Epstein–Barr Virus-Associated Lymphoproliferative Disorders/Lymphoma with Skin Manifestations as the Primary Symptom: A Systematic Review
Abstract
Background. Epstein–Barr virus (EBV) associated skin lesions have been mentioned in case report studies under multiple kinds of lymphoproliferative disorders/lymphoma diagnoses. However, due to the rarity and scattered reporting of cases, it is still unclear whether the related skin symptoms and their pathological findings can guide the clinical diagnosis and treatment of EBV-associated lymphoproliferative disease/lymphoma. Methods. In this review, we summarized the skin symptoms and clinicopathological features mentioned by previously reported cases of EBV-associated lymphoproliferative disorders/lymphoma to assist future clinical diagnosis. The inclusion criteria were based on the population, intervention, comparator, outcomes, and study designs. An electronic search was conducted by September 2023, and the following databases were used: PubMed, EMBASE, Cochrane Library, and Web of Science. Search keywords included “Epstein-Barr Virus Infections,” “Herpesvirus 4, Human,” “Lymphoma,” “Lymphoproliferative Disorders,” and “skin.” Results. The primary outcome was the clinical skin features and pathological findings of EBV-associated lymphoproliferative disease/lymphoma patients. Although it seems unrealistic to differentiate between patients with EBV-related lymphoproliferative disorders/lymphomas with different diagnoses on the basis of cutaneous symptoms and pathological findings alone, based on the evidence summarized in previous case reports, the clinical importance of EBV detection and identification in the differential diagnosis of lymphomas and lymphoproliferative disorders should be recognized. Conclusion. Given the homogeneity of risk factors associated with disease progression found in EBV-associated lymphoproliferative disease/lymphoma patients during the review, future studies can focus on summarizing skin symptoms and pathological outcomes based on possible risk factors for further deterioration in these patients.
1. Introduction
Epstein–Barr virus (EBV) is a member of the herpes virus family and is one of the most common human viruses [1]. About 90% of adults worldwide suffer from EBV infection. It is the leading cause of infectious mononucleosis, most commonly seen in adolescents [2]. EBV infections can lead to a variety of diseases even during latency, including lymphoma and lymphoproliferative disease (LPD) [3]. EBV has a 172 kilobase double-stranded DNA genome [4], infects human B lymphocytes and epithelial cells, and spreads through saliva. 90% of children are infected with EBV before the age of 5, and some patients may progress to infectious mononucleosis, but most are asymptomatic [5, 6]. EBV preferentially infects B lymphocytes through the binding of gp350, a viral surface glycoprotein, to its complement receptor 2 (CR2/CD21) or CR1 (CD35) on B lymphocytes. In addition, the Class II major histocompatibility complex molecule on B lymphocytes binds to gH/gL/gp42 and can act as a coreceptor.
In some individuals, EBV may enhance B-cell transformation and cause a range of EBV-associated lymphoproliferative diseases, and the global burden of cancer deaths due to EBV infection is significant, with approximately 200,000 new cases of EBV-associated malignancies and approximately 143,000 cancer deaths each year [7, 8]. These include Burkitt lymphoma (BL) [9], EBV-positive diffuse large B-cell lymphoma [10], primary effusion lymphoma [11], EBV-positive cutaneous mucosal ulcer [3], lymphomatoid granulomatosis [12], plasmoblastic lymphoma [13], post-transplantation lymphoproliferative disease (PTLD) [14], and classical Hodgkin’s lymphoma [15] and diffuse large B-cell lymphoma associated with chronic inflammation [16]. In addition, EBV-infected B cells may affect surrounding T-cells and natural killer (NK), leading to direct infection of EBV [17, 18] and causing rare EBV-associated T/NK-cell proliferative diseases. These include vascular immunoblastic T-cell lymphoma [19], enteropathy-associated T-cell lymphoma [20], EBV-associated anaplastic large cell lymphoma [21], peripheral T-cell lymphoma [22], systemic EBV-positive T-cell proliferative disease in children [23], extranodal nasal NK/T-cell lymphoma [24], gamma-delta T-cell lymphoma (hepatosplenic and nonhepatosplenic) [25], and aggressive NK-cell leukemia [26]. Meanwhile, significant differences should be noticed in the mode of action of EBV virus in primary and secondary LPD. Primary LPD associated with EBV is more commonly found in the elderly, who are mostly nonsusceptible immunodeficient individuals, suggesting that the development of these processes is related to the damage to the immune system that naturally occurs with age [27]. In contrast, in EBV-associated secondary LPD patients, the specific effect of EBV infection on B-cell transformation and proliferation is more significant, and the disease types and age groups involved are more variable [28]. The hypothesized pathological mechanism is that the immune dysregulation manifested during the treatment of primary cancer may lead to the reactivation of EBV, which may play a role in the proliferation and transformation of B-cell clones into immobile EBV-infected B cells [29].
