On a Stochastic Approach to Extensions of the Susceptible-Infected-Susceptible (SIS) Model Applied to Malaria
Abstract
This work presents a stochastic model of malaria spread. We first calculated the basic reproduction number R0 of the models (ShIhRhSh-SvIv) and (ShLhIhRhSh-SvLvIv) in order to show that the malaria-free equilibrium is asymptotically stable; then, we used a finite Markov chain model to describe the interactions between the different compartments of the model (SeLeIeReSe-SaLaIaRaSa-SvIv). We carried out numerical simulations of our results for two types of transmission zones: a zone with low malaria transmission and an endemic zone. Through these simulations, we first determined the invariant stationary distribution π∗ of the model, and then, we found that the use of the indoor residual spraying (IRS) method by regular application of insecticides is more effective for the elimination of malaria than the use of long-acting impregnated mosquito nets (LLINs).
1. Introduction
Mathematical models of the spread of malaria date back to the beginning of the 20th century by Ross [1]. Several mathematical models have been developed to study the dynamics of malaria spread. We can cite the models (ShIhSh-SvIvSv) of Ross, the models (ShIhRhSh-SvIv) in [2–4], and the models (ShEhIhRhSh) in [5, 6]. The mathematical theory of the spread of malaria was solidified by Reiner et al. [7] in the 1960s. In 2018, Mbogo et al. [2] use the Galton-Watson branching processes to propose an extension of the model formulated by [4]. There are also results in the following works [8–13] that could well explain the literature. In this work, we first present in Section 2 some preliminary results, necessary for the conduct of our work by successively studying the models (ShIhSh-SvIvSv), (ShIhRhSh-SvIv), and (ShLhIhRhSh-SvLvIv). Then, we present an extension of the Markovian SIS model in a hypoendemic and endemic area in Sections 3.2 and 3.3, respectively. And finally, Section 4 is devoted to the work of numerical simulations of our results.
2. Mathematical Preliminaries and Notations
2.1. Ross’s (ShIhSh-SvIvSv) Model
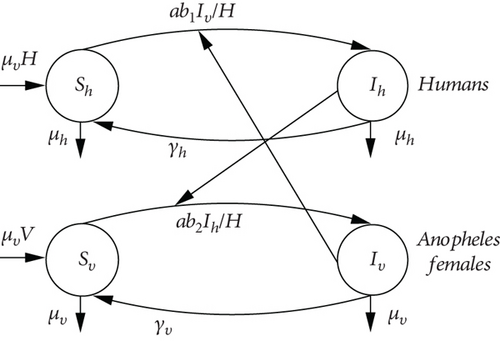
In 1911, Sir Ronald Ross proposed a model which took into account both anopheline and human populations. This model is certainly the starting point for vector-host models. Ross divided hosts (humans) and vectors (anopheles) into two classes, susceptible and infected, respectively. Let Sh be the population of susceptible humans and Ih the population of infected humans. Ross assumes that there is no latency period and that, consequently, an infected person is automatically an infectious person. Similarly, he refers to the population of susceptible anopheles as Sv and the population of infected anopheles as Iv. In his model, Ross assumes that both the human and Anopheles populations are constant and that one mosquito bites “a” humans per unit of time where “a” is constant [14]. The malaria propagation graph in Ross’s (ShIhSh-SvIvSv) model is shown in Figure 1.
Corollary 1.
- (1)
If R0 ≤ 1, then the disease completely disappears from the population after a certain time
- (2)
If R0 > 1, then the disease remains endemic in the population.
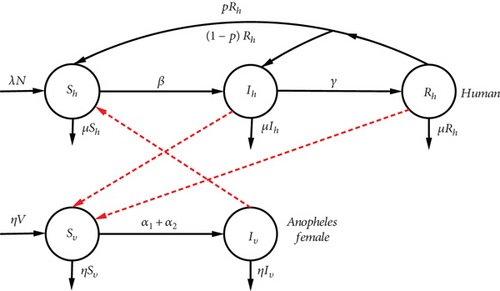
2.2. Model (ShIhRhSh-SvIv)
Since malaria provides temporary immunity and is not lethal if treated, it is possible to use a SIRS (Susceptible-Infected-Recovered-Susceptible) model, since recovered individuals return to the S class with probability p(p > 0) or relapsed individuals become infected again with probability 1 − p. So to Ross’s (ShIhSh-SvIvSv) model, we add the R recovered compartment. These types of model are also solved in [17, 18]. Figure 2 illustrates the scheme of disease progression.
