend on the quality, formulation procedure, mechanisms of action, colonization ability, viability, compatibility, and stability [70]. More trained individuals with required the expertise and collaboration with industries may accelerate the commercialization procedure. The outline of the preparation of PGPR inoculant is given in Figure 3.
5.2. Application of the PGPR Inoculum Reduces the Use of CF Supplements
German scientist von Liebig [71] has given the emphasis on three macronutrients, nitrogen, potassium, and phosphorus for plant nutrition [72]. However, the majority of crop plants cannot get sufficient nutrition from soil. Regular application of the industrially manipulated agrochemicals is required for the compensation of soil nutrient deficiency regarding plant growth to meet the demand of growing population. Nevertheless, large scale use of those synthetic fertilizers affects the natural environment in different ways including leaching of chemical compounds into ground water and resulting eutrophication in aquatic ecosystem [20]. Utilization of PGPR is the beneficiary alternative solution to circumvent this situation. For the sustainable management of soil fertilization regarding agricultural progress, researchers are trying to get maximum benefits from the interaction between holobiont (plants) and rhizomicrobiont (PGPR). It is evident that legume plants require a lesser amount of nitrogen fertilizer than the others as having a symbiotic relationship with nitrogen fixing bacteria grown in root nodules. Bacteria gain carbon and other nutrients from plants and give ammonia (converted from the nitrogen of the air) back to the host [15]. However, a nitrogen fixing bacteria, called Acetobacter diazotrophicus, was found in a nonleguminous Mexican sugarcane cultivar [73]. Azoarcus and Herbaspirillum are the two diazotrophs found in rice root, fix considerable amount of atmospheric nitrogen [74]. A large number of nitrogen fixing bacteria were found in several leguminous and nonleguminous plants maintaining symbiotic relationships. Those provide an alternative resource for nitrogen-based CF supplementation in cultivation processes. A. diazotrophicus and Herbaspirillum sp. contributed to the yield of sugarcane through biological nitrogen fixation [75]. Staphylococcus epidermidis, Erwinia tasmaniensis, Pseudomonas aeruginosa, and B. subtilis were the four phosphate solubilizing bacteria (PSB) with IAA, gibberellic acid (GA), and siderophore production capabilities when applied in legume and rice cultivation with NPK supplementation and showed better results of plant growth and development than the plants grown without PSB [21]. The potassium solubilizing rhizobacteria (KSR), Agrobacterium tumefaciens, Rhizobium pusense, Rhizobium rosettiformans, and Flavobacterium anhuiense, (isolated from different regions of Varanasi, India) when applied along with the recommended dose of potassium resulted considerable increase in yield of maize (in Gangetic planes of India) and validated the significance of KSR [24]. It was observed that some nitrogen fixing bacteria (Azotobacter vinelandii, Azospirillum sp., Brevibacillus formosus, Stenotrophomonas sp.) and K-feldspar solubilizing bacterial strain like Pseudomonas azotoformans enhanced the growth of tomato by improving the NPK availability [76]. Sood et al. [77] characterized Serratia marcesence from the rhizospheric soil and root endosphere of wheat. Indeed, this particular PGPR stain had the potential of IAA, siderophore, ammonia production, and phosphate solubilization. The use of that strain in combination with 80% recommended dose of fertilizer increased the wheat growth significantly and reduced the requirement of CF at the rate of 18 kg of nitrogen and 10 kg of phosphorus per hectare [77]. Burkholderia cepacia, Klebsiella sp., Serratia