Triptolide-Loaded Mesenchymal Stem Cell–Derived Exosomes Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via Induction of Nrf-2/HO-1 Signaling Pathway–Dependent Autophagy and Mitigation of MAPK Signaling Pathway
Abstract
Acute lung injury (ALI) is a severe disease characterized by a pulmonary inflammatory response and oxidative stress. Triptolide has been demonstrated to have anti-inflammatory and antioxidant properties. Herein, we present triptolide-loaded mesenchymal stem cell–derived exosomes (MSC-Exos) as a multifunctional biomimetic delivery system (Tri-Exos) for targeting injured lung tissue. The therapeutic effect of Tri-Exos on ALI was evaluated by measuring inflammatory cytokines content, the expression of myeloperoxidase (MPO), the number of immune cells in bronchoalveolar lavage fluid, malondialdehyde (MDA) content, and superoxide dismutase (SOD) activity. Subsequently, immunohistochemistry and Western blotting were used to assess the expression levels of critical proteins in the nuclear factor erythroid 2-related factor 2 (Nrf-2) signaling pathway, autophagy pathway, apoptosis, and MAPK signaling pathway. The experimental results showed that LPS significantly increased pathological damage, secretion of inflammatory cytokines, and oxidative stress in lung tissue. However, treatment with Tri-Exos significantly alleviated this series of pathological changes. Tri-Exos significantly upregulated the expression of Nrf-2 signaling pathway–related proteins, autophagy, and apoptosis and inhibited the expression of MAPK signaling pathway-related proteins in the ALI model. Our study showed that Tri-Exos exerted a therapeutic effect on LPS-induced ALI by regulating the Nrf-2 signaling pathway–dependent autophagy and MAPK signaling pathway.
1. Introduction
Acute lung injury (ALI) is one of the worldwide public health problems which leads to acute respiratory distress syndrome (ARDS) [1]. ARDS is considered to be a severe inflammatory process in the lungs, caused by various direct and indirect injuries, and still maintains a high mortality rate [2, 3]. ALI is characterized by endothelial and epithelial destruction, which leads to pulmonary inflammatory responses such as neutrophil inflammation, perivascular and interstitial edema, gas exchange disturbances, and surfactant dysfunction [2, 4]. The persistence of inflammation plays a vital role in the development of ALI. There is increasing evidence that oxidative stress is considered a critical lung injury pathway affecting the severity of ALI [5]. Inflammation and oxidative stress are recognized as the highly interrelated event that is involved in the pathological process of ALI [6, 7]. Therefore, focusing on the inhibition of inflammation and oxidative stress may be a potential strategy for preventing and treating ALI. Reactive oxygen species (ROS)-induced oxidative stress, well-established pathogenesis of sepsis-induced ALI, is characterized by an imbalance between oxidants and antioxidants [8]. An increasing number of studies have shown that excessive ROS expression promotes the release of inflammatory mediators, ultimately leading to tracheal inflammation [9, 10].
Nuclear factor erythroid 2-related factor 2 (Nrf-2), a redox-sensitive protein, regulates various antioxidant systems including protein toxicity, oxidative stress, and metabolic stress [11]. Kelch-like ECH-associated protein 1 (Keap1) is a repressor of Nrf-2. Under general conditions, Nrf-2 is degraded by the Nrf-2 inhibitor Keap1-dependent pathway [12]. When activated by ROS, Nrf-2 is dissociated from Keap1 and transported into the nucleus to induce the expression of downstream proteins, including heme oxygenase-1 (HO-1), superoxide dismutase (SOD), and glutathione peroxidase (GSH) [13]. Previous studies have shown that Nrf-2−/− mice are more susceptible to hyperoxia and develop more severe symptoms than Nrf-2+/+ mice in a hyperoxia-induced ALI model [14].
Autophagy is a lysosome-dependent degradation pathway. The autophagy pathway can remove not only abnormal proteins but also remove damaged organelles and decompose them into single components for cells for repetitive use [15, 16]. Some studies have shown that autophagy activation can significantly inhibit the inflammatory response and oxidation stress, thereby playing a therapeutic role in a variety of inflammatory diseases such as osteoarthritis [17].
Most embryonic cells release exosomes (Exos), which are extracellular nanovesicles with high delivery efficiency, good tolerance, and minimal immunogenicity [18, 19]. Particularly, Exos are naturally occurring lipid-based carriers that exhibit a high potential intrinsic aptitude for delivering exogenous medicines to specific organs or cells [20, 21]. Exos are released from a range of cells and take on characteristics from the cells that were donated [22, 23]. Furthermore, mesenchymal stem cell–derived Exos (MSC-Exos) have been shown to reduce proinflammatory mediators including IL-1β, IL-6, and TNF-α [24].
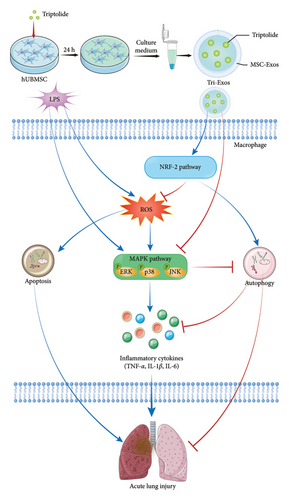
Triptolide, a diterpenoid epoxide extracted from the perennial plant Tripterygium wilfordii [25, 26], has been used to treat arthritis. Triptolide is one of the main bioactive components in Tripterygium wilfordii. Numerous studies have shown that triptolide plays a role in various immune diseases, including lupus nephritis, multiple sclerosis, colitis, and transplant rejection [27–29]. The anti-inflammatory activity of triptolide activates immune cells and resident tissue cells [30, 31]. In our study, MSC-Exos were used to improve the loading efficiency of triptolide. The antioxidant and anti-inflammatory potential of MSC-Exos loaded with triptolide (Tri-Exos) were investigated, and the mechanism of action of Tri-Exos in ALI was explored, which may provide an effective treatment for ALI (Figure 1).