These observations led the researchers to identify EBV as an important vaccine target for cancer prevention. At the same time, it is considered urgent to establish a collaborative network to discover predictive markers and immune-related factors of EBV-associated malignancies to achieve rapid clinical differential diagnosis and treatment [30]. EBV can lead to different skin manifestations depending on the associated disease. Disseminated disease typically presents with multiple subcutaneous nodules, while chronic EBV infection can cause localized blistering, ulcers, and severe reactions to mosquito bites. Analyzing the skin manifestations of various EBV-related diseases can enhance understanding and diagnosis of these conditions. As a result, our objective was to enhance comprehension of EBV-linked lymphoproliferative disorders/lymphomas featuring skin lesions as the primary or initial clinical indication by consolidating the cutaneous presentations and pathological characteristics observed in previously documented cases.
2. Materials and Methods
2.1. Research Design
The present systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement) [31]. The study was registered on PROSPERO with the registration number: CRD42023479805.
2.2. Search Strategy and Data Sources
PubMed, EMBASE, Cochrane, and Web of Science were searched with a predesigned search strategy in September 2023 to retrieve all relevant clinical studies, employing the MeSH terms “Epstein-Barr Virus Infections,” “Herpesvirus 4, Human,” “Lymphoma,” “Lymphoproliferative Disorders,” and “skin,” as well as relevant key words. The comprehensive search strategy for all databases is shown in Table S1. Additionally, references from relevant articles and reviews were screened to identify other eligible studies, and manual screening was also performed. Each study was evaluated by two independent reviewers, and disagreements were resolved by discussion with a third reviewer.
2.3. Inclusion and Exclusion Criteria
The selection criteria were developed based on the PICOS principle as outlined below.
2.3.1. Inclusion Criteria
-
P: EBV-associated lymphoproliferative diseases/lymphomas patients;
-
O: Skin symptoms and pathological mechanisms need to be reported;
-
S: Case reports and case series.
2.3.2. Exclusion Criteria
- (a)
Ineligible study design, such as commentary and conference abstracts;
- (b)
Essential data were absent from studies although emailed authors to obtain it;
- (c)
Older duplicate reports published by the same team based on the same group of participants;
- (d)
Studies included ineligible participants, such as EBV-negative participants;
- (e)
Interested results not available.
- (f)
Unable to obtain full text in English.
2.4. Data Extraction
A predesigned Excel spreadsheet was utilized for data extraction from the included studies. Data extraction was performed by pairs of independent researchers, with inconsistencies resolved through discussion or consultation with a third reviewer [32]. In cases where data were unreported in the studies, the authors were contacted for additional data; the rest of the data were publicly available as reported in the paper. The characteristics of the included studies are summarized as follows: name of the first author, year of publication, study country, study design, sample size, age, gender, clinical diagnosis, past medical history, EBV infection status, clinical skin features, diseased region, pathological findings, prognosis, survival time, and clinical suggestions.
3. Results
3.1. Results of Study Selection
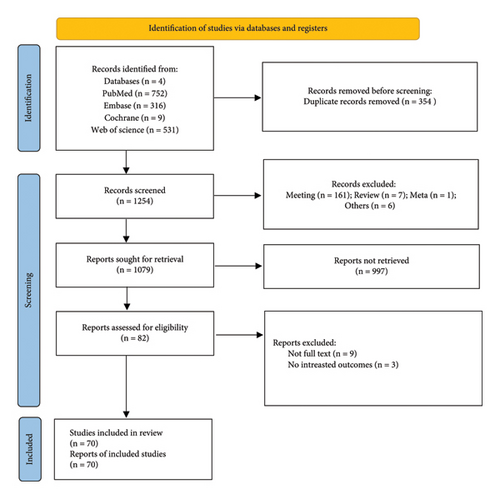
In sum, 1608 articles were identified in electronic and manual searches. However, 354 articles were excluded for duplication. 1526 records were excluded after reviewing the title and abstract, and we excluded 12 records after reviewing the full text of 82 articles. The exclusion reasons were full texts not available. Finally, 70 articles [14, 19, 28, 33–45] were included in this meta-analysis (Figure 1).
