The Influences of Rootstock on the Performance of Pinot Noir (Vitis vinifera L.): Berry and Wine Composition
Abstract
Background and Aims. Rootstocks are widely used in the viticulture industry to manage biotic stress, particularly the infestation of phylloxera, and to improve tolerance to abiotic stress. Grafting grapevines to rootstocks affects the berry quality and may influence the wine composition. This project investigated the impacts of 14 different rootstocks on the berry and wine chemical properties, phenolic profile, and volatile profile of grafted Vitis vinifera L. cv Pinot noir MV6. Methods and Results. This study was conducted at a commercial vineyard located in Mornington Peninsula, Victoria, Australia. The scions of V. vinifera L. cv Pinot noir clone MV6 were grafted onto 14 rootstocks, including 101-14 Millardet et de Grasset (101-14 Mgt), 1103 Paulsen, Selection Oppenheim (SO4), 110 Richter, Schwarzmann, 5C Teleki, 3309 Couderc (3309C), Merbein 5489, Merbein 6262, Merbein 5512, C20, C29, C113, and C114, with own-rooted vines acting as the control group. Berries were collected at harvest in the 2020 and 2021 vintages for small-scale winemaking. The pH, titratable acidity, phenolic content, and antioxidant activity of both the berries and wine were measured using conventional chemical analysis. The phenolic composition of the wine over the two vintages was identified and quantified by high-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (HPLC-QTOF-MS/MS) and HPLC with diode-array detection (HPLC-DAD). The volatile composition was measured by headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometry (GC-MS). Grafting Pinot noir onto 3309C caused a reduction in the berry and wine pH without affecting the titratable acid. Several rootstocks, such as 1103 Paulsen, SO4, Schwarzmann, and 5C Teleki, reduced the total phenolic content (TPC) in both the berries and wine of Pinot noir. Conversely, Merbein 5489 increased the berry and wine TPC by 15% and 63%, respectively, compared to ungrafted Pinot noir MV6. A similar tendency was also found in the condensed tannin content (CTC), where Merbein 5489 increased the berry and wine CTC by 35% and 112%, respectively, compared to ungrafted vines. Several rootstocks, such as 3309C and Merbein 6262, increased the wine anthocyanin content (especially malvidin 3-O-glucoside), which may contribute to enhanced colour intensity. The concentrations of several ethyl ester compounds in wine responsible for the fruity aroma, including ethyl hexanoate, ethyl octanoate, and ethyl butanoate, were significantly higher in scions grafted to Schwarzmann, 3309C, Merbein 5489, and C29, compared to scions grafted to 101-14 Mgt, 1103 Paulsen, and ungrafted vines. This suggests that rootstock plays an important role in altering the texture and flavour of Pinot noir wine. Conclusions. Grafting Pinot noir to any rootstock in this study caused changes in the phenolic and volatile content of Pinot noir wine, likely affecting the perceived wine quality. Wine from Pinot noir grafted onto Merbein 5489, in particular, with relatively high CTC and anthocyanin content, as well as higher concentrations of volatile compounds contributing to the fruity aroma, represents an attractive option for grafting Pinot noir MV6 in cool climates. Significance of the Study. The present study provides results reflecting the impacts of grapevine rootstocks on Pinot noir berry and wine composition, supporting rootstock selection for Pinot noir. The findings offer guidance to vignerons in choosing suitable rootstocks to manage berry and wine acidity, phenolic accumulation and composition, and volatile profile, thereby improving the wine quality. Furthermore, the results could guide future studies in understanding the roles of rootstocks in regulating the metabolic pathways of phenolic and volatile production in the berries of grafted Pinot noir at the molecular level.
1. Introduction
Phylloxera is a significant threat to the Australian wine industry, given that a substantial proportion of grapevines are cultivated on their own roots, thereby increasing their susceptibility to this sap-sucking pest [1]. This is especially true for Victorian wine regions [2]. The Mornington Peninsula, for instance, is adjacent to the Phylloxera Risk Zone, being in relative proximity to phylloxera-infected zones such as the Yarra Valley. The Mornington Peninsula region is a leading cool-climate wine region in Australia and widely recognised for its high-quality Pinot noir production. Any phylloxera invasion could have devastating effects on the local wine industry. Therefore, the adoption of rootstocks is critical to prevent potential detrimental impacts of phylloxera and to maintain the long-term sustainability and resilience of the regional viticultural industry [3].
Apart from contributing to disease management, rootstocks affect the berry quality by altering chemical components. Rootstocks manage the vigour of grafted scions and affect the berry quality by altering physiological responses, including modification of the hydraulic capacities of root systems and the control of stomatal closure to reduce water loss during water stress [4, 5]. Previous research suggested that the adaption of different rootstocks can result in variations in the berry pH and titratable acidity of Shiraz [6], Cabernet Sauvignon [7], and Pinot noir [8]. Certain rootstocks, including Selection Oppenheim (SO4) and 1103 Paulsen, have been shown to affect the accumulation of total phenolic, tannin, and flavan-3-ol contents in berries of Cabernet Sauvignon by managing canopy development during veraison and ripening stages [9].
Pinot noir wine contains a wide range of phenolic compounds, especially anthocyanins, flavonols, flavan-3-ols, and phenolic acids, which contribute to the texture, flavour, and colour of the wine [10–12]. Specifically, gallic acid (13.8–101.3 mg/L) and caftaric acid (12.2–118.0 mg/L) are the most common phenolic acids found in Pinot noir [11, 12]. The most abundant flavonols and flavan-3-ols are quercetin (8.3–91.0 mg/L), catechin (228.0–315.0 mg/L), and epicatechin (89.0–130.0 mg/L), which play important roles in establishing the bitterness and astringency mouthfeel of the wine [10, 11]. The composition of anthocyanins in Pinot noir wine mainly includes malvidin-3-O-glucoside (73.8–219.0 mg/L), cyanidin-3-O-glucoside (11.8–73.4 mg/L), and delphinidin-3-O-glucoside (3.7–12.8 mg/L) [10–12]. These anthocyanin compounds contribute to the blue and purple colour intensity of the wine [13] and enhance the mid- to long-term colour stability of the wine through copigmentation with flavonols [14]. A previous study claimed that grafting Pinot noir onto certain rootstocks, including 110 Richter and SO4, could result in a significantly higher total phenolic content (TPC) and total anthocyanin content in the wine compared to the same scion grafted onto Kober 125 AA and Riparia Gloire de Montpellier [15]. However, none of the previous studies has comprehensively evaluated the influence of rootstocks on the phenolic profile of Pinot noir wine.
Common volatile compounds in Pinot noir wine have also been profiled previously [11, 16]. Several ester compounds in Pinot noir wine, including ethyl butanoate, ethyl 2-methylbutanoate, ethyl 3-methylbutanoate, ethyl hexanoate, ethyl octanoate, and ethyl decanoate, have been shown to contribute to the fruity aroma [11, 16]. Likewise, fatty acids that confer cheese and rancid aromas, especially 3-methylbutanoic acid and octanoic acid, are commonly found in Pinot noir wine [16]. However, how rootstocks alter the accumulation of volatile compounds and contribute to the varietal aromas of Pinot noir wine remains uncertain.
This study evaluated the impact of rootstocks on grape and wine quality as reflected by their chemical properties, composition, and sensory profile. The aim was to determine the suitability of these rootstocks for V. vinifera cv. Pinot noir in the local climate of the Mornington Peninsula. The project investigated the effects of 14 different rootstocks on the grape and wine quality of grafted V. vinifera L. cv Pinot noir MV6. Parameters related to pH, titratable acidity, phenolic composition, and volatile profiles were measured in both berries and wine. The results can be used to evaluate and compare rootstocks regarding their impacts on the berry and wine composition of Pinot noir, providing guidance to vignerons to improve the wine quality by choosing suitable rootstocks for grafting Pinot noir in cool-climate regions.
2. Materials and Methods
2.1. Site Location and Experimental Design
This study was conducted at Robinson Vineyard (38.295 S, 145.058 E, elevation 48 m above sea level, northerly aspect) located in the Mornington Peninsula wine region of Victoria, Australia. The scions of V. vinifera L. cv Pinot noir MV6 were grafted onto 14 rootstocks between 2014 and 2016 (Table 1). The most commonly grafted rootstock for Pinot noir in the Mornington Peninsula, named 101-14 Millardet et de Grasset (101-14 Mgt), and six other conventional rootstocks that have been widely used worldwide, including 1103 Paulsen, Selection Oppenheim (SO4), 110 Richter, Schwarzmann, 5C Teleki, and 3309 Couderc (3309C), have been evaluated in the present study. These aforementioned rootstocks possess divergent genomic backgrounds of V. riparia, V. rupestris, and V. berlandieri. Additionally, three low to moderate vigour rootstocks originating from intraspecies crossbreeding between either V. berlandieri or V. cinerea, namely, Merbein 5489, Merbein 6262, and Merbein 5512, were also included. These three rootstocks may potentially reduce the canopy size of the grafted vines and result in more concentrated chemical compositions in the berries [17]. Furthermore, to evaluate the performance of newly developed rootstocks, four unnamed CSIRO selections of C series rootstocks, temporarily named C20, C29, C113, and C114, were also included in this study. Ungrafted Pinot noir MV6 grapevines were planted as own-rooted controls. A flat block was selected in the vineyard as the experimental site to avoid variance in soil nutrient status and moisture among different treatments. The trial was planted in three rows, with five treatments per row within the block. Each treatment was triplicated, with one panel per replicate and five vines within each panel. The three panels from each treatment were allocated to a single row. The vines were planted with 2.5 m spacing between rows and 1.5 m spacing between vines within the same panel. The trial block was surrounded by commercial grapevine panels to further avoid the influence of inconsistent wind, soil moisture, and soil nutrients. The spatial arrangement of rootstocks was completely randomised. Details related to the spatial arrangement of rootstocks, the training system, pruning method, irrigation schedule, and fertilising schedule have been reported in our previously published work [18, 19].