2. Experimental Procedures
2.1. Materials
Triptolide (HPLC ≥ 98%) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). The primary antibodies of Nrf-2 and HO-1 were obtained from Abcam. Myeloperoxidase (MPO), SOD, and malondialdehyde (MDA) kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The Nrf-2 inhibitor ML385 was purchased from MedChemExpress (New Jersey, USA).
2.2. Tri-Laden Exo Isolation and Characterization
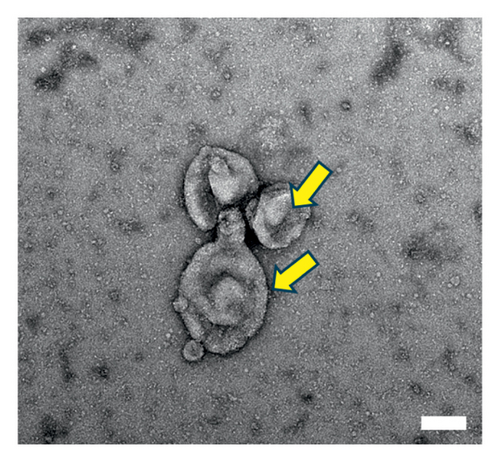
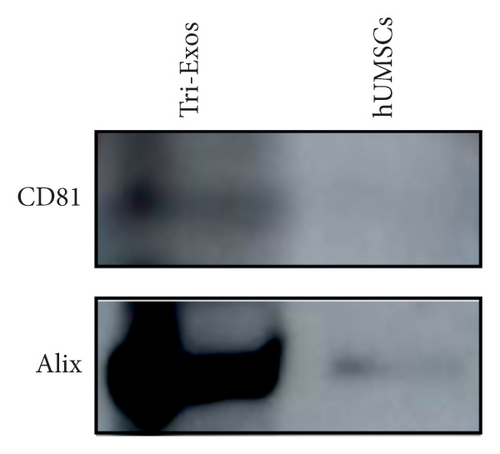
Human umbilical cord MSCs (hUMSC) were primed with triptolide. Briefly, triptolide at 40 nM was added to culture medium of hUMSCs for 24 h. The supernatant was then collected and subjected to ultracentrifugation at 2000 g for 30 min, 10,000 g for 30 min, and 100,000 g for 4 h at 4°C. For further characterization, isolated Tri-Exos were washed once with PBS. Exosomal markers such as Alix and CD81 were identified by Western blotting to determine the identity of Exos. The morphology and size of Exos were observed by electron microscopy (Hitachi H7500, Tokyo, Japan) [32].
2.3. Cell Culture
Raw 264.7 were cultured in RPMI 1640 medium (HyClone, Logan, Utah, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Eggenstein, Germany) and 1% penicillin/streptomycin (FBS; Gibco, Eggenstein, Germany) at 37°C in a 5% CO2 incubator [33]. Cells were purchased from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). During in vitro experiments, Raw 264.7 were treated with 1 μg/mL LPS for 6 h after preincubation with triptolide (40 nM), Tri-Exos (40 μg/mL), or preincubation with ML385 (1 μM) and Tri-Exos for 1 h.
2.4. Animals and ALI Model
The mice (C57BL/6 J, 6–8 weeks old, weighing 20–25 g, male) obtained from Shanghai Sixth People’s Hospital were used to establish the ALI model. All the mice were treated according to the standard guidelines approved by Shanghai Sixth People’s Hospital Ethics Committee. Lipopolysaccharide (LPS, Sigma, Saint Louis, MO, USA) was dissolved in PBS (2 mg/mL) and the LPS-induced ALI model was induced by intraperitoneal injection of LPS (8 mg/kg). The mice were randomized into three groups (n = 3): the control group, the LPS group, the triptolide (50 μg/kg) group, and the Tri-Exos (200 μg) group. The control group received equal volumes of PBS and the triptolide group was administered via a single intraperitoneal injection of triptolide. Triptolide and Tri-Exos were administered 1 h before the LPS challenge, according to a previous study [34]. The lung tissues, bronchoalveolar lavage fluid (BALF), and serum were harvested for analysis at 24 h after being susceptible to LPS.
2.5. Assay of Lung Water Content
After mice were sacrificed, lung tissues were weighted immediately for wet weight and weighted to obtain the dry weight, following treated at 60°C for 48 h in an oven. The ratio of wet weight to dry weight (W/D) was calculated.
2.6. Histopathological Evaluation
After mice were anesthetized with pentobarbital sodium, lung tissues were harvested under standard atmospheric pressure and fixed in 4% paraformaldehyde fixing solution for 24 h. After fixation, samples were embedded in paraffin and cut into 5 μm sections. Then, hematoxylin and eosin (H&E) staining and immunohistochemistry staining were performed to analyze the pathological changes in tissue morphology and composition. The pathological changes of lung tissues were observed by a microscope (Olympus, Tokyo, Japan). The following criterion was performed to evaluate the extent of damage: zero, no injury; one, injury to 25% of the field; two, injury to 50% of the field; three, injury to 75% of the field; four, diffuse injury [33]. The final score was obtained by adding the aforementioned score.
2.7. BALF Collection and Analysis
The BALF was obtained by flushing the right lung (0.3 mL per time, three times). Cells collected from BALF after centrifugation were resuspended in 100 mL cold saline. Then, the total number of cells was counted via hemocytometer. Cell smears were made and stained with Wright–Giemsa staining.
The concentration of BALF protein was detected using bicinchoninic acid (BCA) protein assay kit (Beyotime Biotechnology, Shanghai, China).
2.8. MPO Assay
24 h after LPS treatment, lung tissues were harvested for further analysis. The samples were homogenized in PBS and MPO activity in supernatant was detected using an MPO activity kits purchased from Nanjing Jiancheng Bioengineering Institute (China, Nanjing) according to the manufacturer’s instructions. The supernatant was measured at 460 nm according to the instructions.
2.9. Measurement of MDA and SOD
Content of MDA and activity of SOD in serum were detected using test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions.