2.3. Model (ShLhIhRhSh-SvLvIv)
According to the literature, the average delay between exposure to parasitised blood and the first clinical signs of infection is 4 to 17 days for P. falciparum. There is therefore an additional class of exposed individuals (E) or latent individuals (L) who are not yet infectious. It is therefore necessary to model the dynamics of the spread of malaria using the (ShLhIhRhSh-SvLvIv) model, which would be an extension of the (ShIhSh-SvIvSv) and (ShIhRhSh-SvIv) models previously studied. The disease progression diagram in this type of model can be represented in Figure 3.
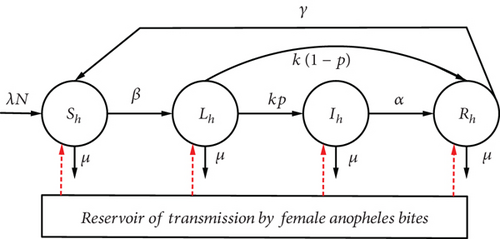
Mosquito ODEs are neglected for simplicity. All parameters and their biological interpretation are recorded in Table 1.
| Parameter | Biological interpretation |
|---|---|
| N | Size of the human population (N = S + L + I + R) |
| λ | Birth rate |
| μ | Death rate |
| k | Incubation rate |
| p | Probability of transition from latent to infectious state |
| 1 − p | Probability of transition from latent to recovered state |
| β | Transmission rate (susceptible to latent) |
| α | Transmission rate (infectious to removed) |
| γ | Immunity loss rate (removed to susceptible) |
The disease-free equilibrium (DFE) of this model is therefore (N, 0, 0). Authors such as [17, 19, 20] have also studied these types of models.
3. Main Results
3.1. Basic Reproduction Rate R0
Theorem 2. The basic reproduction rates R0 of the models (ShIhRhSh-SvIv) in Section 2.2 and (ShLhIhRhSh-SvLvIv) in Section 2.3 are given by
Proof. To determine the basic reproduction rate R0, we apply the Van den Driessche method [15]. The nonlinear ordinary differential equation system (4) integrating the compartments of the model in Figure 2 can be expressed as
The inverse matrix of V is
The set of eigenvalues of FV−1 is denoted Sp(FV−1) and is equal
The basic reproduction number R0 is the spectral radius of the next generation matrix:
The basic reproduction number R01 of our model (4) in Section 2.2 is
Similarly for the model (6) in Section 2.3, we have
Hence,
Therefore,
Then, the basic reproduction rate R02 is given by the spectral radius of FV−1.
Corollary 3. The disease-free equilibrium of the system (6) is locally and asymptotically stable if R01 < 1 and unstable if R01 > 1.
Corollary 4. The malaria-free equilibrium E0 of the system (4) is locally asymptotically stable if R02 < 1 and unstable if R02 > 1.
3.2. Extension of the Markov SIS Model in a Hypoendemic Zone
In this section, we develop a stochastic model for malaria transmission by considering two types of host (“nonimmune” and “semi-immune”) in the human population. The “nonimmune” group comprises human individuals who have never acquired any immunity to malaria. It is assumed that these hosts are vulnerable because they can suffer and/or die from malaria. “Semi-immune” people are those who have acquired or lost at least some immunity to malaria in their lifetime. It is assumed that these hosts are nonvulnerable, so they cannot die from malaria but can only suffer from it. We envisage a model of the type (SeLeIeReSe) for nonimmunes until they enter the semi-immune category and then follow a model of the type (SaLaIaRaSa), where e and a denote the index for nonimmunes and semi-immunes, respectively. For the mosquito population, we use a model of the type (SvIv).
3.2.1. Elaboration of the Model
We consider that it is the gametocyte form of Plasmodium in humans that transmits the infection to mosquitoes and the sporozoite form in the mosquito that transmits the infection to humans. In our model, we adopt a number of operational definitions.