3.2. Study Characteristics
The basic characteristics of the 70 included full-text studies are shown in Table S2. Among 70 studies with 66 case reports and 5 case series published in 17 countries, cases were reported mainly in Japan (15), China (11), USA (10), UK (8), France (6), and Korea (5). A small number of cases have been reported in the Netherlands (3), Germany (2), and Turkey (2). The remaining cases have been sporadic in Italy, Brasil, Poland, Ecuador, Spanish, Kosova, Australia, and Switzerland. Seventy studies reported 174 EBV-positive lymphoproliferative diseases/lymphomas patients with skin symptoms, with a mean age of 46 (1.5 to 86), including 58 women and 116 men. There were 33 pediatric patients younger than 18 years old, 44 patients older than 60 years old, and 37 adult patients’ age from 20 to 59. Clinical diagnoses of 174 patients were divided into six categories: lymphoma (34), LPD (25), B-cell lymphoma combined with T-cell lymphoma (6), hemophagocytic lymphohistiocytosis (2), mucocutaneous ulcer (2), and EBV-associated Kikuchi disease (1). Among patients diagnosed with lymphoma, 15 studies reported patients with T-cell lymphoma (including 6 natural killer (NK) T-cell lymphoma), and 9 studies reported patients with B-cell lymphoma. Of the 25 LPD studies, 5 involved T cells and 4 involved B cells. Three studies reported a previous dermatomyositis of the patients, and another three reported rheumatoid arthritis. No significant similarities were found in the clinical history of other cases. These include hypertension, diabetes, organ transplantation, primary lymphoma, inborn errors of immunity (IEI), rheumatoid arthritis, and mosquito bite sensitivity. Some of the patients who died progressed rapidly (n = 9), with survival greater than one year in only five of the 14 studies, and a minimum survival of two weeks in the remaining patients.
3.3. Clinical Skin Features
The skin symptoms of the patient mainly include skin ulceration and rash, subcutaneous nodules, purpura, papules, granulomatous, and occasionally erythema scales. Severe skin symptoms are manifested as skin erosion or necrosis, and the color of the lesion is mostly red or purple. The back, chest, abdomen, and extremities are the most frequently mentioned lesion locations in case reports. However, we did not find significant differences in skin symptoms between different diagnoses, so we summarized several most frequently reported skin symptoms of EBV-related lymphoproliferative diseases/lymphomas in this part. The detailed skin symptoms and diseased region are summarized in Table S3.
3.3.1. Ulceration
EBV-associated skin ulcers tend to occur on the extremities, face, and neck, with 18 of 24 studies reporting skin ulcers on these sites, 16 of which were found on the legs or arms, and 7 studies reporting patient lesions on the head and neck. There were 3 studies reported rare oral ulcers, in one patient with childhood T-cell lymphoma, one patient with vaccine-like vesicular skin lesions of T-cell lymphoid hyperplasia and subsequent development of aggressive NK/T-cell lymphoma, and one patient with EBV-positive mucocutaneous ulcer.
3.3.2. Nodules
EBV-associated skin nodules are more common in lymphoma patients, and 11 of the 18 studies reported skin nodules in lymphoma patients. The pooled data showed that skin nodules in these patients tended to be on the extremities, and six of the 11 studies reported skin nodules in lymphoma patients on the legs or arms. Multiple skin nodules were reported in 3 additional studies in which 2 patients were diagnosed with T-cell lymphoma and 1 patient with B-cell lymphoma. Four of the six CD56+ NK/T-cell lymphoma patients reported by Child 2003 developed skin symptoms characterized by widespread nodules and plaques, some with characteristic purple color. Another patient with T-cell lymphoma developed multiple, itchy, purplish nodules and excoriations on both of her upper limbs. The only patient with B-cell lymphoma developed multiple skin ulcers and nodules in the torso and arms.