2.10. RNA Isolation and qRT-PCR Analysis
Total RNA was isolated from lung tissues using TRIzol reagents (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Reverse transcription was performed using M-MLV reverse transcriptase (Takara) to synthesize complementary DNA. SYBR Green Premix Ex Taq (Takara) was applied to quantify the target gene mRNAs. The gene primers used are listed in Supporting Table 1 with GAPDH as a housekeeping gene.
2.11. ELISA
The secretion levels of IL-1β, IL-6, and TNF-α were measured using ELISA kits (Anogen) in accordance with the manufacturer’s instructions.
2.12. Western Blot
RIPA (Beyotime Biotechnology, China) was used to extract the proteins from lung tissues and the protein concentrations were detected using a BCA protein assay kit (Beyotime Biotechnology, China). The anti-ERK (Cell Signaling Technology, 4695T), anti-p-JNK (Cell Signaling Technology, 4668T), anti-p-ERK (Cell Signaling Technology, 4370 T), anti-GAPDH (Cell Signaling Technology, 5174), anti-p62 (Abcam, ab56416), anti-Beclin-1 (Abcam, ab207612), anti-Bcl-2 (Abcam, ab59348), and anti-Bax (Abcam, ab32503) were used for Western blot analysis. The Western blotting was performed according to the reported protocol [35].
2.13. Statistical Analysis
All statistical data were shown as mean ± standard deviation. The statistical analysis was performed with IBM SPSS software (version 23.0; IBM, Armonk, NY, USA). One-way ANOVA with Tukey’s post hoc method was used to compare means of values of groups. The difference was considered statistically significant when the p value was less than 0.05.
3. Results
3.1. Identification and Characterization of Tri-Exos
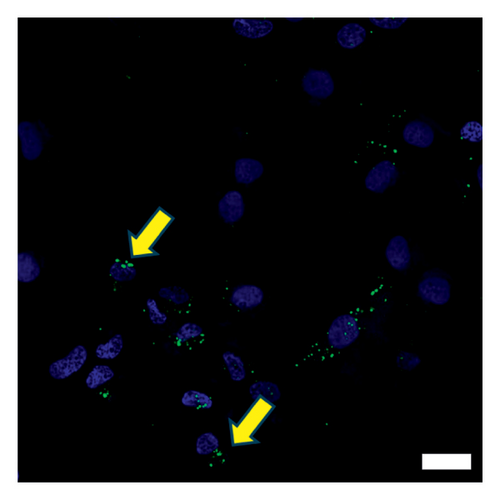
The morphology of the Exos was observed using an electron microscope, as shown in Figure 2(a). Additionally, Western blot analysis revealed that exosomal specific markers such as CD81 and Alix were positive (Figure 2(b)). The above findings show that they were Exos. Furthermore, PKH67 labeling of Tri-Exos for cellular uptake observation demonstrated that Raw 264.7 successfully assimilated Tri-Exos (Figure 2(c)).
3.2. Tri-Exos Treatment Attenuated LPS-Induced Lung Injury in Mice
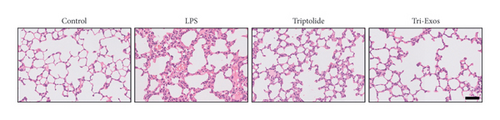
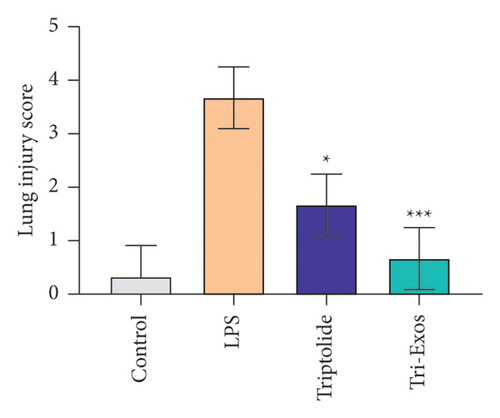
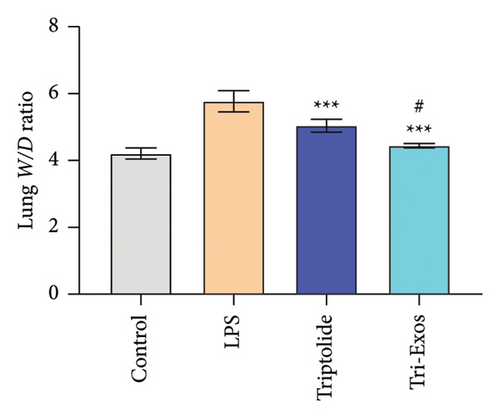
First, to evaluate the effect of Tri-Exos on ALI, histopathological changes of the lung tissues were detected by H&E staining (Figure 3(a)). In the LPS group, significant histopathological alterations were found, including inflammatory cell infiltration, alveolar damage, and pulmonary edema. However, treatment with triptolide and Tri-Exos markedly attenuated histopathological changes induced by LPS in ALI mice. The results of the lung injury score also showed similar changes (Figure 3(b)). The score increased markedly after treatment with LPS. The injection of triptolide and especially Tri-Exos significantly downregulated the lung injury score. The results indicated that Tri-Exos plays a protective role in the development of ALI. Then, to further evaluate the protective effect of Tri-Exos in LPS-induced ALI, lung W/D ratio was detected among all groups (Figure 3(c)). The results demonstrated that LPS challenge significantly upregulated lung W/D ratio compared to the control group, and the ratio was reduced by Tri-Exos treatment. These results confirmed the protective effect of Tri-Exos against LPS-induced ALI.