- (i)
Susceptible (S): an individual bitten by infectious mosquitoes and capable of contracting malaria
- (ii)
Latent (L): individual who has contracted malaria but does not transmit it initially
- (iii)
Infectious (I): an individual who harbours a high level of parasites in gametocyte form. They can suffer and/or die from malaria
- (iv)
Immune (R): an asymptomatic carrier of the parasite. This is a state of protection against the disease, but not against the parasite; it is a healing stage
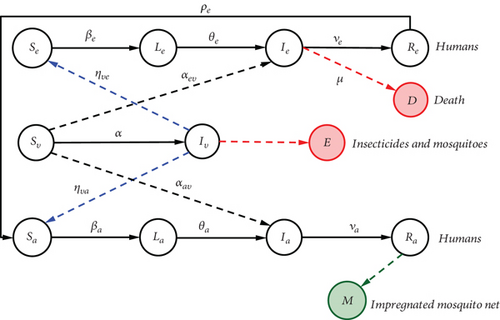
A human being is said to be ill with malaria if he or she is either in a latent or infectious state. In order to write the model, the nonimmune human host types are subdivided into four (4) compartments: susceptible (Se), latent (Le), infectious (Ie), and immune (Re). Semi-immune human host types are also subdivided into four (4) compartments: susceptible (Sa), latent (La), infectious (Ia), and immune (Ra). The mosquito population is made up of susceptible (Sv) and infectious (Iv) mosquitoes. We are studying two heterogeneous populations made up of several pathological classes, in which individuals can move from one class to another. There are ten different disease states. The pattern of disease progression in this model is illustrated in Figure 4.
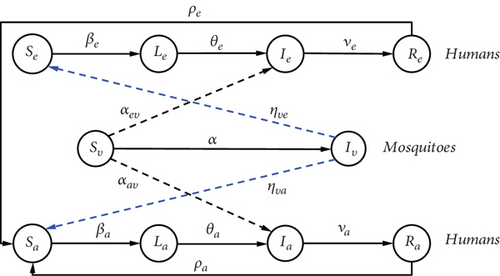
The solid arrows indicate the direction of propagation of Plasmodium, thus creating states of health in the subjects.
The red dotted arrows indicating the direction of infection from an infectious mosquito (Iv) to a nonimmune susceptible human individual (Se) or a semi-immune susceptible human individual.
The black dotted arrows indicate the direction of infection of a susceptible mosquito (Sv) on an infectious nonimmune human (Ie) or an infectious nonimmune human (Ia).
3.2.2. Model Assumptions
A1: the probabilities ηve, ηva, αev, αav, and α are in the interval [0, 1[.
A2: the parameters βe, θe, ηe, ρe, βa, θa, ηa, and ρa are assumed to be strictly positive in the interval ]0, 1[ and represent the probabilities of moving from a subfund i to a subfund j.
A3: these passage probabilities and parameters designate the conditional probabilities ℙ(X1 = compartment j|X0 = compartment i) satisfying the Markov property. These conditional probabilities linking two consecutive pathological states are between ]0, 1[ if the transition has a biological meaning and 0 otherwise.
3.2.3. Interaction between Humans and Mosquitoes
Initially, infectious mosquitoes (Iv) bite susceptible humans (Se) with a ηve probability. The latter may remain susceptible (Se) with probability r for some time or become latent (Le) with probability βe. Individuals can remain latent for a given time with probability q or become infectious (Ie) with θe as the transition probability. In this infectious state (Ie), humans can die with a probability μ or become immune with a probability equal to νe, or even remain infectious with a probability p for an interval of time. Having completed the 1st cycle, the immune (Re) becomes susceptible (Sa) to the ρe immunity rate, and through the intermediary of the infectious mosquito (Iv), the cycle resumes as shown in Figure 4. At stage (Ra), having acquired a certain immunity, the individual re-enters the susceptible class (Sa) at the rate ρa. The susceptible mosquitoes (Sv) inoculate the parasite from a blood meal on the infectious (Ie) and Ia with probabilities αev and αav, respectively; then, they become infectious (Iv) with probability α.