marcescens, and Enterobacter sp. were applied with NPK fertilizer in the cultivation of ginger. Those strains increased the soil microbial biomass and thereby enhanced yield [78]. Azospirillum and Azotobacter inoculation reduced the half of N-fertilizer use in sesame and also increased the yield and oil quality [79]. A similar type of result was also found in the case of Brassica carinata (mustard) and Carthamus tinctorius (safflower) [80, 81]. Use of rhizobacterial strains of Pseudomonus putida, B. cepacia, and Burkholderia sp. in the cultivation of mustard with a reduced dose of CF increased plant height, biomass, and yield in comparison with uninoculated control set where full recommended dose of NPK fertilizer was used [82]. Dhandapani and Nadu [83] examined the biofertilizing effect of four nitrogen fixing bacteria Azotobacter, Azosprillium, Phosphobacter, and Rhizobacter on Helianthus annuus (sunflower) plant and observed 40.95%–45.87% increase in growth and 60%–90% increase in yield. Coinoculation of Azotobacter chroococcum, B. megaterium, and Bacillus mucilaginous with arbuscular mycorrhizal fungus Glomus mosseae or Glomus intraradices in maize has given a result of increase in biomass, seedling height, nutritional (total N, P, K) assimilation of plant and soil fertility improvement which is comparable to the effect after the application of half dose of CF [84]. Rhizobacteria like Sinorhizobium meliloti, Bradyrhizobium japonicum and P. putida have functioned to increase shoot and root system dry weight in soybean plant. For this, the authors accredited nitrogen fixation and potassium solubilizing abilities of those strains, in their study [85]. Mesorhizobium mediterraneum inoculation in soil during plantation of barley and chickpea enhanced the plant growth with the events like phosphate solubilization and nitrogen fixation. However, an increase in the total nitrogen, potassium, calcium, and magnesium content of treated plants was also noticed [86]. The growth promotion of Pisum sativum (pea) by the inoculation of P. fluorescens was attributed to the significant phosphate solubilization capacity (~400−1300 mg L−1) of that bacterial strain [87]. Paenibacillus polymyxa, P. putida, and Rhodobacter capsulatus were suggested for using in sustainable agriculture as they increased root weight (2.8%–46.7%) and yield (9.8%–20.8%) of sugarbeet in greenhouse condition [88]. Recently, Wang et al. [25] have used Bacillus sp., Agrobacterium tumifaciens, Klebsiella varicola, R. larrymoorei, Pseudomonus sp., and Klebsiella pneumonae in wheat cultivation and observed remarkable increase in nitrogen (49.46%), phosphorus (99.51%), and potassium (19.38%) in soil. Likewise, NPK contents in wheat plants were also elevated by 97.7%, 96.4%, and 42.1%, respectively. Requirement for the CF supplements was decreased about 25% in wheat cultivation after using those microbial inoculum. A nonpathogenic strain of Erwinia sp. enhanced seed germination, seedling development, and root elongation in tomato and lettuce with significant phosphate solubilization, IAA production, and siderophore production [89]. Successful application of those types of PGPR in crop development may solve the problem of frequent application of synthetic fertilizers. Kobua, Jou, and Wang [90] applied CF and PGPR strains conjointly in four different combinations like (i) 100% CF, (ii) 75% CF + 25% PGPR, (iii) 50% CF + 50% PGPR, and (iv) 100% PGPR. In their study, 25%–50% reduction in CF compensated with PGPR significantly increased grain yield and crop biomass production in rice. Bacterial strains used here are Bacillus aryabhattai, Burkholderia ambifaria, Stenotrophomonas maltophilia, Sphingomonas sp., Burkholderia caribensis, Sphingobium yanoikuyae, and Paenibacillus glucanolyticus [90]. The information given here are summarized in Table 2.