3.3. Tri-Exos Treatment Inhibited Inflammatory Cell Count and Protein Content in the BALF of ALI Mice
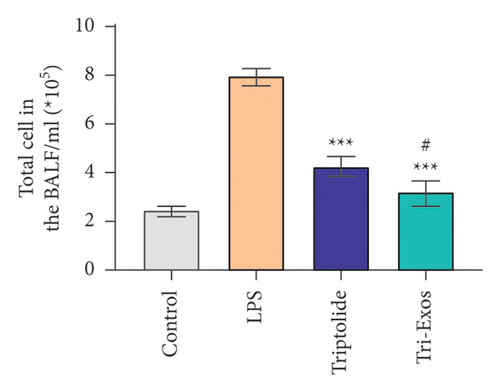
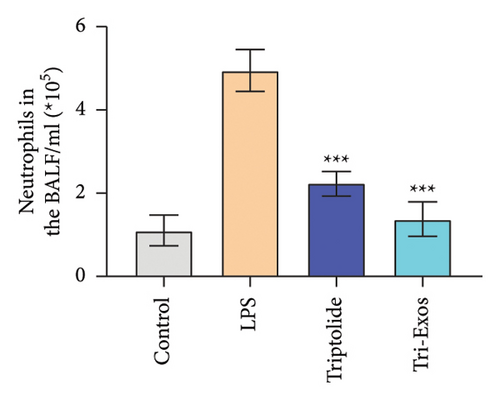
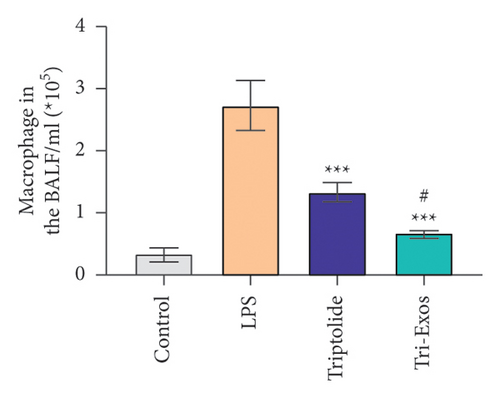
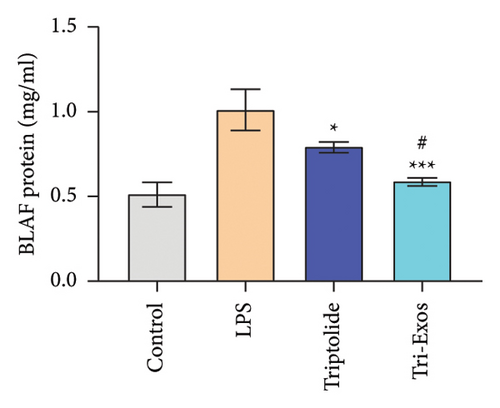
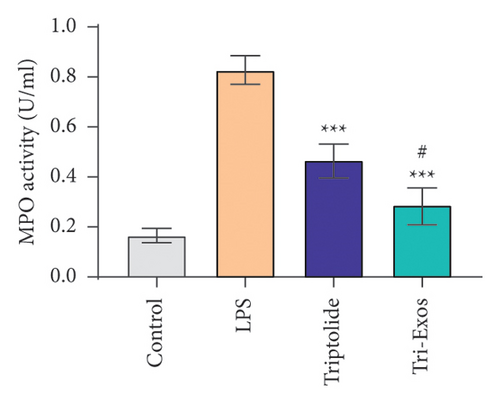
Capillary leakage and inflammatory cell infiltration are characteristic changes in ALI. The number of inflammatory cells in BALF was detected after LPS challenge (Figures 4(a), 4(b), 4(c)). LPS challenge significantly upregulated the number of total cells, neutrophils, and macrophages compared to the control group. However, the number of total cells, neutrophils, and macrophages was markedly reduced by treatment with Tri-Exos. Protein content indicates the lung permeability. Treatment with Tri-Exos significantly downregulated the level of protein in the BALF compared to the LPS group (Figure 4(d)). MPO activity, a marker of neutrophil activation, was detected to investigate the inflammation of lung tissue (Figure 4(e)). Similarly, treatment with Tri-Exos markedly reduced MPO activity compared to the LPS group.
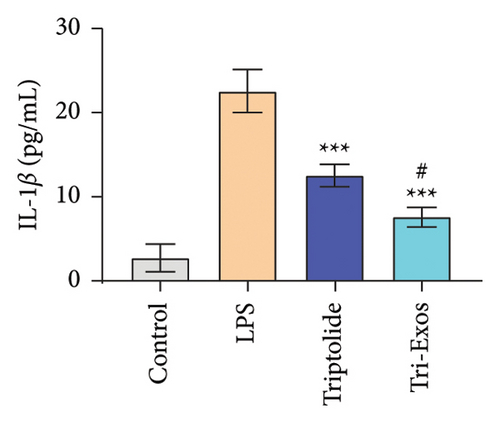