Tables 2–4 summarise the parameters that will be described in the model.
Proposition 5. Let be a probability space and X = {Xn, n ≥ 0} the process of the mode of transmission of plasmodia with values in the discrete state space . Then, the process X defined on with values in is an irreducible and aperiodic homogeneous Markov chain with initial distribution and transition matrix H, where is the probability that the process X is in state i at time 0. And this Markov chain has a stationary distribution π∗ such that π∗ = limk⟶∞π0Hk.
Proof. We consider that the ten compartments Se, Le, Ie, Re, Sa, La, Ia, Ra, Sv, and Iv in Figure 4 represent the ten states of a Markov chain. These ten states are denoted {i1, i2, i3, i4, i5, i6, i7, i8, i9, i10}, respectively. Let be the set of ten states of the Markov chain. Let X = {Xn, n ≥ 0} be the process of the mode of transmission and evolution of Plasmodium. Let denote the probability that the process is in state j at time n + 1 knowing that it is in state i at time n. Furthermore, the probability is assumed to be independent of n. For all , pij is called the transition probability from state i to state j. The probabilistic transition graph of the Markov chain model is given in Figure 5, where the xi, i ∈ {1, ⋯, 23} correspond to the different parameters of the model and represent the transition probabilities of the chain. The chain is irreducible because its representative graph (Figure 5) is strongly connected.
| Param. | Meaning | Unity |
|---|---|---|
| βe | Rate of transition from the susceptible state Se to the latent state Le. | Without Dim. |
| θe | Rate of transition from latent state Le to infectious state Ie. | Without Dim. |
| νe | Rate of transition from infectious state Ie to immune state Re. | Without Dim. |
| Param. | Meaning | Unity |
|---|---|---|
| na | Number of times a mosquito could bite a human being per unit of time, if human beings were freely available | Time–1 |
| ηev | Probability of transmission of an infection from an infectious mosquito (Iv) to a susceptible nonimmune human (Se) | Without Dim. |
| ηva | Probability of transmission of an infection from an infectious mosquito (Iv) to a susceptible nonimmune human (Sa) | Without Dim. |
| αev | Probability of transmission of infection from an infectious human being (Ie) to a susceptible mosquito (Sv), given that there has been contact between the two | Without Dim. |
| αav | Probability of transmission of infection from an infectious human being (Ia) to a susceptible mosquito (Sa), given that there has been contact between the two | Without Dim. |
| Param. | Meaning | Unity |
|---|---|---|
| βa | Rate of transition from the susceptible state Sa to the latent state La | Without Dim. |
| θa | Rate of transition from latent state La to infectious state Ia | Without Dim. |
| νa | Rate of transition from infectious state Ia to immune state Ra | Without Dim. |
| ρe | Rate of transition from the immune state Re to the susceptible state Sa | Without Dim. |
| ρa | Rate of transition from the immune state Ra to the susceptible state Sa | Without Dim. |
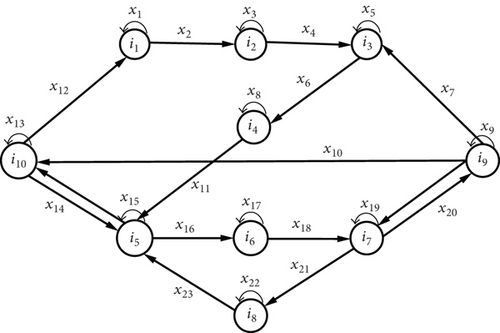

The H matrix thus constructed is stochastic by extension of the SIS model.
3.3. Extending the SIS Markov Model to an Endemic Area
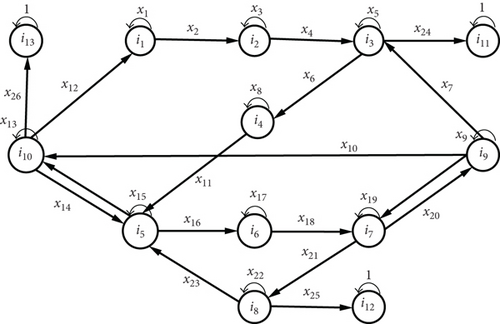
Children under 5 years old are the most vulnerable to malaria in endemic areas [21–24]. They have not yet developed their own immunity and are therefore considered nonimmune. We do not take into account the age of the individual but rather his or her immunological status, because according to studies [25, 26], children and adults have the same probability of being infected by malaria. It is assumed that there has been one case of death in the infectious nonimmune human compartments. Then, the Plasmodium propagation diagram takes the form of Figure 6.