| No. | Rootstock | Parent species | Planting time |
|---|---|---|---|
| 1 | 101-14 Millardet et de Grasset | V. riparia × V. rupestris | 06/11/2014 |
| 2 | 1103 Paulsen | V. berlandieri × V. rupestris | 06/11/2014 |
| 3 | SO4 | V. berlandieri × V. riparia | 06/11/2014 |
| 4 | 110 Richter | V. berlandieri × V. rupestris | 06/11/2014 |
| 5 | Schwarzmann | V. riparia × V. rupestris | 06/11/2014 |
| 6 | 5C Teleki | V. berlandieri × V. riparia | 06/11/2014 |
| 7 | 3309C | V. riparia × V. rupestris | 06/11/2014 |
| 8 | Merbein 5489 | V. berlandieri var. helleri “Resseguier #1” × V. berlandieri var. helleri Mazade | 06/11/2014 |
| 9 | Merbein 6262 | V. cinerea “B 58” × V. cinerea B 194-1 | 06/11/2014 |
| 10 | Merbein 5512 | V. berlandieri var. helleri “Resseguier #1” × V. berlandieri var. helleri 7651 | 06/11/2014 |
| 11 | C20 | V. champinii × V. rupestris × V. riparia | 01/11/2016 |
| 12 | C29 | V. champinii × V. rupestris × V. riparia | 01/11/2016 |
| 13 | C113 | V. champinii × V. cinerea | 01/11/2016 |
| 14 | C114 | V. champinii × V. berlandieri | 01/11/2016 |
| 15 | Own roots | Vitis vinifera L | 29/09/2015 |
Evaluation of the grafted grapevines was conducted in the 2020 and 2021 vintages. Thirty bunches of berries (approximately 100 g per bunch) were randomly selected and collected from each replication when the total soluble sugar (TSS) content in berries reached approximately 22 °Brix in both vintages (13 March in the 2020 vintage and 7 March in the 2021 vintage, respectively). Samples were cooled with dry ice and stored at 4°C prior to small-scale winemaking and lab-based chemical analysis. Weather data for the 2020 and 2021 vintages were collected from the nearest Australian Bureau of Meteorology Cerberus weather stations (temperature data from Station No. 086361, precipitation data from Station No. 086079). The 2020 vintage experienced higher precipitation during veraison to harvest and lower temperatures close to harvest (mean max temperature (MMXT) in April 2020: 18.6°C, MMXT in April 2021: 19.9°C) (Table S1).
2.2. Small-Scale Winemaking Trial
The experiment was conducted following the small-scale fermentation protocol established by the Irymple Research Centre of the Department of Jobs, Skills, Industry and Regions of the Victorian Government (DJSIR), as previously described with modifications [20]. Briefly, 2 kg of berries was destemmed from each replication and crushed in sanitised 2-L containers, with three replications per treatment. Then, 0.25 g/L commercial yeast (Lalvin RC212, Lallemand Wine, Montreal, Quebec, Canada) was added to the juice, followed by the addition of diammonium phosphate (10% m/v) at 0.1 mL/L and potassium metabisulphite (10% m/v) at 0.5 mL/L. Primary fermentation was carried out at 18°C in a temperature-controlled aerobic incubator for 7 days until dry (Baume readings <1) and then pressed using a ratchet-style press. Wines were transferred into clean 750-mL sealed glass wine bottles, with the addition of potassium metabisulphite (10% m/v) at 0.5 mL/L and commercial malolactic bacteria (PN4, Lallemand Wine, Montreal, Quebec, Canada) at 0.1 g/L. The wines were placed in a temperature-controlled aerobic incubator at 18°C for 28 days to perform malolactic fermentation and were then racked into 750-mL bottles filled with CO2 and placed in a 4°C cool room for stabilisation until analysis.
2.3. Analysis of Berry and Wine Chemical Properties
2.3.1. Berry Total Soluble Sugar
Soluble sugar in juice (°Brix) was measured at the initial stage of small-scale winemaking. Approximately 1 g of supernatant was collected from each replication prior to yeast fermentation. The Brix degree was then measured using a digital refractometer (HI96811, HANNA Instruments, Smithfield, RI, USA) at 20°C. The refractometer was calibrated with distilled water before measuring the samples.
2.3.2. Juice and Wine pH and Titratable Acidity
The pH and titratable acidity of the juice were measured at the initial stage of small-scale winemaking prior to the addition of commercial yeast. The measurement of pH and titratable acidity for wine was conducted 28 days after the completion of small-scale winemaking. For each replication, 10 mL of sample was precisely measured and transferred into a 50-mL vial, where the pH was then measured using a digital pH meter (HI5221, HANNA Instruments, Smithfield, RI, USA). The 10-mL samples were then titrated with 0.1 M NaOH to pH 8.2, and the volume of the NaOH solution used was recorded. The results were converted to g/L tartaric acid (g/L H2T).
2.4. Analysis of Phenolic Contents and Antioxidant Activity
2.4.1. Sample Preparation
Extraction of phenolic compounds from berries was performed following an established protocol with modifications [21]. Approximately 50 g of berries collected from each replication was frozen with liquid nitrogen and then blended into powder using a grinder (MultiGrinder II, Sunbeam Products, Boca Raton, FL, USA). Samples were then transferred into 50-mL vials and stored at −20°C for 24 hours. Subsequently, the samples were freeze-dried at −50°C for 72 hours using a freeze drier (Table Top Freeze Dryer BK-FD10S, BIOBASE, Jinan, Shandong, China). Two grams of the freeze-dried sample from each replicate was dissolved in 20 mL of methanol with 0.1% HCl (v/v) and kept at 20°C for 24 hours under shaking conditions at 150 rpm for phenolic extraction. The extracted samples were then centrifuged at 7000 rpm (4°C) for 15 minutes using a centrifuge (Allegra X-12R, Beckman Coulter Australia Pty Ltd, Glen Waverley, VIC, Australia). The supernatant was collected and stored in 15-mL centrifuge tubes at −20°C in the dark until further analysis, with the headspace of the centrifuge tubes being flushed with nitrogen gas.
2.4.2. Determination of Total Phenolic Content (TPC) in Berry and Wine
TPC was measured using the Folin–Ciocalteu method described by Liang et al. [22] with modifications. Wine and extracted berry samples were diluted fivefold using methanol. A 30-μL diluted sample was mixed with 750 μL of 0.2 N Folin–Ciocalteu reagent solution and incubated for 5 minutes at room temperature, followed by the addition of 600 μL of 7.5% (w/v) sodium carbonate solution. The mixture was incubated at room temperature for 1 hour in the dark and then transferred to a 96-well flat-bottom microplate. The absorbance was measured using a UV spectrophotometer (Multiskan GO, Thermo Fisher Scientific Australia Pty Ltd, Scoresby, VIC, Australia) at 765 nm. Gallic acid was used as the standard, and methanol as the blank. The results were expressed as mg gallic acid equivalents (GAE)/g dry mass (DM) for berries or as GAE/mL for wine.
2.4.3. Determination of Condensed Tannin Content (CTC)
CTC was determined using the vanillin-HCl assay method described by Luo et al. [23] with modifications. Wine and extracted berry samples were diluted 25-fold using methanol. The vanillin-HCl reagent was prepared daily by mixing equal volumes of 2% vanillin in methanol solution (w/v) with acidified methanol solution (8% HCl, v/v). An aliquot of 160 μL of the diluted sample was mixed with 140 μL of vanillin-HCl reagent in a 96-well microplate and incubated for 20 minutes at room temperature in the dark. The absorbance was then measured at 500 nm using a UV spectrophotometer. Catechin was used as the standard, and methanol was used as the blank. The results were expressed as mg catechin equivalents (CAE)/g DM for berries or as CAE/mL for wine.
2.4.4. Determination of Ferric Reducing Antioxidant Power (FRAP)
The FRAP assay was conducted according to the method of Liang et al. [22] with slight modifications. Wine and extracted berry samples were diluted 25-fold using methanol. FRAP reagents A and B were freshly prepared daily. Specifically, FRAP A was prepared by mixing acetate buffer (300 mM, pH = 3.6), 10 mM TPTZ solution, and iron (III) chloride anhydrous solution in a ratio of 10 : 1 : 1 (v/v/v), while FRAP B was prepared by mixing acetate buffer (300 mM, pH = 3.6), 10 mM TPTZ solution, and distilled water in a ratio of 10 : 1 : 1 (v/v/v). For the assay, 10 μL of diluted wine or berry extract sample was added to a 96-well microplate prefilled with 90 μL of distilled water and 200 μL of FRAP reagent A, followed by a 5-minute incubation at 37°C in the dark. The absorbance was then measured at 593 nm using a UV spectrophotometer. A standard curve was prepared by mixing 100 μL of 0–1.5 mM iron (II) sulphate heptahydrate with 200 μL of FRAP B reagent. The results were expressed as mM Fe2+ equivalents/g DM for berry or mM Fe2+ equivalents/mL for wine.