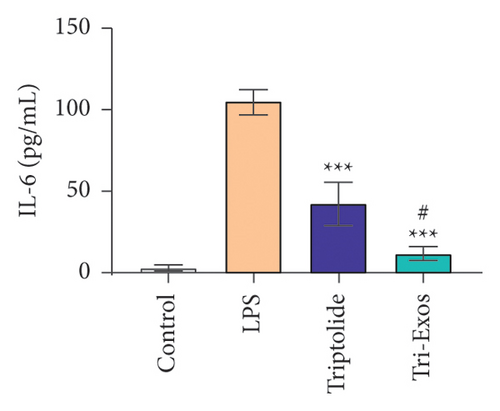
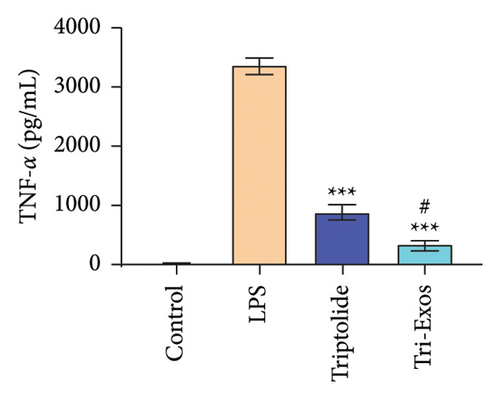
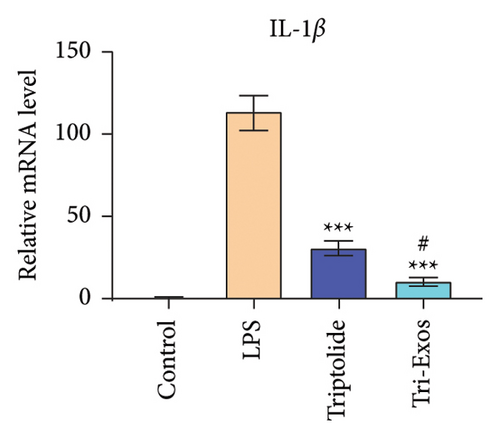
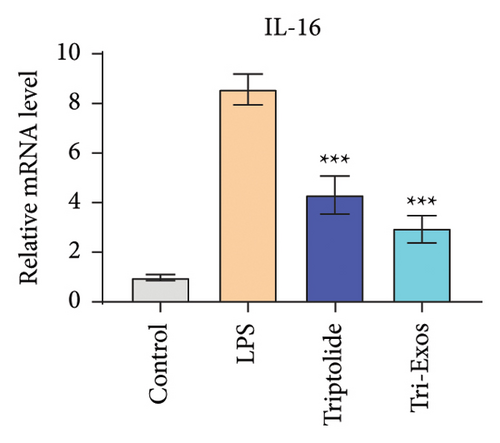
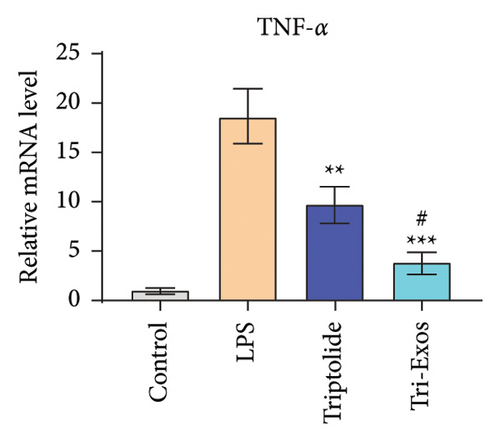
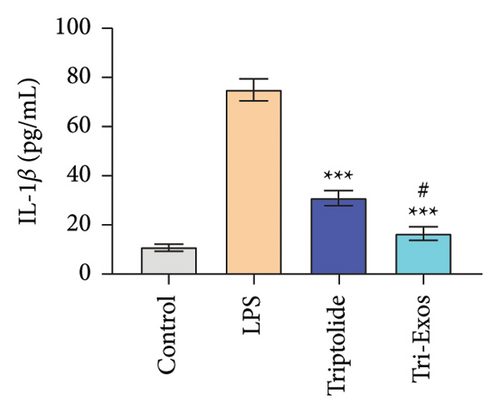
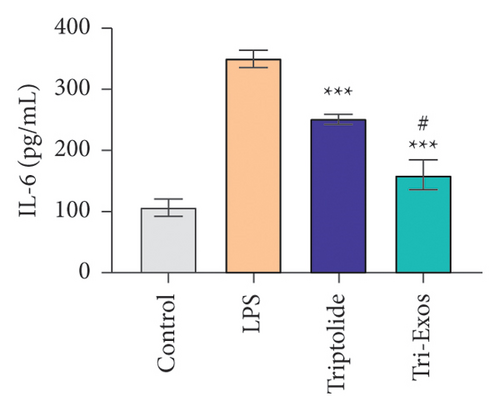
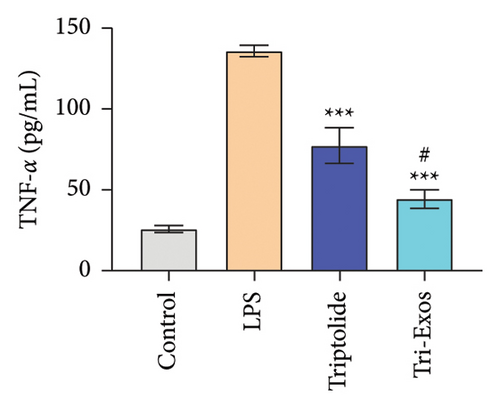
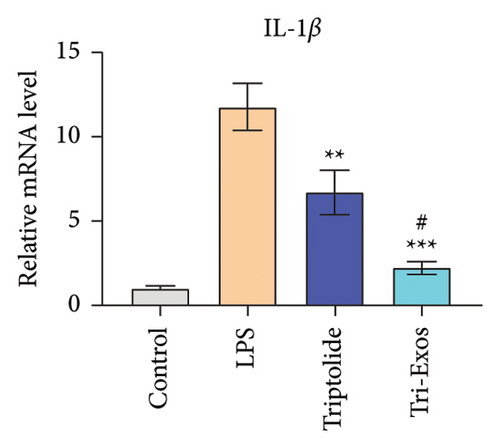
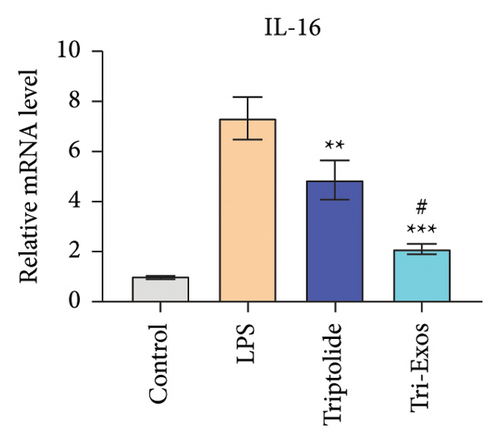
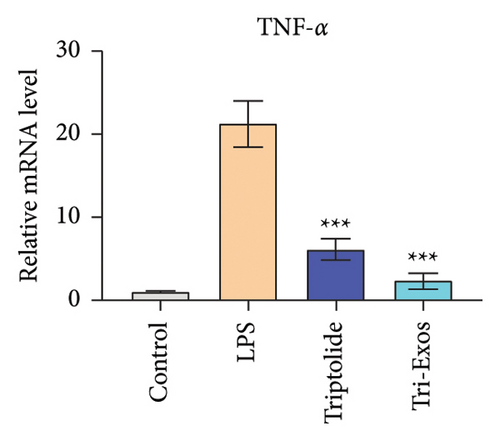
3.4. Tri-Exos Treatment Alleviated LPS-Stimulated Inflammatory Cytokines In Vitro and In Vivo
The progression of inflammation response was closely associated with the proinflammatory cytokines [36]. To evaluate the anti-inflammatory effect of Tri-Exos, the ELISA and qRT-PCR (Figures 5 and 6) were performed to detect the level of proinflammatory cytokines in macrophages and lung tissues. As shown in Figures 5 and 6, the expression levels of IL-1β, IL-6, and TNF-α were upregulated in the LPS group in both in vitro and in vivo compared to the control group. However, treatment with Tri-Exos significantly decreased the expression of proinflammatory cytokines. The results indicated that Tri-Exos had anti-inflammatory effect in ALI mice.