| Name of PGPR strains | Plant | Function | References |
|---|---|---|---|
| Acetobacter diazotrophicus | Mexican rice cultivar | Nitrogen fixation | [73] |
| Azoarcus sp., Herbaspirillum sp. | Rice | Nitrogen fixation | [74] |
| Serratia marcesence | Wheat | Production IAA, siderophore, and ammonia. Phosphate solubilization | [77] |
| P. azotoformans, A. vinelandii, Azospirillum sp., B. formosus, and Stenotrophomonas sp. | Tomato | Potassium solubization, nitrogen fixation, and increase NPK availability | [76] |
| A. vinelandii, Azospirillum sp., B. formosus, and Stenotrophomonas sp. | Tomato | Nitrogen fixation | [76] |
| S. epidermidis, Erwinia tasmaniensis, P. aeruginosa, and B. subtilis | Rice, legumes | Phosphate solubilization, production of IAA, GA, and siderophore | [21] |
| Bacillus sp., Agrobacterium tumifaciens, Klebsiella varicola, Pseudomonus sp., and Klebsiella pneumonae | Wheat | Enhancement of nitrogen, phosphorus, and potassium content | [25] |
| B. cepacia, Klebsiella sp., S. marcescens, and Enterobacter sp. | Ginger | Increase in soil microbial biomass | [78] |
| Pseudomonus putida, B. cepacia, and Burkholderia sp. | Mustard | Phosphate solubilization, IAA production | [82] |
| Acetobacter diazotrophicus and Herbaspirillum sp. | Sugarcane | Nitrogen fixation | [75] |
| A. tumefaciens | Maize | Potassium solubilization | [24] |
| Azospirillum and Azotobacter | Sesame, mustard, safflower | Nitrogen fixation | [79–81] |
| Azotobacter, Azosprillium, Phosphobacter, and Rhizobacter | Sunflower | Nitrogen fixation | [83] |
| A. chroococcum, B. megaterium, and Bacillus mucilaginous | Maize | Nitrogen fixation, potassium solubilization, and phosphate solubilization | [84] |
| Sinorhizobium meliloti, B. japonicum, and P. putida | Soybean | Nitrogen fixation, phosphate solubilization | [85] |
| P. polymyxa, P. putida, and R. capsulatus | Sugar beet | Nitrogen fixation, phosphate solubilization | [88] |
| P. fluorescens | Pea | Phosphate solubilization | [87] |
| M. mediterraneum | Chickpea, barley | Nitrogen fixation, phosphate solubilization, increasing total nitrogen, potassium, calcium, and magnesium content | [86] |
| Erwinia sp. (nonpathogenic) | Tomato, lettuce | Phosphate solubilization, IAA production, and siderophore production | [89] |
| Bacillus aryabhattai, B. ambifaria, S. maltophilia, Sphingomonas sp., B. caribensis, S. yanoikuyae, and P. glucanolyticus | Rice | Increase in chemical fertilizer activity | [87] |
- Abbreviations: GA, gibberellic acid; IAA, indole-3-acetic acid; PGPR, plant growth promoting rhizobacteria.
5.3. Challenges Regarding PGPR Application and Possible Ways for Improvement
Laboratory screening-based PGPR inoculum development mostly relies on specific functions (like auxin production, phosphate solubilization, nitrogen fixation, etc.) of pure culture isolates. Manipulation of the rhizospheric microbial population through the PGPR inoculation has given a propitious output under green house and laboratory conditions but varied results were found in the field cultivation [91]. Rhizospheric colonization of PGPR inoculants depends on pH, temperature, soil texture, aeration, organic matter, microaggregation, water availability, ion exchange capacity, competition with soil grown micro- and macro-fauna, and a few more factors [92]. Addition of biochar to the soil may improve the rhizobacterial colonization by providing suitable ambience including water, oxygen, nutrients, niche space, etc., and increase soil fertility [93]. In several areas, soil fumigation is a regular practice for controlling pest before the cultivation of high-value crops. Repeated application of fumigants also affects the beneficial biocommunity of the soil and its interaction with the plants [94]. Therefore, too much fumigation should be avoided to maintain the soil health and fertility. Assemblage of microorganisms attached to the surface sometimes forms biofilm being enclosed within an extracellular polymeric substance matrix. Formation of biofilm on plant tissue surface is of great importance in the maintenance of plant–microbe interaction. Enhancement of PGPR activities (e.g., mineral solubilization) through the inoculation of seeds with biofilm was reported by some authors [95, 96]. Sometimes, more than one organism contributes to plant growth improvement with their coactivities. Plant root-associated symbiotic mycorrhizal fungi enhance the root surface area and facilitate acquisition of a larger amount of soil nutrients [97]. Combined inoculation of PGPR