2.5. Analysis of Wine Phenolic Compounds by HPLC
2.5.1. HPLC-DAD Quantitative Analysis of Wine Phenolic Compounds
For each replication, a 1-mL wine sample was filtered through a 0.45-μm pore size filter and dispensed into 2-mL chromatography vials. The quantification of phenolic compounds in wine was completed using an Agilent 1260 series HPLC system equipped with diode-array detection (Agilent Technologies, Santa Clara, CA, USA). Mobile phase A was 0.1% formic acid in distilled water, and phase B was 0.1% formic acid in LC-MS grade acetonitrile. The separation was achieved on a Synergi Max-RP 80A LC column (4 μm, 250 × 4.6 mm) protected by a C12 guard column (4.0 × 3.0 mm) (Phenomenex, Lane Cove, NSW, Australia). The LC conditions were set following the method described by Xiong et al. [24]. Briefly, the column temperature was maintained at 30°C, the injection volume was set at 10 μL, and the flow rate was 0.5 mL/minute. The DAD scan range was set from 190 to 720 nm with monitoring wavelengths at 280 nm for hydroxybenzoic acids, 325 nm for hydroxycinnamic acids, 365 nm for flavonols, and 520 nm for anthocyanins. The elution program was set as follows: 5% B (0 min), 5–15% B (10 min), 15–20% B (25 min), 20–25% B (15 min), 25–40% B (10 min), 40–100% B (5 min), 100% B (15 min), 100–5% B (0.1 min), and 5% B (4.9 min). The concentration of phenolic compounds was quantified by comparison with external standards (Table S6), and the data were analysed using OpenLab software (Agilent Technologies, Santa Clara, CA, USA).
2.5.2. HPLC-QTOF-MS/MS Qualitative Analysis of Wine Phenolic Compounds
Phenolic identification was conducted using an Agilent 1200 series high-performance liquid chromatography system equipped with a vacuum degasser, autosampler, binary pump, and Agilent 6520I Accurate-Mass Q-TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA). The same column (maintained at 40°C) and LC setup were applied as previously described in section 2.5.1. The QTOF-MS/MS analysis was based on Xiong et al. [24] with modifications. Briefly, phenolic compounds were analysed twice in both positive and negative modes separately, via a dual electrospray ionisation source (ESI). The MS acquisition parameters were as follows: drying gas N2, temperature 325°C, gas flow 9 L/minute, nebuliser 45 psi; capillary voltage 3500 V, fragmentor 175 V; and MS scan range 90–1000 m/z. The MS/MS was performed in the automode with an MS/MS scan range of 60–1000 m/z and collision energy of 15–30 eV.
2.6. Analysis of Volatile Compounds by HS-SPME-GC-MS
Wine samples were prepared for wine volatile analysis based on the protocol proposed by Luo et al. [25] with some modifications. For each replication, 10 mL of wine sample was transferred into a HS-SPME vial (Agilent Technologies, 20 mL) with the addition of 2 g of sodium chloride and 20 μL of 4-octanol (internal standard; 100 mg/L). The vial and its contents were shaken at 220 rpm and heated to 35°C. A polydimethylsiloxane (PDMS, Agilent) 100 μm fibre was exposed to the headspace and agitated for 10 minutes.
An Agilent Technologies 6850 Series II (GC; Agilent Technologies, Santa Clara, CA) equipped with an Agilent PAL 120 multipurpose autosampler and coupled with an Agilent 5873 mass selective detector (MSD) was used for volatile assessments. The instruments were controlled using Agilent G1701EA MSC ChemStation software in conjunction with Agilent PAL Sampler Software Control B.01.04 for ChemStation. The GC was fitted with a J&W DB-wax column (30 m × 0.25 mm, 0.25 μm film df) with helium as the carrier gas (ultrahigh purity, BOC Australia, North Ryde, NSW, Australia), and the flow rate was 2.0 mL/minute in a constant flow mode. The GC inlet was fitted with a borosilicate glass SPME inlet liner (Agilent, 6.3 mm o.d., 78.5 mm length) held at 220°C.
The SPME fibre was desorbed in the pulsed splitless mode with the splitter set at 50 : 1, which was opened after 30 seconds. The fibre was baked in the inlet for 10 minutes. The oven was initialised at 40°C, held for 4 minutes, then increased to 220°C, and held for 20 minutes. The MS source, quadrupole, and transfer line were maintained at 230°C, 106°C, and 250°C, respectively. The MS was operated in the positive EI mode at 70 eV, scanning over a mass acquisition range of 35–350 m/z. The standard solutions of 132 commonly found compounds in wine were prepared in 13 scale dilutions in a model wine solution (13% alcohol, titrate buffer, pH 3.2) and analysed using the GC as wine samples to generate standard calibration curves for volatile quantification. Wine volatiles were identified by comparing the mass spectra and retention indices with the NIST library in ChemStation, the NIST Chemistry WebBook database, and the obtained standard solutions. All compounds were quantified based on the GC peak ratio of individual compounds to the internal standard and the calibration curves generated from the standard solutions. Blank SPME runs and blank internal standards were checked regularly.
2.7. Statistical Analysis
All primary data processing was performed using Microsoft Excel™, with Grubb’s test performed [26] to exclude outliers using Minitab 18 (Minitab, Pennsylvania State University, State College, PA, USA). One-way and two-way ANOVA were performed at p < 0.05 (Fisher’s pairwise least significant difference) using Minitab 18 (Minitab, Pennsylvania, USA). Partial least squares-discriminant analysis (PLS-DA), z-scores, and scatter violin plots were performed using R Studio (R Studio 2022.12.0 + 353, Boston, MA, USA). Bar charts and radar charts were plotted using Microsoft Excel™. Figures for PLS-DA, heat maps, and scatter violin plots were created using the “ggplot2,” “gplots,” “PCAtools,” “ropls,” and “grafify” R packages.
3. Results and Discussion
3.1. Effect of Vintage and Rootstock on Berry Chemical Properties
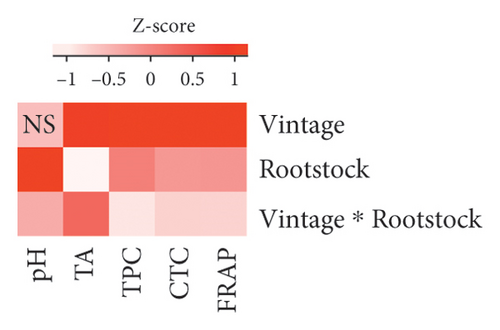
The two-way ANOVA on the chemical properties of berries (Figure 1(a) and Table S2) showed that vintage did not significantly affect the berry pH (p = 0.802). However, it significantly influenced the berry titratable acidity (F value = 10.964, p = 0.002), TPC (F value = 52.935, p < 0.001), CTC (F value = 130.705, p < 0.001), and FRAP (F value = 125.35, p < 0.001). On the other hand, rootstocks showed a significant influence on all properties, particularly on berry pH (F value = 23.678, p < 0.001).
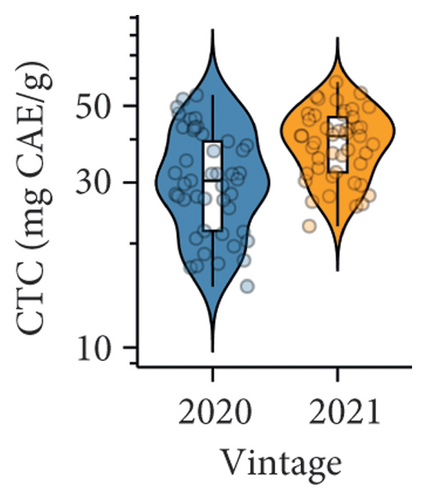
3.1.1. Effect of Vintage on Berry Quality
The general compositions of berries in the 2020 and 2021 vintages are presented in Table S3. The average TSS of berries was 21.6 ± 0.6°Brix in the 2020 vintage and 20.8 ± 0.8°Brix in the 2021 vintage, with no significant difference found between TSS of berries harvested in the 2020 and 2021 vintages.