3.5. Tri-Exos Treatment Attenuated Oxidative Stress in LPS-Induced ALI Mice
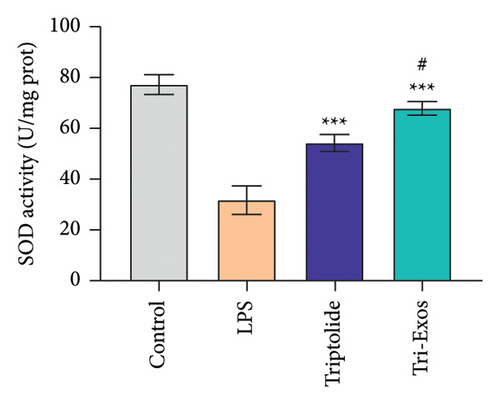
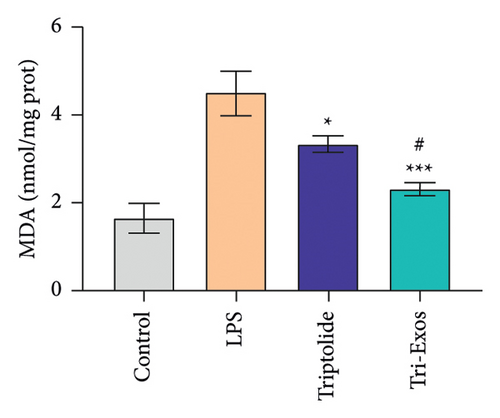
Oxidative stress plays a crucial role in a wide range of diseases [37]. Therefore, the activity of SOD and MDA in serum was detected (Figures 7(a) and 7(b)). LPS challenge significantly reduced the expression of SOD, an antioxidant, and increased the expression of MDA, a marker of oxidative stress, in serum compared to control group, while Tri-Exos treatment markedly upregulated the activity of SOD and suppressed the expression of MDA compared to the LPS group. The results indicated that Tri-Exos had the antioxidative effect in ALI mice.
3.6. Nrf-2/HO-1 Signaling Pathway Was Associated With the Antioxidative Stress Effect of Tri-Exos
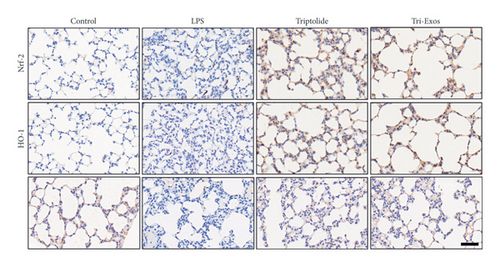
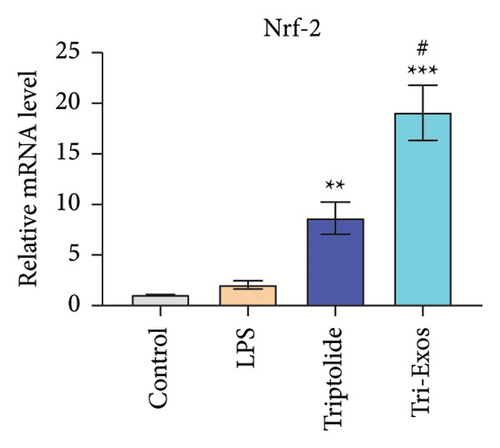
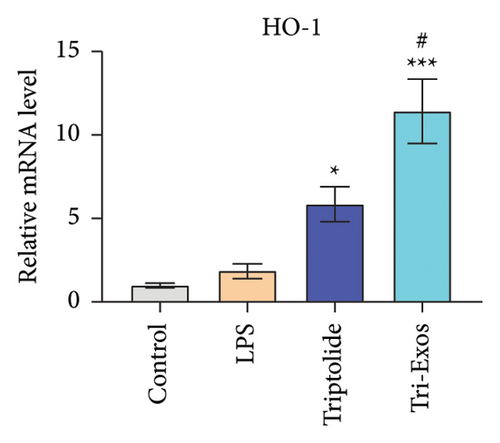
Nrf-2/HO-1 signaling pathway has been reported to be involved in the regulation of oxidative stress and inflammatory responses [38]. To evaluate the mechanism by which Tri-Exos exerts its antioxidative stress, immunohistochemical staining (Figure 8(a)) qRT-PCR (Figures 8(b), 8(c), and 8(d)) was used to detect the protein and mRNA expression of Nrf-2 and HO-1 in lung tissues. The results showed that LPS challenge slightly upregulated the protein expression levels and the mRNA expression levels of Nrf-2 and HO-1 compared to the control group. However, Tri-Exos treatment significantly increased the protein expression levels and the mRNA expression levels of Nrf-2 and HO-1 in lung tissues.