The solid arrows indicate the direction of propagation of Plasmodium, thus creating states of health in the subjects.
The blue dotted arrows indicate the direction of infection from an infectious mosquito (Iv) to a nonimmune susceptible human individual (Se) or a semi-immune susceptible human individual.
The black dotted arrows indicate the direction of infection of a susceptible mosquito (Sv) on an infectious nonimmune human (Ie) or an infectious nonimmune human (Ia).
- (i)
of infectious nonimmune human (Ie) thus entering compartment D, where μ represents the probability of dying from malaria knowing that the nonimmune human is infectious
- (ii)
mosquitoes by intradomiciliary spraying
The green dotted arrow indicates the use of long-lasting impregnated mosquito nets.
3.3.1. Model Assumptions
We supplement the list of assumptions in the previous Section 3.2 with two more:
A4: infectious mosquitoes Iv are eliminated by intradomiciliary insecticide spraying with a probability p, (p ∈ ]0, 1[).
A5: humans use long-acting impregnated mosquito nets with a probability of 1 − p, and for reasons of model simplification, environmental factors that could have more or less significant effects are neglected.
Proposition 6. Let be a probability space and X = {Xn, n ≥ 0} the process of the mode of transmission of plasmodia with values in the discrete state space . Then, the process X defined on with values in E is a homogeneous absorbing Markov chain with transition matrix P.
Proof. The graph representing the Markov chain model is given in Figure 7, where the xi, i ∈ {1, ⋯, 26} correspond to the different parameters of the model and represent the transition probabilities of the chain.
There are three absorbing states (i11, i12, i13) on the graph. As a result, the chain is absorbent. And under the assumptions A1,…,A5 and under the same interactions between humans and mosquitoes described in the previous section, we group the pij in a matrix P whose canonical form is represented by the relation

The matrix of transient states Q and that of absorbing states R are given by the relations


The fundamental matrix will be determined by application.
Proposition 7. The matrix elements ni,j≔(I − Q)i,j give us the expectation of the total number Ni(j) of stays in j before passing beyond the transient states, and the i line of (I − Q)−1 gives us the “expected life balance,” with a given initial state i.
Proof. Let Ik be the indicator of being present in the transient state at time k. We pose
By changing the order of summation, we obtain
4. Numerical Simulations
4.1. Values Assigned to Transition Probabilities
In this section, we numerically analyse the stochastic matrices (18) and (22). We consider two malaria transmission zones: zone A corresponding to a low transmission zone and zone B corresponding to a high transmission zone. We begin by determining the values of the biological parameters equivalent to the transition probabilities in each zone. Most of these data are obtained from the literature, in particular from models developed by [27–31].
4.1.1. The Transition Probabilities between the Three Types of Host
The probability of effective transition between the three host types is the average number of contacts per unit of time (in this case the day) that could lead to infection of a specific host type.
na: we estimate that the average number of mosquito bites a human being can suffer per day is 0.28 for the low transmission zone and 0.38 for the high transmission zone. This average number na is a function of the exposed surface area of the human and any vector control interventions used by humans to reduce exposure to mosquitoes [27, 30].
ηve: we estimate that the probability of transmission of an infection from an infectious mosquito (Iv) to a susceptible nonimmune human being (Se), knowing that there has been contact between the two, is 0.021 for the low transmission zone and 0.07 for the high transmission zone [30].
ηva: we estimate that the probability of transmission of an infection from an infectious mosquito (Iv) to a susceptible semi-immune human being (Sa), knowing that there has been contact between the two, is 0.012 for the low transmission zone and 0.022 for the high transmission zone [28, 31].