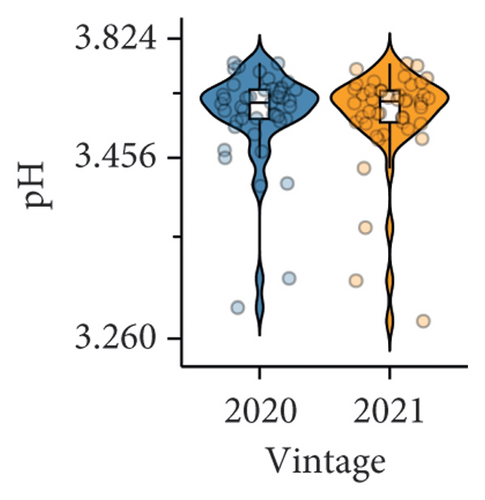
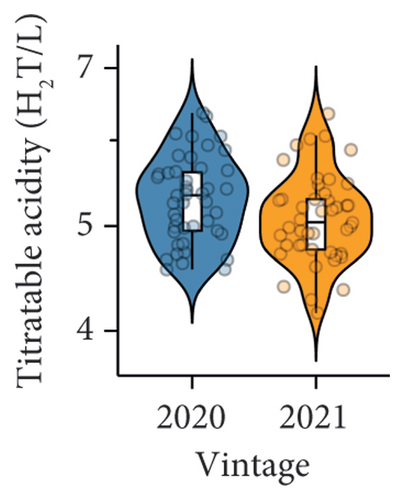
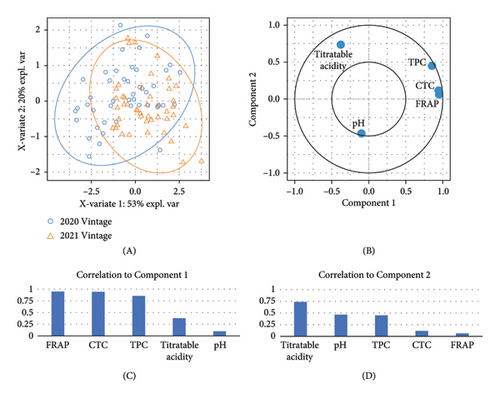
The difference in berry pH was nonsignificant between the two studied vintages (Figure 1(b), Table S3). However, significantly lower titratable acidity was observed in the 2021 vintage compared to the 2020 vintage (Figure 1(c) and Table S3), which was in accordance with previous studies showing that the acidity of berry juice can vary considerably across different vintages [6, 27]. The result of the berry titratable acidity in our study was also supported by PLS-DA (Figure 1(f)). The majority of samples collected in the 2020 vintage were displayed on the positive side of component 2 on the loading plot, which aligned with the direction of the titratable acidity on the correlation circle, suggesting their positive correlation. The inconsistency of the berry titratable acidity over the two vintages is likely due to the influence of abiotic factors. For example, increasing the exposure of Pinot noir berries to sunlight has been proven to raise the titratable acidity of juice [28]. This may explain why the 2021 vintage, with a higher volume of precipitation and therefore less exposure to sunlight from September to January, resulted in lower titratable acidity of grape juice compared to the 2020 vintage in our study. Similarly, the potassium content in berries can also affect the acidity of juice. High concentrations of potassium generally decrease the proportion of free acids (e.g., tartaric acid) in berry juice, which may cause an undesirable taste in wine due to an imbalanced sugar/acid ratio [29]. Our previous study showed that the petiole potassium content in the 2020 vintage was significantly higher than in the 2021 vintage [18], which could, to some extent, reflect the difference in potassium uptake by grafted Pinot noir between the two vintages. Nonetheless, the potassium content specifically in berries was not measured in the presented work, suggesting a future research area.
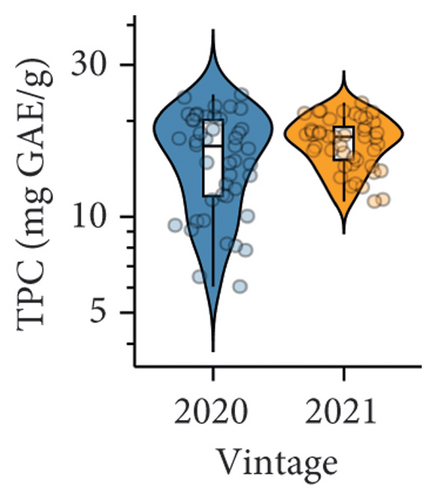
An overall higher TPC and CTC in berries were found in the 2021 vintage compared to the 2020 vintage (Figures 1(d) and 1(e) and Table S3). In our study, both TPC and CTC were the predominant factors distinguishing berry composition between the two vintages, as revealed by PLS-DA (Figure 1(f)). Specifically, both parameters were located on the positive side of component 1 on the correlation circle, which was highly consistent with the distribution of berries collected in the 2021 vintage on the PLS-DA loading plot. The increased accumulation of condensed tannins and other phenolic compounds in the 2021 vintage was likely due to the slightly higher average temperature between November 2020 and February 2021 compared to the 2020 vintage (Table S1), where a warmer environment during the period of anthesis and veraison may facilitate the accumulation of tannins in berries [30].
3.1.2. Effect of Rootstock on Berry Quality
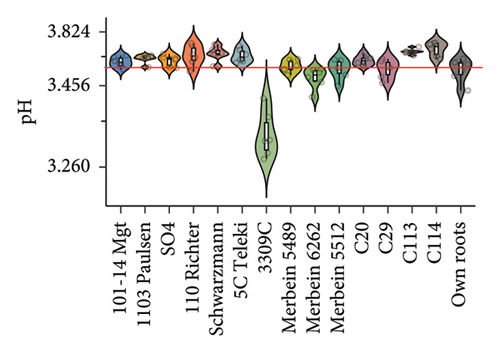
Our study suggested that no significant difference was found between the TSS of berries from vines grafted to different rootstocks. This result is generally consistent with previous studies showing that rootstocks, including Schwarzmann, 101-14 Mgt, 110 Richter, and SO4, had a negligible effect on the berry TSS of red varieties [15, 31]. Grafting Pinot noir onto 3309C (pH 3.32 ± 0.05) significantly reduced berry pH compared to ungrafted vines (pH 3.52 ± 0.05; Figure 2(a) and Table S3). Similarly, relatively low berry pH was also observed in scions grafted to Merbein 6262 (Figure 2(a) and Table S3). However, consistently higher berry pH compared to ungrafted vines was found in scions grafted to C114, C113, 5C Teleki, and Schwarzmann (Figure 2(a), Table S3). The ability of 3309C to reduce the berry pH has been previously reported, with significantly lower berry pH found in Shiraz and Merlot grafted to 3309C compared to ungrafted vines [8]. However, the effects of several other rootstocks, including 101-14 Mgt, 1103 Paulsen, 110 Richter, Schwarzmann, 5C Teleki, Merbein 5489, Merbein 6262, and Merbein 5512, in managing the berry pH were not consistent [6, 8, 27].
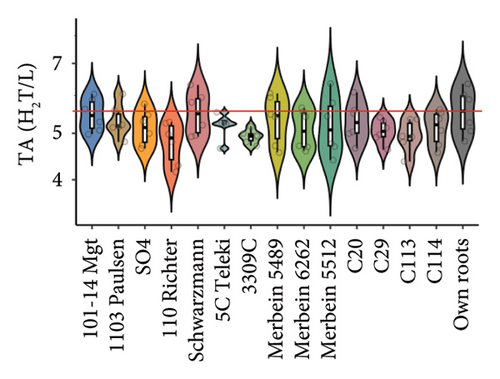
Scions grafted to all rootstocks had lower berry juice titratable acidy over the two studied vintages compared to own roots (Figure 2(b) and Table S3). Interestingly, scions grafted to 3309C, which had low berry pH, showed an extremely low titratable acid content (4.9 ± 0.2 g/L H2T; Figure 2(b) and Table S3). Our results contradict previous research on grafting Shiraz, Chardonnay, and Merlot to different rootstocks, which showed that rootstocks have insignificant influences on the titratable acidity of berries [8]. The same study also suggested that scion variety and vintage were the predominant factors affecting berry titratable acidity. This finding is supported by our study, where the two-way ANOVA demonstrated a higher F value for vintage (10.964) compared to the F value for rootstock (3.256; Figure 1(a) and Table S2).
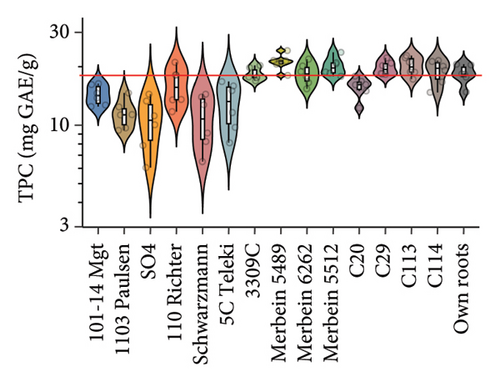
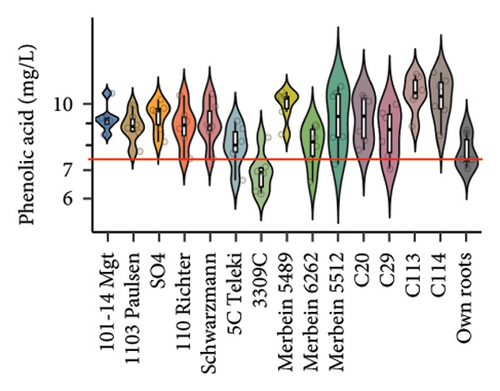
We noted that the TPC of the berries was significantly different between vines grafted to different rootstocks. Significantly higher TPC in berries was found in scions grafted to Merbein 5489, Merbein 5512, and C113 compared to own roots (Figure 2(c) and Table S3). Conversely, consistently lower berry TPC was observed in scions grafted to 1103 Paulsen, SO4, and Schwarzmann (Figure 2(c) and Table S3).
A variance in rootstocks was also found for the berry CTC. Our study suggested that over the two vintages, grafting Pinot noir MV6 onto 3309C, Merbein 5489, Merbein 5512, C113, and C114 led to significantly increased CTC compared to own-rooted vines (Figure 2(d) and Table S3). In contrast, lower grape CTC was found in vines grafted onto 1103 Paulsen, SO4, Schwarzmann, and 5C Teleki (Figure 2(d) and Table S3). The effect of rootstocks in regulating tannin accumulation in the berries of Pinot noir was evaluated in a recent study, which observed a higher tannin content in berries from Pinot noir grafted onto SO4 compared to those grafted onto 101-14 Mgt [15]. Such a tendency was not observed in our study. Overall, previous studies have suggested that the vintage and scion variety, rather than rootstocks, are the main factors affecting the tannin content of berries, which is in accordance with our current results [8, 15].