3.7. Tri-Exos Upregulated Autophagy in LPS-Induced ALI Mice via Nrf-2/HO-1 Signaling Pathway
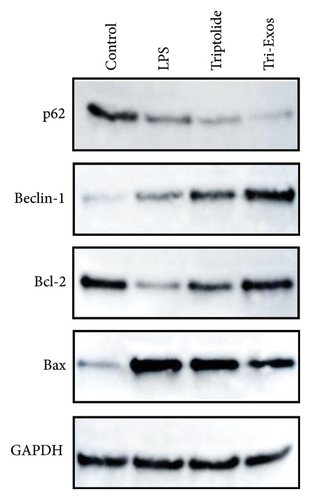
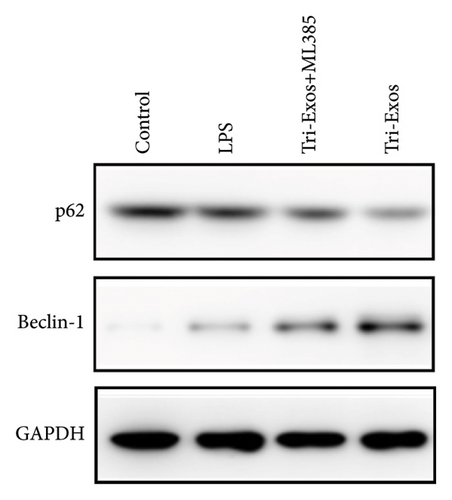
Given the established protective effect of autophagy on lung tissue in ALI [39], we further investigated the induction of autophagy by Tri-Exos. The results (Figure 9(a)) showed a significant increase in the expression of Beclin-1 and an obvious decrease in the expression of p62 in the LPS group, demonstrating that autophagy was significantly activated in LPS-induced ALI. However, the expression of Beclin-1 was significantly upregulated, and the expression of p62 was obviously downregulated in the Tri-Exos group compared with the PBS group. These results indicated that Tri-Exos could significantly activate autophagy in LPS-induced ALI, thereby exerting a therapeutic effect. Recently, many studies have shown a close relationship between the Nrf-2/HO-1 signaling pathway and autophagy [40]. To further explore the role of Nrf-2/HO-1 signaling pathway and autophagy, ML385 (Nrf-2 inhibitor) was used to perform rescue experiments to evaluate the effect of Nrf-2/HO-1 signaling pathway on autophagy activation. In our experiment, the results (Figure 9(b)) showed an obvious upregulation of Beclin-1 and a significant downregulation of p62 in the Tri-Exos group. However, after being treated with ML385, the autophagy induced by Tri-Exos was significantly inhibited. In general, Tri-Exos could induce autophagy by promoting Nrf-2/HO-1 signaling pathway.
3.8. Tri-Exos Attenuated Apoptosis in LPS-Induced ALI Mice
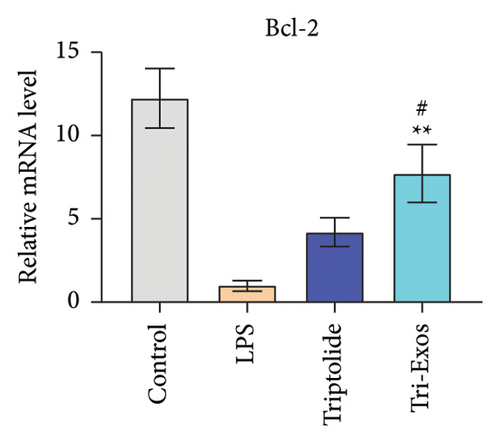
The level of Bcl-2 and Bax was measured to evaluate the effect of Tri-Exos on apoptosis in ALI mice. The results (Figure 9(a)) indicated that Tri-Exos significantly upregulated the expression of Bcl-2, while markedly downregulated the level of Bax compared with the PBS group. These results demonstrated that Tri-Exos inhibited apoptosis in LPS-induced ALI mice.
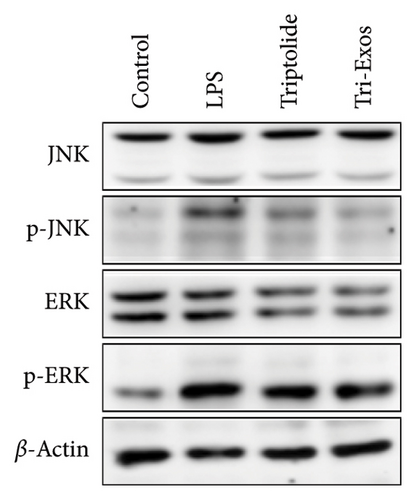
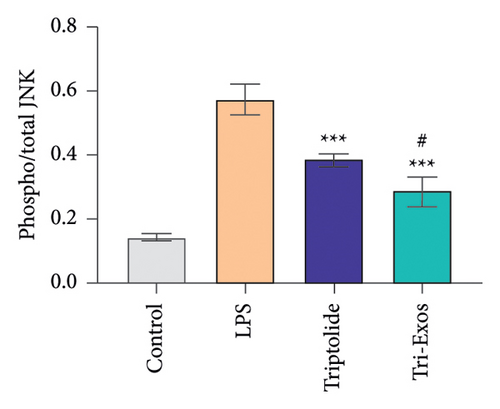
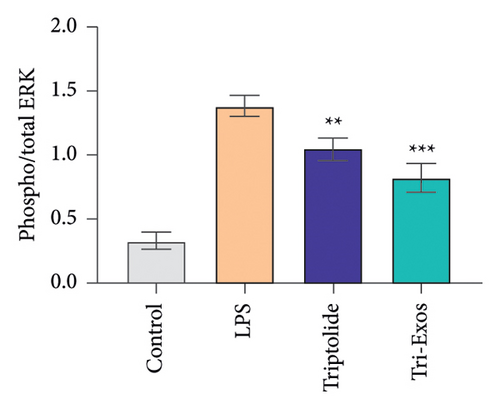
3.9. Tri-Exos Inhibited Inflammation via Activating the MAPK Signaling Pathway
MAPK signaling pathway is found to play a crucial role in the inflammatory process [41]. The phosphorylation activities of JNK and ERK signals are key processes in the MAPK pathway during the inflammatory response [42]. Therefore, to further evaluate the anti-inflammatory mechanism employed by Tri-Exos in the treatment of ALI, the expression of p-JNK and p-ERK in lung tissues were detected via Western blot analysis (Figures 10(a), 10(b), and 10(c)). LPS challenge significantly increased the ratio of phosphorylated JNK to total JNK protein (p/t) compared to the control group, which is consistent with the trend of lung tissue destruction. However, Tri-Exos treatment showed a markedly inhibition on the expression of p-JNK. The expression levels of p-ERK were consistent with the trend of the p-JNK expression in all groups. The results demonstrated that Tri-Exos suppressed LPS-induced inflammation by attenuating MAPK signaling pathway.