Two of the four LPD patients with skin nodules had a history of significant influence on immune function, namely, AIDS and late after transplantation. Another 14-year-old patient with T/NK-cell lymphoproliferative disorders, who developed a rare recurrent spontaneous perforation of the gastrointestinal tract, died as a result of an early diagnosis of IBD and delayed treatment. The researchers suggest that infectious and malignant diseases should be considered in patients with IBD who present atypically and do not improve after appropriate treatment. Nodules, ruptures, and escharosis of the extremities and buttocks in this case have the potential to be key to early identification.
3.3.3. Papules
Papules associated with EBV infection were reported in 9 of 70 studies, with scalp, trunk, both lower limbs and face all likely to occur. Skin papules were painless in six of the nine studies, but painful erosive papules were reported in three of the studies. Two cases of T-cell lymphoma occur on the face. A patient with T-cell lymphoma mimicking dermatomyositis developed a rare eye condition with erythema swelling on both eyelids. Another patient with aggressive NK/T-cell lymphoma developed erosive facial papules and small oral ulcers.
In addition, sporadic skin symptoms such as papules and granulomatous skin lesions, diffuse nonpruritic papules, vesicles, bullosa and healing erosion, and viral leakage have been reported in EBV-positive cases. This also indicates that it is difficult to make a reliable diagnosis based on skin symptoms alone, but the study suggests that patients with congenital immunodeficiency should consider the possibility of viral infection even if the skin lesions and/or lymphocytosis symptoms are not obvious [26]. Furthermore, two studies reporting recurrent EBV-associated lymphoproliferative skin lesions suggest that such skin symptoms may be an early sign of lymphoma or leukemia, and the importance of accurate pathological diagnosis by peripheral blood EBV DNA testing and EBER testing of infected tissue should be recognized [34, 38].
3.4. Pathological Findings
In this part, we refer to the revised 5th Edition of the WHO classification of tumours of the hematopoietic system [46] to be included EBV-positive lymphoproliferative diseases/lymphomas were classified and the skin pathological features of patients with different diagnoses were summarized. Among the 70 studies, 63 provided the skin pathological characteristics of the patients, and the specific pathological results are shown in Table S4.
3.4.1. EBV-Associated T-Cell Lymphoma
EBV-positive and skin symptoms were identified for 10 T- and NK-cell-associated lymphomas, six of which were extranodal NK/T-cell lymphoma, two were cutaneous NK/T-cell lymphoma, and one was aggressive NK/T-cell lymphoma [47]. The remaining one was the use of a related EBV-positive primary cutaneous NK/T-cell lymphoma with methotrexate [48].
(1) T/NK-Cell Lymphoma. Patients with extranodal T/NK-cell lymphoma are characterized by vascular injury and destruction, significant necrosis, cytotoxic phenotype, and are associated with EBV (e.g., cases involving the liver and spleen). Skin biopsies from the patients included in the analysis showed dense lymphatic infiltration of the dermis and subcutaneous tissue, predominantly perivascular and periadnexal, with no significant vasculitis or fibrinoid necrosis. The infiltration is mainly composed of small- and medium-sized lymphocytes, and the tumour cells are atypical medium-sized cells. The histological changes of the patient’s skin showed infiltration of subcutaneous lobe lymphoid tumour cells. Tumour cells infiltrate secretory glands. Atypical, medium, and pleomorphic lymphoid neoplasms proliferative with a clear cytoplasm, comma-shaped nuclei, and microscopic chromatin. Compared with pediatric patients, fever frequency was significantly reduced in adult patients, and the incidence of skin erythema was higher. Vaccine-shaped hydrops and mosquito bite allergy are less common in adult-onset patients. Laboratory results at the time of initial diagnosis showed that elevated liver enzymes were more common in pediatric patients and hemophagocytic syndrome in bone marrow biopsies in adult patients [49].
(2) EBV-Positive Nodal T- and NK-Cell Lymphoma. Both patients showed EBV coding RNA (EBER) and gene rearrangement in situ hybridization and were free of pancytopenia and hemophagocytophagic syndrome. Patients exhibit an extended clinical course of disease, culminating in an invasive phase characterized by coinfection and disease progression [24, 50].
Aggressive natural killer cell leukemia (ANKL) is a systemic NK cell tumour that is almost always associated with EBV [51]. Histological examination of the skin biopsy of the case reported by Toksoy 2016 revealed variable dense lymphatic infiltration. In situ hybridization with EBV RNA, molecular cloning analysis, and lymph node and bone marrow biopsies showed the same EBV+ T-cell proliferation.