Berries with high TPC and CTC could contribute to the stronger antioxidant activity potential observed in our study. In particular, berries collected from vines grafted onto Merbein 5489, Merbein 5512, C113, and C114 showed significantly stronger FRAP compared to grapes from own-rooted vines (Table S3), which was consistent with the results for TPC and CTC. In contrast, the lowest FRAP was observed in the berries from Schwarzmann (39.7 ± 10.7 mM Fe2+/g DM; Table S3).
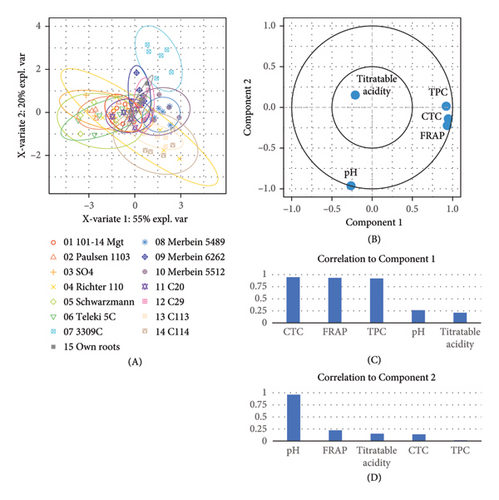
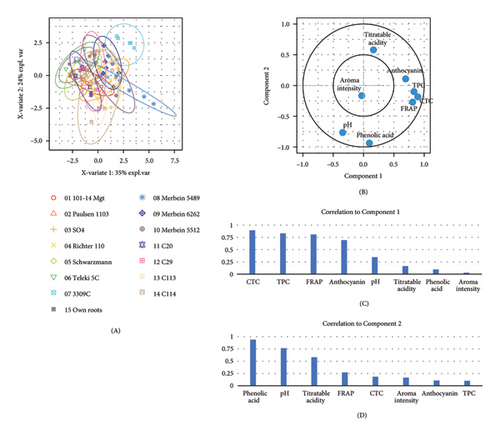
PLS-DA displays the overall variation in the berry composition among different rootstocks, where most attributes including TPC, CTC, and FRAP are located on the positive side of component 1, while pH is located on the negative side of component 2 (Figure 2(e)). 3309C and Merbein 5489 were well separated from 101-14 Mgt, 1103 Paulsen, and Schwarzmann along component 1, while 3309C was clearly separated from most rootstocks excepting Merbein 6262, Merbein 5512, and own-rooted vines along component 2.
3.2. Effect of Vintage and Rootstock on Wine Chemical Properties and Wine Composition
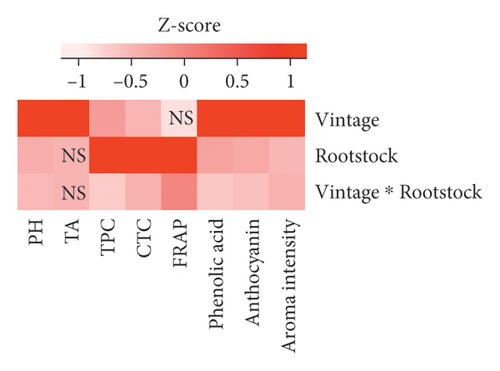
The two-way ANOVA on the chemical compositions of Pinot noir wines indicated that both the vintage and rootstock significantly affect the wine quality, with vintage acting as the predominant factor influencing the pH, titratable acidity, total phenolic acid content, total anthocyanin content, and overall aroma intensity, as suggested by the high F values (Figure 3(a) and Table S4). In contrast, rootstocks showed considerably higher F values for TPC (F value = 12.101, p < 0.001), CTC (F value = 17.060, p < 0.001), and FRAP (F value = 82.640, p < 0.001), indicating their impact on the phenolic compound accumulation and antioxidant activity.
3.2.1. Effect of Vintage on Wine Quality
Wines from the 2021 vintage tend to have significantly higher pH and lower titratable acidity compared to the 2020 vintage (Table S5). The differences in the wine pH and titratable acidity over the two studied vintages were consistent with the tendencies observed in berries, aligning with results observed in Shiraz, Merlot, and Chardonnay in previous studies [8, 27]. This suggests that the chemical properties of berries can directly affect the quality of wine.
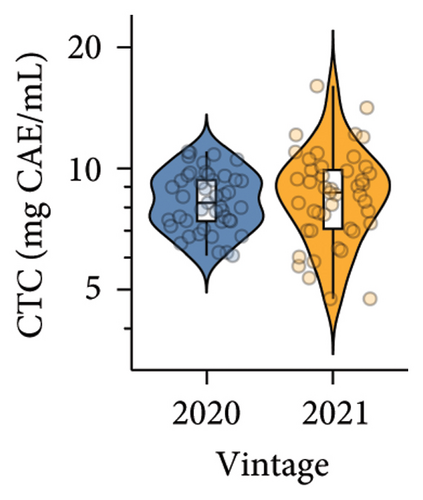
Slightly higher CTC was observed in wine produced in 2021 compared to 2020, which was consistent with the tendency of CTC accumulation in berries. Previous studies have suggested that most condensed tannins in red wine are extracted from the skin and seeds of berries (up to 4 mg/mL) during the winemaking process [32, 33]. Specifically, skin tannins, which consist of long polymeric chains (up to 83 subunits) of flavonol subunits and gallocatechin-derived units linked by acid-labile interflavan bonds, are extracted during the early stages of maceration [32, 34, 35]. On the other hand, seed tannins, which consist of short chains of catechin, epicatechin, and galloylated units, dominate the extraction of tannins in the later stages of yeast fermentation [34, 35]. This could explain the consistent CTC results in the grape and wine. The selection of yeast could also affect CTC in wine due to their roles in biochemical reactions, enzymatic reactions, and adsorption of tannin [36]. Nonetheless, the effect of different yeast strains on wine CTC was not investigated in the current study.
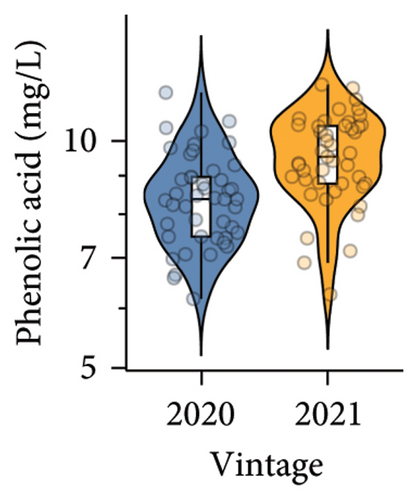
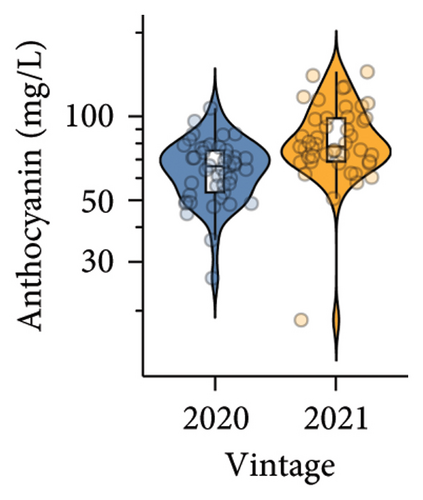
Higher phenolic acid and anthocyanin contents were observed in wines produced in the 2021 vintage compared to the 2020 vintage. Four phenolic acids, including gallic acid, vanillic acid, syringic acid, and caffeic acid, were detected (Table S6, Table S7), and a total of five anthocyanins were quantified. The concentration of malvidin 3-glucoside was the highest in both vintages, which is consistent with previous studies on Pinot noir wine [37, 38]. The anthocyanins in red wine are primarily extracted from the skins of berries during crushing and alcoholic fermentation, reaching maximum concentrations after approximately 2–3 days of alcoholic fermentation [10]. Studies on various red V. vinifera grape cultivars found that warm climates with sufficient sun exposure could improve the accumulation of phenolics [10, 39]. Furthermore, the transcription levels of several structural genes involved in anthocyanin biosynthesis, including chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), dihydroflavonol 4-reductase (DFR), leucoanthocyanidin dioxygenase (LDOX), and UDP-glucose:flavonoid 3-O-glucosyltransferase (UFGT), decreased in darkness or in shade [40]. This could explain why the 2021 vintage, with more sunlight projection and less precipitation from veraison to harvest (Table S1), resulted in a higher anthocyanin content in wine.