αev: we assume that the probability of transmission of infection from an infectious nonimmune human being (Ie) to a susceptible mosquito (Sv), given that there has been contact between the two, is 0.11 for a low transmission zone and 0.45 for a high transmission zone [31].
αav: we assume that the probability of transmission of infection from an infectious semi-immune human being (Ia) to a susceptible mosquito (Sv), given that there has been contact between the two, is 0.08 for an area of low transmission and 0.35 for an area of high transmission.
4.1.2. Transitions in Nonimmune Host Compartments
θe: the probability of transition from the latent state (Le) to the infectious state (Ie). We assume that θe = 0.10 for both types of zone.
νe: the probability of transition from the infectious state (Ie) to the immune state (Re). We have assumed that this rate is 0.005 for the zone of low transmission and 0.001 for the zone of high transmission zone.
ρe: the probability of transition from the immune state (Re) to the susceptible state (Sa). This is a phase of change of status from nonimmune to semi-immune hosts. We have assumed that this probability is 5.5 × 10−4 for the low transmission zone and 2.7 × 10−3 for the high transmission zone [27, 29, 31].
4.1.3. Transitions in the Compartments of Semi-immune Hosts
θa: the probability of transition from the latent state (La) to the infectious state (Ia). We assume that θa = 0.09 for both types of zone.
νa: the probability of transition from the infectious state (Ia) to the immune state (Ra). We have assumed that this rate is 0.01 for both types of zone.
ρa: the probability of transition from the immune state (Ra) to the susceptible state (Sa). Here, the immune cells heal and return to the susceptible compartment. We assume that ρa = 0.0083 for the low transmission zone and ρa = 0.033 for the high transmission zone [30].
For i = j, the pii are obtained by stochastic effect. The other transition probabilities not quoted here are obtained by using the Markov property.
All these estimated probabilities are summarised in Table 5.
| Param. | Zone A | Zone B | Intervals |
|---|---|---|---|
| na | 0.25 | 0.38 | 0.13-0.47 |
| ηve | 0.021 | 0.07 | 0.01-0.27 |
| ηva | 0.012 | 0.022 | 0.01-0.27 |
| αev | 0.11 | 0.45 | 0.072-0.64 |
| αva | 0.08 | 0.35 | 0.072-0.64 |
| θe | 0.10 | 0.10 | 0.067-0.20 |
| θa | 0.09 | 0.09 | 0.067-0.20 |
| νe | 0.005 | 0.001 | 0.0014-0.017 |
| νa | 0.01 | 0.01 | 0.0014-0.017 |
| ρe | 5.5 × 10−4 | 2.7 × 10−3 | 1.1 × 10−2 − 5.5 × 10−5 |
4.2. Study of the H Matrix in a Zone A