and mycorrhiza during the cultivation process would increase the accessibility of nutrients by their synergistic action [98]. Coinoculation of Glomus fasiculatum (mycorrhiza), B. megaterium (PSB), and Azotobacter (diazotroph) significantly increased NPK uptake [99]. Glomus intraradices is another mycorrhizal fungus when inoculated with two PGPR strains B. amyloliquefaciens and B. pumilus induced significant improvement in yield [100]. Improvement of nitrogen nutrition in rice plant was observed in the presence of a Basidiomycetous yeast strain named Rhodotorula mucilaginosa JGTA-S1. However, the ability to convert nitrate or nitrite to ammonia has not been found directly in that yeast strain. Here, the nitrogen enrichment was contributed by the diazotrophic endobacteria Pseudomonus stutzeri harbored by that yeast strain (R. mucilaginosa JGTA-S1) [101]. Utilization of those mycorrhiza will certainly improve the colonization and activity of PGPR strains. Hence, efficient strategies should be applied for the successful colonization and consistent performance of PGPR inoculum under field conditions.
6. Discussion on the Comparative Study of Cited Literatures
The plant growth improvement by rhizospheric microorganism is found very much significant as per the analysis of relevant literatures. The valuable information on nutritional enrichment through those microbial inoculation indicates the great importance of PGPR. However, sufficient reports are not available to develop a sustainable model for PGPR application in cultivation field. Their thought about the way of application should be mentioned briefly in the respective articles. The total number of plants that have been taken during the experiment is an important factor but was not mentioned in most of the cases and questions are created regarding the reliability. So, total number of plants considered for the study is an important thing which is needed to be documented. Collection of authentic plant samples and representation of statistical data for the respective experimental works are needed in well-structured reports but not present in all. Those issues should be kept in mind in next studies for making them more comprehensive. During the compilation of data interpretation of different experimental works for writing this review all the cited articles are critically analyzed in terms of the methodologies of the works and results. Prominent differences have been noted by the authors of this review article in several aspects like types of PGPR strains, mode of inoculation, tenure of the experimental works, statistical evidences, etc. It is crucial to mention the overall percentage of success after a large-scale survey but still, it is lacking in many cases. It is also needed for the further improvement of the way of reporting. Comparative study between the same plants with well growth and poor growth collected from different soil zones (which may contain different microbial association) is needed to be conducted also. This may provide a better understanding for the bioactivities of PGPR trains. Isolation, characterization, and identification of different PGPR stains were reported in several literatures. However, more specific information is needed here to ascertain their contribution behind their roles claimed in the reports. In many reports, it was found that soil bacteria secret several bioactive compounds which were thought to fight against other pathogenic organisms. In this context, in silico analysis (of interaction between two compounds, one from microbial source and another from plant source) may provide useful data. Antagonism can be explained through the interaction between bioactive compounds secreted from PGPR strains and toxic compound secreted from pathogenic strains. Little conflict of concepts has been found in different studies. Standardization of the dosage of application of commercially available PGPR to obtain the maximum efficacy is necessary but the considerable information is not available in previous reports. Whether the activity of PGPR strains depends upon their population doubling time or not is not discussed anywhere. Information about the maintenance and handling of useful strains is also missing. It should be observed finely and hypothesized whether the performance of PGPR strains is affected by different environmental factors like temperature, humidity, air flow, precipitation, etc. Addition of those information after further studies may enlighten this new avenue of organic farming process.