4. Discussion
ALI is associated with significant morbidity and mortality. It is a severe disease characterized by acute inflammation in the lung tissues [43]. In the LPS-induced ALI model, immune cells such as neutrophils and macrophages were stimulated by LPS to infiltrate into lung tissue and produced many inflammatory factors in BALF, serum, and lung tissue, including TNF-α, IL-6, and IL-1β [44–46]. Polymorphonuclear neutrophils are recruited to inflammatory lesions under the induction of inflammatory factors and play an essential role in the pathogenesis of ALI [47, 48]. In this study, to evaluate the protective effect of Tri-Exos, the LPS-induced ALI mice were treated with Tri-Exos. Tri-Exos ameliorated the pathological changes and W/D ratio of lung tissues in ALI models. Tri-Exos significantly suppressed LPS-induced IL-1β, IL-6, and TNF-α production in vitro and in vivo. The MPO activity and the number of neutrophils and macrophages were markedly downregulated by Tri-Exos treatment. These results indicated that Tri-Exos had a therapeutic effect on LPS-induced ALI by alleviating the inflammatory response.
It was reported that oxidative stress also played an essential role in LPS-induced ALI [34]. In this study, LPS challenge significantly upregulated the expression of MDA and downregulated the expression of SOD. However, the expression of MDA was markedly decreased and the expression of SOD was increased in the Tri-Exos group. The results indicated that Tri-Exos markedly attenuated oxidative stress in ALI mice.
Nrf-2 is an essential transcriptional regulator that is upregulated by ROS and promotes the expression of antioxidant genes, including HO-1 and NQO-1, to protect cells and tissues from oxidative stress damage [49]. Under normal conditions, Nrf-2 binds to cytoplasmic Keap1 to promote proteasomal degradation. However, under oxidative stress conditions, the conformation of Keap1 changes and leads to the release of free Nrf-2. Then, Nrf-2 translocates into the nucleus and promotes the expression of antioxidant genes [49, 50]. Previous studies have shown that Nrf-2 has an obvious protective effect on LPS-induced lung injury in mice [34]. Similarly, the results of this study showed that Tri-Exos treatment significantly promote the expression of Nrf-2 and HO-1. These results indicated that Tri-Exos alleviated oxidative stress in the pathological process of LPS-induced ALI by activating the Nrf-2 signaling pathway.
Numerous studies have found that autophagy inhibits activation of key proteins of the NLRP3 inflammasome, thereby alleviating the production and release of IL-1β [51, 52]. Autophagy could promote the polarization of macrophages from M1 phenotype to M2 phenotype and inhibit apoptosis. And autophagy could also relieve the symptoms caused by sepsis [51, 52]. In terms of antioxidative stress damage, the autophagy pathway promotes the defense mechanism of cells against oxidative stress by selectively removing misfolded proteins and damaged organelles in cells, thereby alleviating oxidative damage [53, 54]. Our study demonstrated that Tri-Exos could alleviate LPS-induced ALI by inhibiting inflammation and oxidative stress via promoting Nrf-2 and autophagy signaling pathways.
Apoptosis is the process of programming cell death. Bcl-2 and Bax are key proteins participating in this process. Bcl-2 has the ability to resist apoptosis, while Bax participates in the induction of apoptosis [55]. Some studies have shown that the autophagy can reduce the activation of apoptosis by removing damaged organelles [56]. In this study, we found that apoptosis was significantly reduced in the Tri-Exos group compared with LPS-induced ALI mice. Our results indicated that Tri-Exos inhibited apoptosis in LPS-induced ALI mice by promoting autophagy signaling pathway.
The proinflammatory cytokines, including IL-6, IL-1β, and TNF-α, are important components in the development of inflammatory response [57]. Previous studies have shown that the MAPK signaling pathway plays a vital role in regulating the inflammatory response [58]. In this study, Tri-Exos significantly reduced the expression of IL-6, IL-1β, and TNF-α in vitro and in vivo. Tri-Exos also significantly inhibited the expression of essential proteins in the MAPK signaling pathway in ALI mice, such as p-JNK and p-ERK. Therefore, the results indicated that Tri-Exos alleviated the LPS-induced inflammatory response by inhibiting the MAPK signaling pathway. Although this study comprehensively investigated the therapeutic effect and mechanism of Tri-Exos on ALI in mice, it has not been validated in large animals such as goats, which is a limitation of this study. We will further investigate this in future studies.
5. Conclusion
In summary, our study showed that LPS induced inflammation and oxidative stress leading to ALI in mice. As a ROS scavenger and an inflammatory inhibitor, Tri-Exos played a therapeutic role by regulating the Nrf-2 signaling pathway–dependent autophagy and MAPK signaling pathways to relieve oxidative stress and inflammatory responses in the pathological process of ALI. This study provides a potential strategy for the clinical treatment of ALI.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the National Natural Science Foundation of China (81772062).
Supporting Information
See Supporting Table 1 in the Supporting Information for the gene primers used in qRT-PCR analysis.
Open Research
Data Availability Statement
The data that support the findings of this study are available from corresponding author upon reasonable request.