Patients with EBV-positive primary cutaneous NK/T-cell lymphoma reported by Parker [48] had received oral methotrexate for long-term rheumatoid arthritis. Tissue analysis revealed large tumor cells that were surface CD2- and CD3-positive; T-cells restricted intracellular antigen-positive, and immunophenotypic features suggest atypical natural killer/t cell lymphoma [48].
3.4.2. EBV-Associated T or NK Cell Lymphoproliferativer Disorders
Of the 26 studies that reported EBV-associated LPD patients, 9 reported T-cell and/or NK-cell-associated lymphoproliferative disorders, 4 reported B-cell-associated LPD, 4 reported LPD after organ transplantation, 3 reported Hydroa vacciniforme-like (HVL) LPD, and 2 reported EBV-positive mucocutaneous ulcer (EBVMCUs). The remaining four studies involved two studies of LPD after methotrexate treatment, one study of iatrogenic infection EBV plus low-dose methotrexate [52], and one study of EBV-associated cutaneous lymphatic hyperplasia in children with AIDS [53].
(1) EBV-Positive T- and NK-Cell Lymphoproliferative Disorders. Twelve of the 14 cases of EBV-positive T- and NK-cell lymphoproliferative disorders occurred in minors and young patients in their twenties, including 1 child patient who was sensitive to mosquito bites [54], 2 patients had recurrent lymphoproliferative skin lesions associated with EBV [55, 56], 1 had previously received antitumor mab [57], and 1 had recurrent gastroenteroid perforation [58]. There was no significant homogeneity in pathological results.
(2) EBV-Positive T- and NK-Cell Lymphoid Proliferations and Lymphomas of Childhood. As one of the hotspots of current research, this family includes chronic active EBV disease (CAEBVD) and systemic EBV-positive T-cell lymphoma in childhood. CAEBVD has a wide range of clinical features, ranging from local and/or inert forms (severe mosquito bite allergy and vaccine-like vesicular lymphoproliferative disease [HVLPD] classical forms) to systemic disease with or without skin manifestations accompanied by fever, hepatosplenomegaly, and lymphadenopathy [46]. Four studies have reported on children with HVLPD, and only two have performed skin biopsies, all suggesting a dense infiltration of lymphocytes and histiocytes [25, 59]. Three studies reported on four adolescent patients with Hydroa vacciniforme-like lymphoproliferative disorder (HV LPD). Atypical lymphocyte infiltrates were found in all skin pathological biopsies, with distribution around blood vessels, adnexa, and nerves. Clonal TCR gene rearrangement detection in two of the three studies suggested that EBV DNA is elevated in the blood in all cases. One 3-year-old patient reported a 3-year history of recurrent skin lesions, with fever for 3 months and cough for 8 days prior to this recurrence [60]. Another patient, a 16-year-old woman, presented with an indolent clinical course with episodes of remission and recurrence [61]. Skin biopsy results from a systemic EBV-positive T-cell lymphoma of childhood study showed dense, superficial to deep perivascular, peri adnexal, perineural, and panniculitis-like lymphoid infiltrates, as well as a sparse interstitial infiltrate with irregular and pleomorphic medium to large nuclei. Lymphoid cells showed mild epidermotropism, with tagging to the basal layer. Some vessel walls were infiltrated by similar cells [50]. However, mosquito bite hypersensitivity, which is more common in children, has been reported in two adult lymphatic cancer studies, and no associated cases in children have been reported in the included studies.
3.4.3. EBV-Associated B-Cell Lymphoma
Of the case reports included in the analysis, nine studies reported 13 patients with EBV-positive or associated B-cell lymphoma. The patient’s clinical diagnosis including precursor B-cell lymphoblastic lymphoma (B-LBL) (n = 1), methotrexate treatment-associated B-cell lymphoma (n = 2), and diffuse large B-cell lymphoma (n = 7).
(1) Precursor B-Cell Lymphoblastic Lymphoma. Alhaji 2011 [33] reported a rare case of primary cutaneous precursor B-LBL in the elderly. Histologically, the diagnostic clues for B-LBL usually include histochemical staining, immunocytochemistry, and flow cytometry. Embryos are almost always terminal deoxytransferase (TdT)-positive and express different B-cell markers such as CD22, CD20, CD79a, and CD10. T-cell markers are usually negative (e.g., CD3).