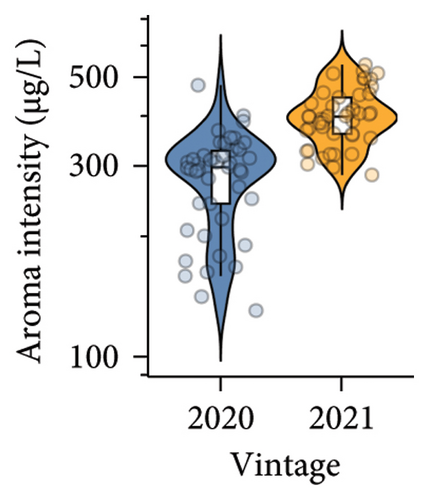
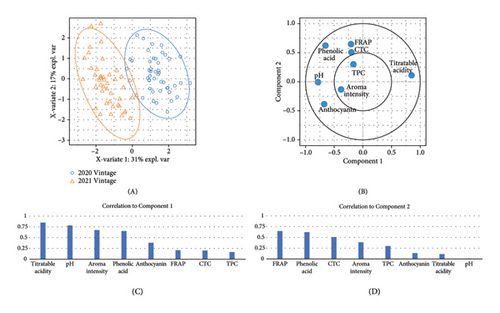
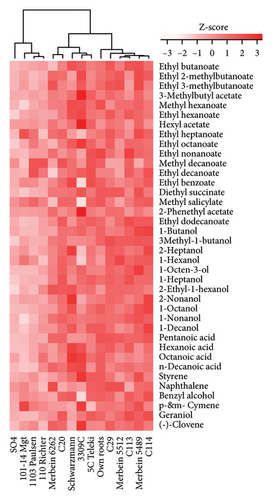
A total of 38 volatile compounds were identified and quantified by HS-SPME-GC-MS in both vintages in the present study (Table S8). These compounds were distributed across six chemical groups: esters (17), alcohols (11), acids (4), benzenoids (3), monoterpenes (2), and one sesquiterpene. Among them, the concentrations of eight compounds were above the published odour activity value (OAV), including ethyl butanoate, ethyl 2-methylbutanoate, ethyl 3-methylbutanoate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, hexanoic acid, and octanoic acid (Table S9). A significantly lower aroma intensity was found in wines made in the 2020 vintage (Figure 3(e), Table S5), perhaps due to the higher amount of precipitation during January to April 2020 compared to the same period in 2021. Increased rainfall and reduced sunlight during the grape maturation period are significantly negatively correlated with the accumulation of volatile compounds, especially alcohols, in Cabernet Sauvignon berries [41]. In our study, wines made in the 2021 vintage were more abundant in several ethyl esters, including ethyl butanoate, ethyl hexanoate, and ethyl octanoate (Table S8). These compounds contribute apple and berry aromas to wine [42, 43]. Similarly, the higher concentration of octanoic acid in wine observed in the 2021 vintage (OVA 3.7 in the 2020 vintage and OVA 9.0 in the 2021 vintage; Table S9) would confer a cheese aroma to wines [43]. Considerably higher hexanoic acid was found in the 2021 vintage wines (OVA 9.6) compared to the 2020 vintage wines (OVA 3.7; Table S9), which confer a grass and vegetable aroma [43, 44]. Overall, there is a clear vintage effect on the quality of Pinot noir in this study, where wine from the two vintages can be clearly separated by pH, titratable acidity, and anthocyanins in PLS-DA analysis (Figure 3(f)).
3.2.2. Effect of Rootstock on Wine Quality
The pH and titratable acidity of Pinot noir wines were more affected by vintage than rootstock in the present study (Figure 3(a)). The majority of rootstocks (except for 3309C and Merbein 6262) showed similar wine pH compared to ungrafted vines (Table S5). 3309C and Merbein 6262 significantly reduced the pH of wine, consistent with their low berry pH (Table S3, Table S5). The low pH of wine from 3309C did not correspond to higher titratable acidity, and no significant difference in wine titratable acidity was observed among different rootstocks (Figure 3(a) and Table S4). As pH and titratable acidity are important factors affecting the aging potential and acidity mouthfeel of wine [45], we conclude that the choice of rootstock has a relatively limited impact on these parameters.
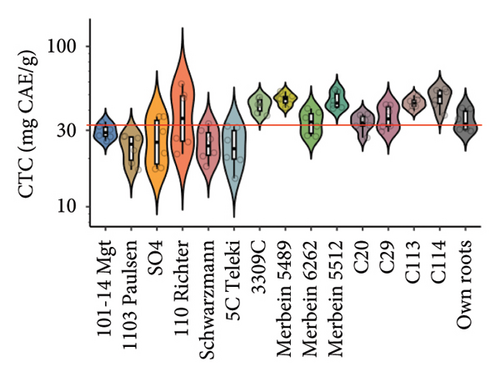
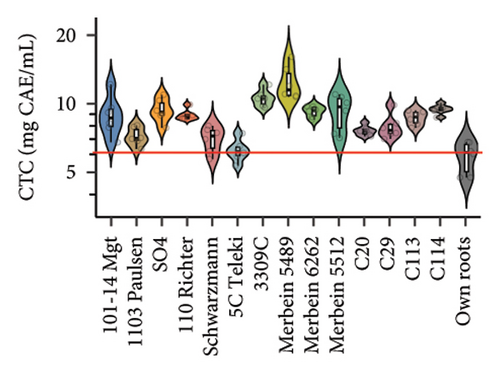
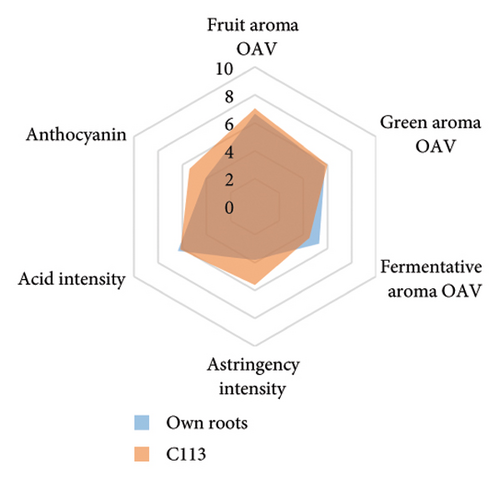
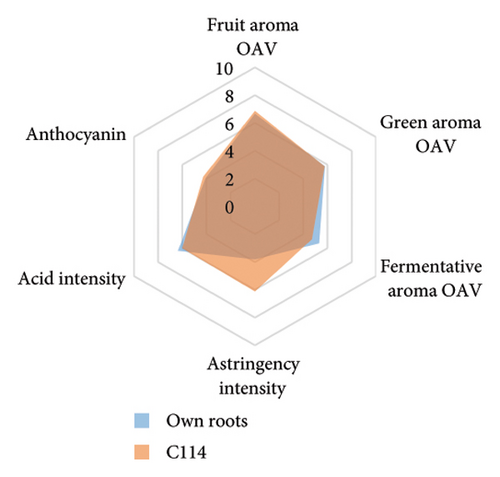
Rootstocks significantly affected the wine CTC (p < 0.001; Figure 3(a) and Table S4). Almost all rootstocks, except for 5C Teleki, resulted in a significantly increased CTC, with the highest wine CTC found in the scion grafted to Merbein 5489 (12.4 ± 2.2 mg CAE/mL, Figure 4(b) and Table S5). The results obtained in the current study align with previous Pinot noir rootstock research, which showed that rootstocks tend to increase the tannin content in wine [15, 27]. Tannins in red wine contribute to the sensory perception of astringency by binding with salivary proteins to form insoluble tannin-protein precipitates [46, 47]. Furthermore, certain types of tannins, especially flavan-3-ol glycosides, can interact with salivary proteins as nonbound and “free” astringent stimuli [48]. Hence, increasing the wine CTC by grafting Pinot noir onto rootstocks, including 3309C, Merbein 5489, C113, and C114, may result in higher wine astringency intensity (Figures 5(g), 5(h) 5(m), and 5(n)). Thus, the rootstock choice may allow winemakers to fine-tune this aroma attribute in high-quality winemaking.