7. Authors’ Own Opinion About the Significance of the Study
After consulting the previous paradigm of describing PGPR, different aspects are included in one write up which are not so common in other articles. A comprehensive story of PGPR mentioning its discovery, growing condition, affinity to the host, as well as trading off relation from host side, necessity, utility, and sustainable activity as fertilizer in comparison with traditional agrochemicals has been given here. This review briefly draws a clear idea that PGPR strains are involved in transporter gene expression of the host plants and therefore can be marked as the forerunners of an advanced weapon in genetic engineering for host gene function alteration regarding productivity improvement. Commercial development of PGPR bioinoculant existing shortcomings along with their possible solutions is discussed and that may encourage further research on this. Prospective line of thoughts toward the universal use of PGPR as biofertilizer has been shared in the next section.
8. Future Prospect
In the face of global climate change and the rapidly increasing population, it is time for the agricultural scientists to think about how to feed this 7.7 billion world population [102]. The green revolution saved a large fraction of global population from starvation and underfeeding by increasing agricultural production, triggering CF development, and other necessary requirements. Taking into account the negative impacts of the CFs on the ecosystem, a tendency to restrict their overuse has been grown among scientists. As the complete elimination of the use of CFs with avoiding drastic drop in food production is not possible in the present situation, an urgent need to find alternative measures necessitates extensive studies on PGPR. The market value of PGPRs is still little in amount as compared to CF but the demand for these reliable and cost-effective products is increasing day by day. Sometimes, multifaceted activities of a single type strain remain unexplored but need a great deal of attention for clarifying the regulation of those functions. The PGPR inoculum having the ability to control disease together with plant growth promoting activities has a great potential with notable market value [69]. Reduction of adverse effects of abiotic stresses like salinity, drought, heavy metals, etc., through PGPR mechanisms is also a prospective route in sustainable agriculture [59]. Using modern approaches of microencapsulation and nanoencapsulation (i.e., coating the bacterial cells with polymer sheaths) may improve the viability range of PGPR inoculum [54]. Reports that are presently available regarding the simultaneous activation of multiple signals during plant–microbe interaction are still not sufficient enough to understand the event, completely. Also, the comprehensive information regarding plant-microbe–microbe interaction found in previous reports is negligible. Interpretation of the interconnection among multiple signals generated in plants in response to PGPR attributes has not been found anywhere in detail. Focusing on those issues in an integrated manner may resolve some unanswered questions and open a new avenue for the development of a holistic approach including transcriptomics, metabolomics, and proteomics. Moreover, in-depth knowledge of soil microorganisms through interdisciplinary research, large-scale production, and advanced business management strategies are required for successful marketization of PGPR products. Employment of the remarkable impacts of PGPR in relation to biofertilization, bioremediation, and biocontrolling in agriculture with technological advancement can uplift the economic condition both in the country and worldwide.
9. Conclusion
Proper understanding of the rhizospheric microenvironment of the plant of interest will help to guide the choice of PGPR strains for inoculant formulation and fruitful application. Genetic conservation of novel strains for rhizospheric management regarding crop cultivation is very important here. Use of advanced tools may lead to increase the effectiveness of the inoculant along with their survival and colonization in root surface. In this study, we have presented the descriptive information about rhizospheric microorganism and their association with plant root surface along with different necessary parameters for successful interaction, PGPR strains showing bioactivities favoring the crop cultivation and their potentialities for biofertilization, and steps for the promising commercial use of PGPR strains mainly. It may help others to gain clear idea about the possibility of PGPR application in traditional cultivation system instead of vigorous use of CFs. Hope that the further improvement of techniques will direct the sustainable PGPR application toward the development of ideal agroecosystem.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Dew Biswas drafted the entire manuscript. Amit Kumar Chakraborty and Vikas Srivastava checked the manuscript. Arunava Mandal conceived the idea and thoroughly checked and finalized the manuscript.
Funding
The authors’ work is supported by Start-up Research Grant of Science and Engineering Research Board, Department of Science and Technology, Government of India (File no. SRG/2020/001618). Dew Biswas acknowledges CSIR for providing fellowship (File no. 09/028(1152)/2020-EMR-I).
Open Research
Data Availability Statement
There are no data to report. We have discussed the research outcomes of several research groups working in this field.