(2) Methotrexate Treatment-Associated B-Cell Lymphoma. Giard et al. [62] and Tournadre et al. [63], respectively, reported one case of B-cell lymphoma caused by methotrexate injection. Patients were treated with methotrexate for dermatomyositis for 4 weeks and 2 months, respectively. Biopsies of skin lesions showing dense subcutaneous lymphocytic hyperplasia with a B-cell phenotype, or a large B-cell dermal infiltration with CD30 expression, suggest polymorphic centroblastic B-cell lymphoma. EBV-derived LMP-1 and EBV-encoded RNA were expressed by tumour cells. The whole body CT scan and bone marrow biopsy were normal. In all cases, skin lesions began to shrink 15 days after the last dose of methotrexate, and no lesions were observed 4 months later.
3.4.4. Large B-Cell Lymphomas
DLBCL is the most common form of non-Hodgkin’s lymphoma (NHL), accounting for 40% of adult NHL [66]. 7 studies reported eight patients with diffuse large B-cell lymphoma (DLBCL) of different causes, including seven elderly patients (mean age 75.66) and one young patient [67]. A large number of mitotic maps and significant apoptosis and necrosis of tumor cells were observed in the pathological findings of 5 patients. Histological images of a diffuse large B-cell lymphoma following EBV-positive nasopharyngeal carcinoma show a malignant B-cell sheet composed mainly of large cells. This is similar to the pathological findings of a patient with diffuse large B-cell lymphoma with persistent paraneoplastic symptoms after eradication of the “primary” tumor [34]. The patient had an overall good prognosis, with symptoms involving only the skin [67].
3.4.5. EBV-Positive Mucocutaneous Ulcer
EBVMCUs have been considered as definitive entities [64]. The relevant criteria emphasize that these are isolated lesions that occur primarily in the oropharyngeal area and are usually self-limiting, painless processes [3]. One of the 2 elderly EBVMCU patients reported in this report had lesions in the oral cavity, and the symptoms disappeared after local immunotherapy [26]. An additional elderly patient at a rare site (neck) was suggested to have synchro or heterochronous tumours due to partial positive atypical cells CD45, CD20, CD79a, CD30, B-cell lymphoma 2 (Bcl-2), and latent membrane protein 1 (LMP1) [65].
3.4.6. B-Cell Lymphoma Combined with T-Cell Lymphoma
Six studies reported cases of T-cell lymphoma combined with B-cell lymphoma. The results of three studies on cutaneous B-cell lymphoma secondary to angioimmunoblastic T-cell Lymphoma and one study on T-cell-rich B-cell lymphoma indicate that immunodysregulation in T-cell lymphoma patients after chemotherapy may lead to reactivation of EBV, which may play a role in B-cell proliferation and transformation into EBV-infected permanent B-cell clones (the frequency and number of HRS-L cells were significantly increased). This supports the specific role of EBV infection in B-cell transformation and proliferation.
In contrast, Take 1996 reported on a patient with a rare indolent type of EBV-associated T-cell-Rich B-cell lymphoma. Skin tumour biopsies diffuse contain double-clonal EBV genomes in polyclonal T-cell and monoclonal B-cell infiltrations. The clinical course of disease is quite mild compared to classical EBV-related diseases [68].
3.4.7. EBV-Positive Polymorphic B-Cell Lymphoproliferative Disorder
EBV + B-cell LPD has been observed to occur in different clinical settings with a variety of pathogenesis, including post-transplantation, primary immunodeficiency, methotrexate treatment, and immune deficiency due to aging. Monoclonal IGH gene rearrangement [36] and rare oral B-cell lymphoproliferative symptoms [69] have been reported in several studies. Genetic alterations were rare, but studies have reported generally poor outcomes for patients.
3.4.8. EBV-Associated Hemophagocytic Lymphohistiocytosis
Two of the 70 studies included patients with hemophagocytic lymphohistiocytosis who reported death. There were no similarities between the two patients in age (1.5 vs 59), pathological findings, or prior medical history (adult patients had a prior history of large B-cell lymphoma). EBV-positive and acute adverse outcomes were the only similarities between the two patients. This suggests that EBV-positive screening may serve as an early skin warning sign for life-threatening hemophagocytic lymphohistiocytosis.