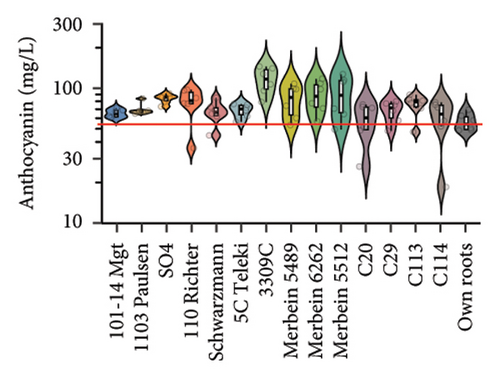
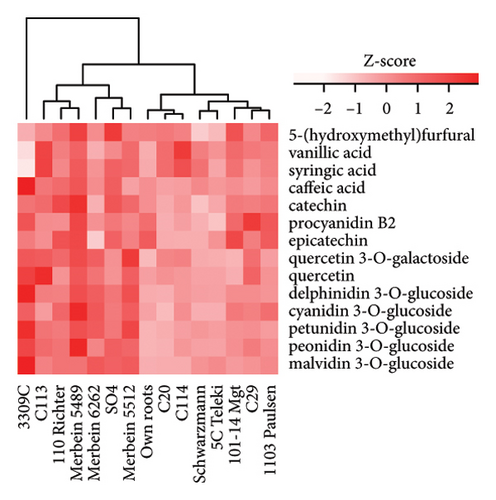
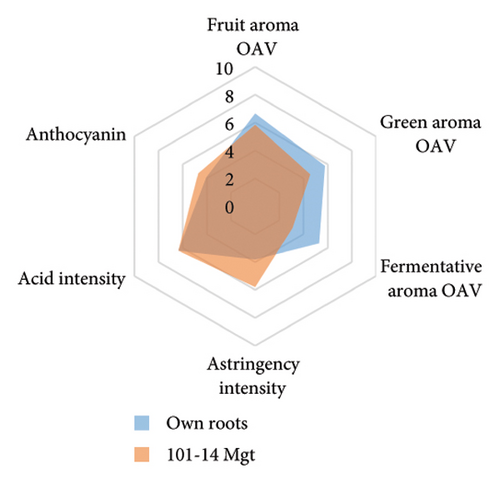
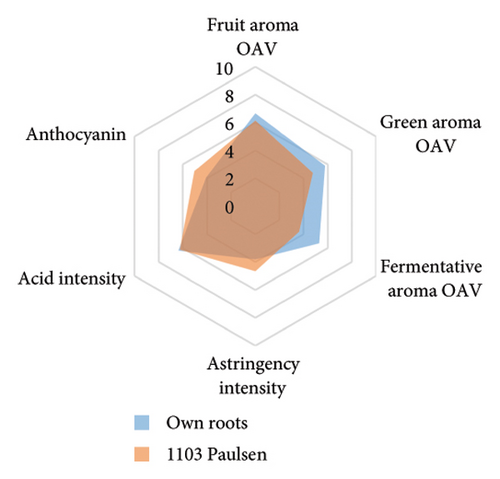
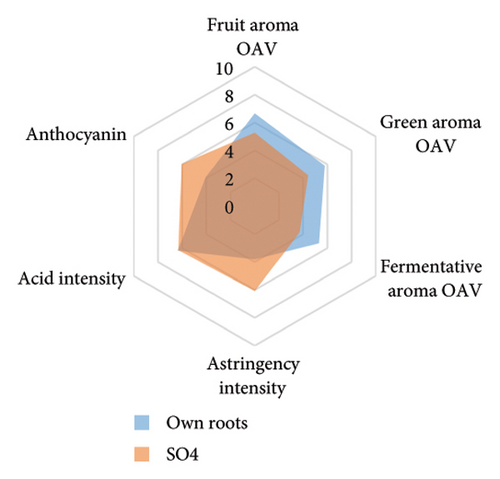
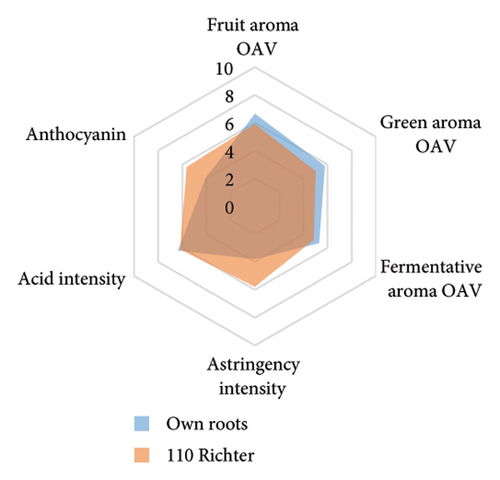
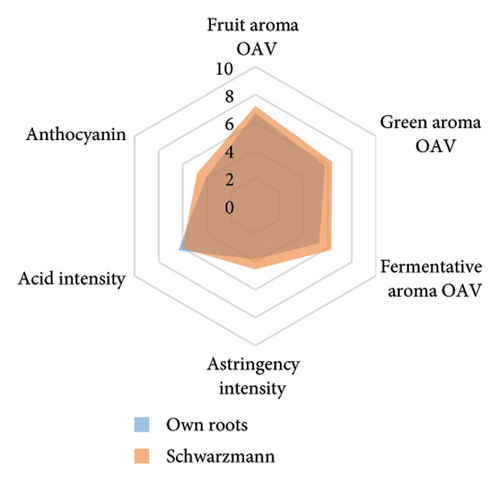
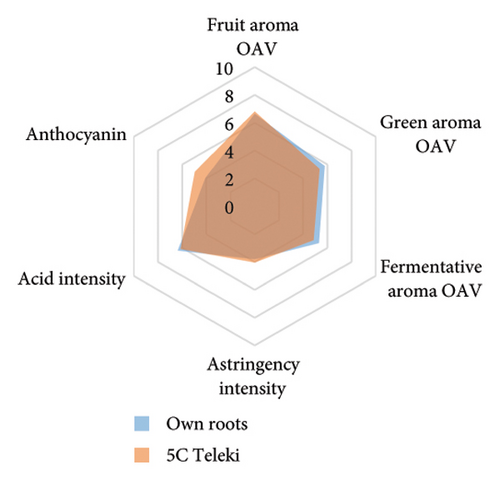
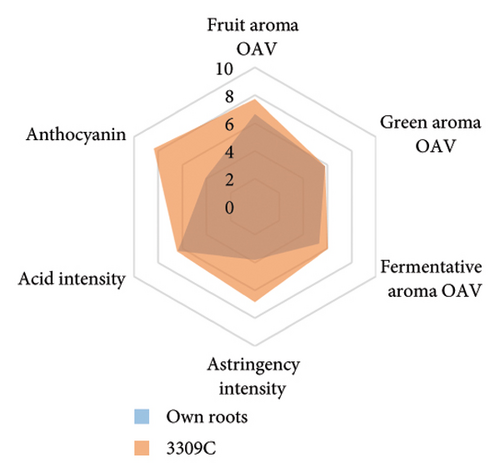
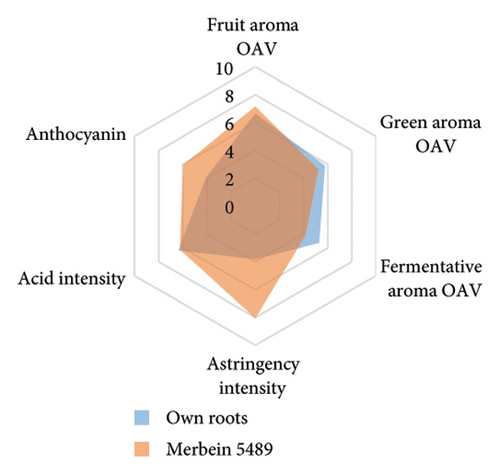
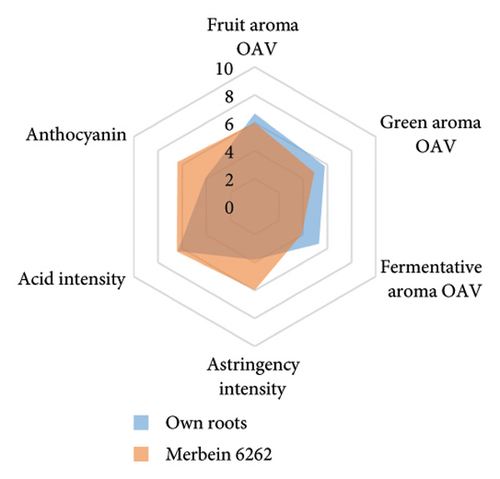
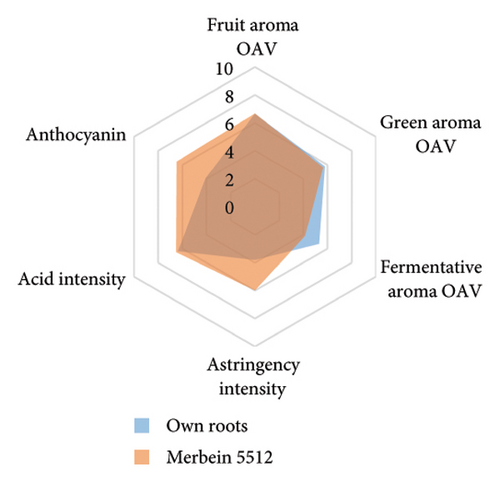
The anthocyanin concentration in wine varied among different rootstocks in our study (Figure 4(c) and Table S5). The total anthocyanin concentrations (the sum of five different anthocyanins quantified by HPLC) in wines from 3309C, Merbein 5512, Merbein 6262, and Merbein 5489 were considerably higher than that found in wine made from ungrafted vines (Figure 4(c) and Table S5). A relatively high wine anthocyanin concentration was also found in vines grafted to 110 Richter, which was in accordance with previous study [15]. This is likely due to the higher nitrogen uptake and translocation capacity of rootstocks. Grapevines with lower leaf nitrogen status tend to upregulate the phenylpropanoid pathway and promote anthocyanin production [49]. Notably, a significantly higher malvidin 3-O-glucoside concentration in wine was found in 3309C and Merbein 6262 compared to 101-14 Mgt, C20, C114, and ungrafted vines (Figure 4(f) and Table S6). This effect is similar to a previous study on Merlot, where ungrafted vines and scions grafted to 101-14 Mgt showed significantly lower concentrations of malvidin derivatives in berries compared to the same scion grafted to SO4 and 110 Richter [50]. High concentrations of malvidin 3-O-monoglucosides contribute to the colour intensity of wine by enriching the blue and purple pigments [13]. Furthermore, these anthocyanins can conjugate with certain flavonols, such as kaempferol and quercetin [14]. This process, known as copigmentation, enhances the mid- to long-term colour stability of red wine [14]. This effect may be more pronounced in wines from 3309C, Merbein 5512, and Merbein 5489 due to their relatively higher concentrations of both malvidin 3-O-glucoside and quercetin, as observed in this research (Figure 4(f) and Table S6).