that is, 20% of susceptible nonimmune, 15% of latent nonimmune, 10% of infectious nonimmune, 12% of recovered nonimmune, 9% of susceptible mosquitoes, 11% of infectious mosquitoes, 5% of susceptible semi-immune, 8% of latent semi-immune, 6% of infectious semi-immune, and 4% of recovered semi-immune. We use R version 4.2.1 (2022-06-23 ucrt)-“Funny-Looking Kid” for the simulation results. Tables 6 and 7 give the probabilities πn = π0Hn for n = {1, 2, ⋯, 21}. They illustrate the evolution of the different stages of malaria over a period of twenty-one malaria episodes, using the initial distribution π0 of the relationship (32).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Se | 0.167 | 0.138 | 0.114 | 0.095 | 0.081 | 0.072 | 0.067 | 0.064 | 0.063 | 0.064 |
| Le | 0.118 | 0.093 | 0.074 | 0.059 | 0.047 | 0.039 | 0.032 | 0.028 | 0.024 | 0.022 |
| Ie | 0.121 | 0.127 | 0.125 | 0.119 | 0.110 | 0.102 | 0.094 | 0.088 | 0.083 | 0.080 |
| Re | 0.118 | 0.117 | 0.116 | 0.115 | 0.113 | 0.110 | 0.106 | 0.102 | 0.099 | 0.095 |
| Sv | 0.087 | 0.098 | 0.116 | 0.137 | 0.158 | 0.177 | 0.193 | 0.207 | 0.218 | 0.226 |
| Iv | 0.085 | 0.070 | 0.063 | 0.062 | 0.065 | 0.070 | 0.076 | 0.082 | 0.087 | 0.092 |
| Sa | 0.044 | 0.039 | 0.036 | 0.033 | 0.030 | 0.028 | 0.027 | 0.026 | 0.025 | 0.024 |
| La | 0.099 | 0.112 | 0.122 | 0.129 | 0.133 | 0.135 | 0.136 | 0.136 | 0.136 | 0.135 |
| Ia | 0.121 | 0.162 | 0.188 | 0.205 | 0.214 | 0.219 | 0.220 | 0.219 | 0.218 | 0.216 |
| Ra | 0.042 | 0.044 | 0.046 | 0.047 | 0.048 | 0.048 | 0.048 | 0.048 | 0.047 | 0.047 |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Se | 0.065 | 0.067 | 0.069 | 0.071 | 0.072 | 0.074 | 0.075 | 0.076 | 0.077 | 0.078 | 0.078 |
| Le | 0.021 | 0.020 | 0.019 | 0.019 | 0.019 | 0.019 | 0.019 | 0.019 | 0.020 | 0.020 | 0.020 |
| Ie | 0.077 | 0.076 | 0.075 | 0.075 | 0.076 | 0.076 | 0.077 | 0.078 | 0.078 | 0.079 | 0.079 |
| Re | 0.091 | 0.088 | 0.085 | 0.082 | 0.080 | 0.078 | 0.077 | 0.076 | 0.075 | 0.074 | 0.074 |
| Sv | 0.233 | 0.238 | 0.241 | 0.243 | 0.245 | 0.246 | 0.246 | 0.247 | 0.247 | 0.247 | 0.247 |
| Iv | 0.096 | 0.099 | 0.101 | 0.103 | 0.104 | 0.105 | 0.106 | 0.106 | 0.107 | 0.107 | 0.107 |
| Sa | 0.023 | 0.023 | 0.023 | 0.022 | 0.022 | 0.022 | 0.220 | 0.022 | 0.022 | 0.022 | 0.022 |
| La | 0.134 | 0.133 | 0.132 | 0.130 | 0.130 | 0.129 | 0.128 | 0.127 | 0.127 | 0.126 | 0.126 |
| Ia | 0.214 | 0.212 | 0.210 | 0.209 | 0.208 | 0.207 | 0.206 | 0.205 | 0.205 | 0.205 | 0.205 |
| Ra | 0.046 | 0.045 | 0.045 | 0.044 | 0.044 | 0.044 | 0.044 | 0.043 | 0.043 | 0.043 | 0.043 |
Graphical representations of the results of Tables 6 and 7 are listed in Figure 8.