3.4.9. EBV-Associated Kikuchi’s Histiocytic Necrotizing Lymphadenitis
The only case of EBV-associated patient with Kikuchi’s histiocytic necrotizing lymphadenitis was reported by Yen 1997 [70]. Skin lesions and affected lymph nodes showed histiocyte aggregation, atypical lymphoid cells, nuclear tangle fragments, and patchy necrosis. It resolves spontaneously after 2 months. Serological studies, immunoperoxidase staining of EBV latent membrane protein, EBER-1 RNA in situ hybridization, and EBV EBNA-1 DNA polymerase chain reaction indicated that EBV was the pathogenic agent.
3.5. EBV-Associated Skin Manifestations with Different Immune Status
EBV infection can lead to various skin manifestations, particularly in individuals with different immunological statuses, including congenital, acquired, and age-related immune deficiencies.
Patients with congenital immune deficiencies are highly susceptible to EBV infections. Grześk et al. reported a case of a pediatric patient with common variable immunodeficiency (CVID) and chronic active EBV infection presenting with vesicles, bullae, healing erosions, and papulopustules on the neck and forehead [59]. Organ transplant recipients are typically prescribed immunosuppressive drugs to prevent organ rejection, which can lead to acquired immune deficiencies. Six of the 70 studies included in this study reported that organ transplant patients developed LPD, including four kidney transplants, one single lung transplant, and one bone marrow transplant. Although there is no significant homogeneity in the pathological test results of each study, 9 patients in 5 studies report the occurrence of erythematous plaque in skin symptoms. Methotrexate is widely used for its immunosuppressive and anti-inflammatory properties in treating various autoimmune diseases. Six studies have reported various EBV-associated skin manifestations in patients treated with methotrexate, in which four patients presented with skin ulcers, one patient with a hypopigmented patch, and one patient with erythematous plaque.
4. Discussion
EBV-positive skin symptoms may appear in patients with multiple clinical diagnoses, and there is great heterogeneity in the clinical scene, symptom range, disease course, prognosis, pathological results, and even the morphology and location of the skin symptoms themselves. By pooled analysis, we concluded that it is not realistic to distinguish patients with EBV-positive skin symptoms by skin symptoms and pathological features. Previous similar studies have also reached similar conclusions to ours, and it is challenging to distinguish EBVMCUs from other EBV-positive LPD based solely on pathological features [71].
Skin involvement disorders are frequently observed in individuals with EBV-related lymphoproliferative disorders/lymphoma, such as EBV + mucocutaneous ulcer (EBVMCU), which has been recently recognized by the World Health Organization (WHO) as being closely related to immunodeficiency and EBV immune surveillance deficiency. The patient’s lesions involved skin and mucosa [3]. In addition to this, other entities in the B-cell group with cutaneous manifestations include lymphomato-like granulomatosis, which can directly involve the skin or present as “paraneoplastic” lymphohistiocytic systemic inflammation, and EBV-driven LPD, which can appear as a form of post-transplant LPD and HIV settings and involve the skin [30]. In summary, EBV may lead to a variety of lymphoproliferative disorders/lymphoma, and there are significant differences in treatment, disease progression, and prognosis among different diagnoses. For example, unlike EBV-associated LPD and EBV-positive lymphoma, EBVMCU has a favorable prognosis. Previous studies have shown that nearly all EBVMCU patients achieve remission by reducing or discontinuing immunosuppressants or by watching and waiting. However, recent case report studies have reported that in EBV-associated B-cells, T-cell lymphoproliferative disorder, hemophagocytic lymphohistiocytosis, invasive progression, and death were observed within 2–5 months of the onset of clinical skin symptoms [28, 33, 54, 72].
It is undeniable that since EBV-associated B-, T-, and NK-cell lymphoproliferative disorders have been clearly defined, it is important to recognize the importance of EBV DNA testing in peripheral blood and EBER testing in infected tissues if there are patients who have skin symptoms that deviate from the conventional definition or who have failed conventional therapy and have accompanying skin symptoms. Although a clear association and pathological mechanism have not been demonstrated, several studies have supported the role of EBV virus in early screening and early warning in lymphoma or lymphoproliferative diseases [56, 70, 73], and some studies have even suggested evaluating EBV expression in all primary cutaneous lymphoma [74]. Moreover, based on current research, EBV infection in EBV-positive DLBCL, lymphomatoid granulomatosis, chronic active EBV disease (CAEBVD), and systemic EBV-positive T-cell lymphoma of childhood also played a decisive role. Among these, hydroa vacciniforme-like lymphoma is thought to be a proliferation of clonal T cells or less frequently NK cells related to EBV infection. Defects in genes necessary to regulate lymphocyte activation and proliferation may affect the function of virus-specific or nonspecific lymphocytes and allow EBV-infected T cells or NK cells to expand. In addition, distribution of EBV subtypes with high tumorigenic potential or low immunogenicity and mutated expression of EBV-associated antigens such as LMP-1 may contribute to the prevalence of EBV lymphoma. Although there are differences in pathological changes and prognosis between EBV-positive and EBV-negative patients in these cancer types, it is unclear whether this can be explained by differences in EBV infection alone. It is important to note that even in EBV-positive patients, age differences can lead to clinical differences in patients, for example, many adult-onset CAEBV patients do not have a history of mosquito bites and vaccine-like polyp allergy, but these symptoms are common in pediatric patients.