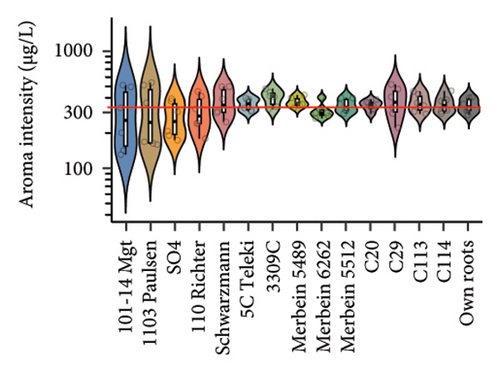
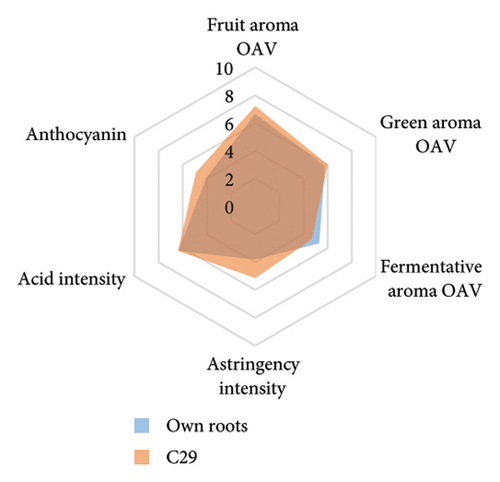
Differences in the wine aroma intensity were observed among different rootstocks in our study (Figure 4(d) and Table S5). Grafting vines onto 3309C (398.4 ± 61.6 μg/L) and C29 (372.7 ± 111.9 μg/L) resulted in the increased aroma intensity compared to ungrafted vines (344.3 ± 49.2 μg/L). In contrast, considerably lower aroma intensity was shown in wines from 101-14 Mgt (300.4 ± 170.8 μg/L) and SO4 (272.7 ± 95.9 μg/L; Figure 4(d) and Table S5). Rootstocks also significantly affected the wine volatile composition (Figure 4(g) and Table S8). Wines produced from grafted 3309C were richer in esters, especially 3-methylbutyl acetate, hexyl acetate, ethyl octanoate, and 2-phenethyl acetate, compared to all other treatments. By contrast, grafting Pinot noir onto Schwarzmann, Merbein 5489, and C114 increased the proportion of alcohols, such as 2-heptanol, 2-nonanol, 1-octanol, and 1-decanol (Figure 4(d) and Table S5).
The overall variation in the wine composition among different rootstocks was explained by PLS-DA (Figure 4(e)). Attributes including TPC, CTC, FRAP, and anthocyanin were located on the positive side of component 1, while pH and phenolic acid were located on the negative side of component 2. 3309C was well separated from Schwarzmann, 5C Teleki, C20, and ungrafted vines along component 1, while a clear separation between 101-14 Mgt, Merbein 5489, and 3309C was observed along component 2.
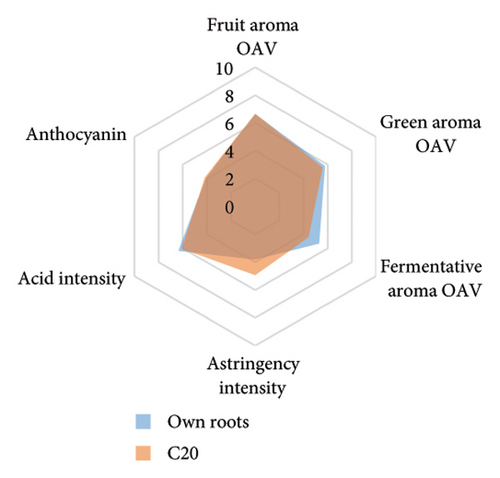
Wine characteristics of individual rootstocks have been visualised in spider charts based on the OVA values, tannin, and anthocyanin content (Figure 5). The results suggest that wine from 3309C exhibited the strongest fruity aroma intensity (Figure 5(g)), predominantly due to the high concentrations of ethyl hexanoate and ethyl octanoate. Similarly, C29, Schwarzmann, and Merbein 5489 also tended to increase the fruity aroma intensity compared to ungrafted grapevines (Figures 5(e), 5(h), and 5(l)). In contrast, SO4, 101-14 Mgt, and 110 Richter exhibited lower fruity aroma intensity compared to ungrafted vines (Figures 5(a), 5(c), and 5(d)). This finding contrasts with a previous study, where Shiraz grafted onto 101-14 Mgt showed stronger wine fruity aroma compared to grapes derived from vines grafted onto Merbein 6262 and Merbein 5512 [6]. Almost all rootstocks, excluding Schwarzmann and 3309C, reduced the fermentative aroma as indicated by the octanoic acid content in wine compared to ungrafted vines, with the lowest concentration found in vines grafted onto 101-14 Mgt. A few other volatile compounds responsible for dairy and fermentative aromas were also detected in our study, but their OAV was less than 1. Schwarzmann and 3309C imparted a stronger vegetative odour for hexanoic acid compared to own-rooted vines. Wine produced from Merbein 5489 and 5C Teleki had lower concentrations of hexanoic acid. These two rootstocks could be good options for producing wine with higher fruity aroma intensity and less vegetative aroma. Wine from rootstocks 101-14 Mgt and SO4 had low concentrations of hexanoic acid compared to wine from ungrafted vines, while their fruity and fermentative aroma intensity was also lower (Figures 5(a), 5(c)). Due to the inconsistency between our study and the result from the previous study on Shiraz [6], we conclude that there is an interaction between the scion variety and rootstock, which affects the composition of berries, leading to distinct aromas in wine. Understanding these interactions allows winemakers to craft wine with particular characteristics.
4. Conclusion
This study evaluated the berry and wine composition of Pinot noir clone MV6 scion grafted onto 14 different Vitis rootstocks. Clear differences in berry and wine chemical properties associated with rootstocks, such as pH, titratable acidity, TPC, CTC, and wine volatile profile, were identified.
Grafting Pinot noir onto 3309C caused a significant reduction in the berry and wine pH but did not increase titratable acidity. Several rootstocks, such as 3309C, Merbein 5489, Merbein 5512, and C29, increased phenolic compounds in berries and wine compared to ungrafted Pinot noir. In contrast, 1103 Paulsen, SO4, Schwarzmann, and 5C Teleki reduced the phenolic content in grapes and wine. The TPC, CTC, and anthocyanin intensity (predominantly malvidin 3-O-glucoside) in wine were improved by 3309C, Merbein 5489, Merbein 6262, Merbein 5512, C29, C113, and C114 compared to ungrafted Pinot noir. The profile of volatile compounds in wine was also altered by rootstocks. Among them, 5C Teleki and Merbein 5489 may provide a more balanced aroma to Pinot noir wine due to their positive contribution to compounds related to the fruity aroma and their negative influence on the concentrations of compounds contributing to the green aroma. Therefore, these two rootstocks are recommended as good options for producing Pinot noir wine. Apart from the rootstock, vintage also played an important role in managing the accumulation of phenolic and volatile compounds. Specifically, significantly higher TPC and CTC were found in the 2021 vintage in both grapes and wine. Similarly, the concentration of anthocyanin and ethyl esters was also higher in 2021 compared to 2020, likely due to the higher average temperature and less precipitation close to harvest in 2021.
Our study highlights the importance of rootstock and vintage interaction in optimising the berry and wine composition of Pinot noir, thereby improving the aroma, flavour, and mouthfeel of Pinot noir wine. The rootstock performance reported in this paper provides guidance for grape growers and winemakers in selecting suitable rootstocks for Pinot noir, with the aim of maintaining sustainable, high-quality Pinot noir wine production in cool-climate regions. Furthermore, the results could guide future studies in understanding the roles of rootstocks in regulating the metabolic pathways of phenolic and volatile production in the berries of grafted Pinot noir at the molecular level.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors’ Contributions
Yipeng Chen contributed to methodology, formal analysis, investigation, data curation, visualisation, and the writing of the original draft. Zijian Liang helped with methodology, validation, investigation, and data curation. Mark Krstic and Peter Clingeleffer contributed to conceptualisation, methodology, resources, writing, review, and editing. Kate Howell and Deli Chen helped with conceptualisation, methodology, supervision, writing, review, and editing. Pangzhen Zhang contributed to conceptualisation, methodology, investigation, resources, supervision, project administration, writing, review, and editing.
Acknowledgments
We would like to thank Mr Tyson Lewis, Ms Olivia Barrie, and Ms Cheryl Lee from Mornington Peninsula Vignerons Association and Mr Hugh Robinson from Peninsula Vinecare for providing experimental sites and technical support. We also thank Yalumba Nursery for providing studied rootstocks and the technical advice from Mr Adam Hall and Mr Nick Dry. We would like to thank the Mass Spectrometry and Proteomics Facility at the Bio21 Institute for providing facility and technical support for HPLC-QTOF-MS/MS qualitative analysis of wine phenolic compounds. This research was partially funded by Australian Government-Australian Grape and Wine Authority (Wine Australia-Incubator Initiative; UM 1801 and UM1901, Themis Agreement ID: 104778), Victoria State Government—Wine Victoria (Wine Growth Fund; Themis Agreement ID: 301637), and the PhD Program of the University of Melbourne. Open access publishing facilitated by The University of Melbourne, as part of the Wiley - The University of Melbourne agreement via the Council of Australian University Librarians.
Open Research
Data Availability
The data used to support the findings of this study are included within the article or supplementary materials attached.