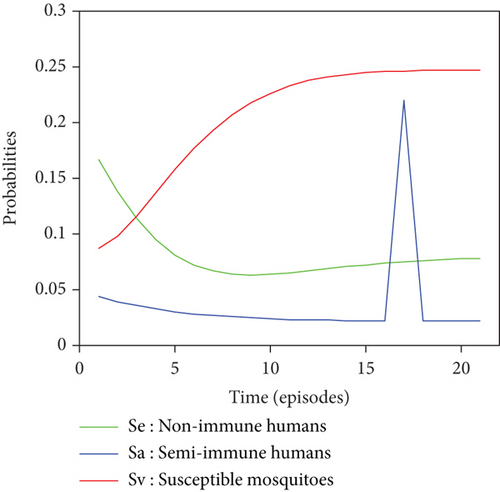
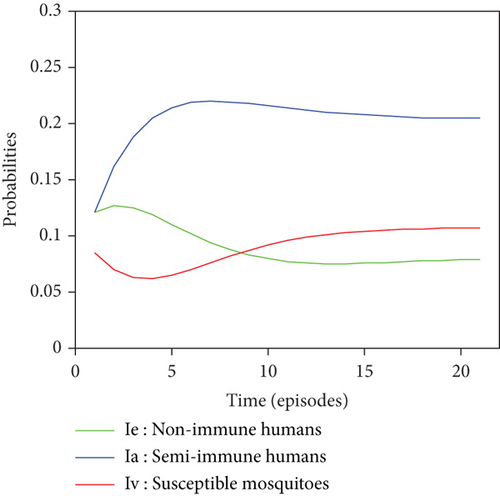
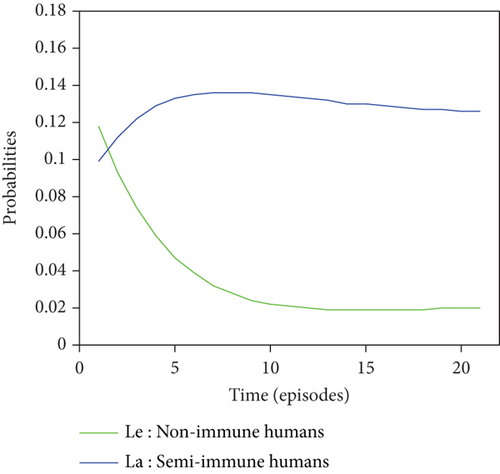
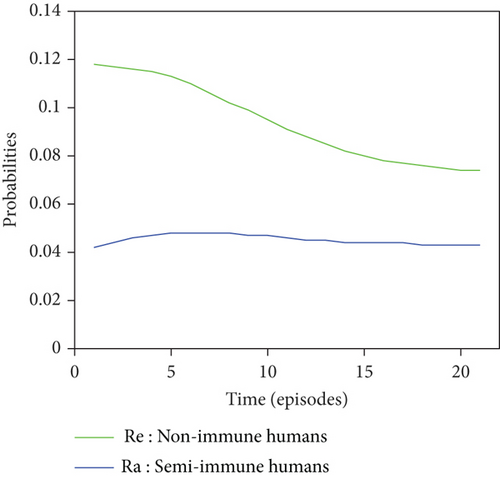
For a given initial distribution, the population of susceptible Anopheles mosquitoes increases and stabilises at around 25%. There has also been an increase in the number of infectious mosquitoes. The rapid decline in the susceptible nonimmune curve led to rapid growth in the susceptible semi-immune curve, creating a peak of probability 0.22 which stabilised after the twentieth episode. After several episodes of malaria, nonimmune individuals gradually migrate to the semi-immune class to acquire a certain level of immunity, leading to a high level of semi-immune individuals, which stabilises from the twentieth episode onwards.
4.3. Study of the P Matrix in an Endemic Area



4.3.1. Fundamental Matrix


According to these results, the probability of an individual Se, Le, Ie, Re, Sa, La, Ia, and Ra dying from malaria is equal to 0.006, 0.006, 0.046, 0.000, 0.005, 0.002, and 0.001, respectively. It can be seen that the probability of an infectious individual Ie dying from malaria is consistent with the clinical results. Susceptible mosquitoes Sv (infectious Iv, respectively) are absorbed either by insecticides E at 95.3% (at 82.9%, respectively) or by LLINs at 04.6% (at 16.6%, respectively). The results show that to eliminate malaria, it is preferable to use indoor residual spraying (IRS) by regular application of insecticides rather than long-acting impregnated mosquito nets (LLINs). Susceptible individuals Se and Sa are absorbed by IRS at 62.1% and 84.5% and by LLIN at 37.4% and 14.8%, respectively. This means that susceptible humans prefer IRS rather than LLINs to protect themselves against parasite infection.
5. Conclusion
In this study, we proposed a Markovian stochastic approach to a compartment model regarding malaria transmission. This Markovian model (SeLeIeReSe-SaLaIaRaSa-SvIv) generalizes the host-vector compartment models of types (ShIh-SvIv), (ShIhRhSh-SvIv), and (ShLhIhRhSh-SvLvIv). The study of this model made it possible to identify a technique suitable for combating malaria. Our model constitutes a valuable tool for the stochastic modeling of epidemics: it is used to predict the evolution of the dynamics of malaria in a human and anopheline population. In the forthcoming paper, we will study the geometric V-ergodicity of the model by including the effect of abiotic factors such as temperature.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Open Research
Data Availability
All the data is in the article.