The development of the classification of lymphoid neoplasms began in 1994 [75], which was validated globally in 1997 and led to the WHO Classification in 2001 [76]. Extranodal nasal NK/T-cell lymphomas were categorized primarily based on their presentation in the nasal cavity and their association with EBV. The 2001 WHO Classification recognized “extranodal NK/T-cell lymphoma, nasal type” as a distinct entity, characterized by its presentation in the nasal region and its aggressive clinical course [76]. As research progressed, it became evident that these lymphomas could also present in other extranodal sites, leading to a broader categorization. The 2008 WHO Classification expanded the definition to include non-nasal presentations, emphasizing the disease’s extranodal nature regardless of the primary site [77]. This led to the term “extranodal NK/T-cell lymphomas, nasal type,” encompassing both nasal and non-nasal presentations. In the most recent 2022 WHO Classification, the primary cutaneous extranodal nasal type EB + lymphomas were abolished and included in the larger group of EB + NK/T-cell lymphomas, recognizing the overlap and need for a more unified classification [46].
There are some limitations in this study. We used systematic retrieval and data extraction methods to comprehensively collect all relevant publications published so far. While it has the advantage of being inclusive, the potential downside is that this approach combines multiple sources of heterogeneous datasets and includes so many different clinical diagnoses that it is not easy to integrate and comb through these data. This also highlights the need for more clearly defined diagnostic criteria to standardize the understanding of these types of diseases. In addition, this paper does not carry out quantitative analysis, so it is difficult to summarize specific rules from the complex qualitative description. In addition, because of the particularity of the disease, all that can be retrieved are case reports, and the cases themselves are also special in clinical practice, which may also hinder the discovery of rules. Nevertheless, the included studies represent a variety of patient populations and clinical situations, including aggressive EBV-associated hemophagocytic syndrome, primary cutaneous EBV positive diffuse large B-cell lymphoma in young patients, ocular symptoms of T-cell lymphoma, as well as a variety of complex medical needs, and it is considered to be broadly representative of the reality of skin symptoms and pathological outcomes of EBV-related lymphoproliferative diseases/lymphomas.
At the same time, future studies can focus on summarizing skin symptoms and pathological outcomes of EBV-related lymphoproliferative diseases/lymphomas based on possible risk factors for further deterioration in these patients. A number of relevant factors were observed in this study, such as NK cell C56+, methotrexate therapy, immune deficiency, age, recurrent skin lesions, adalimumab and infliximab therapy, and history of previous lymphoma. This study found that the skin manifestations of EBV infection in immunosuppressed post-transplant patients were predominantly erythematous plaques, whereas patients treated with methotrexate mainly exhibited skin ulcers. However, the sample size in this study was small, and further large-scale studies are needed to confirm these findings. Previous studies have also found that skin B-cell PTLD typically presents as single or multiple erythema nodules [78]. Therefore, we believe that further summary of the clinical characteristics of patients in the subdivision diagnosis under this topic, combined with specific risk factors, may be more beneficial to the implementation of differential diagnosis.
In summary, this study did not find obvious commonalities in skin symptoms and pathological features of EBV-positive lymphoproliferative diseases/lymphomas’ patients, we searched and summarized all relevant and special relevant case information published in the past, which is the systematic review study with the largest number of included studies so far. It is hoped that the contents of this study may help future researchers and clinical practitioners to better understand the differences between disease processes in relevant entities with EBV and skin symptoms and help clinicians and pathologists develop disease-specific treatment and follow-up plans.
Ethical Approval
This is a systematic review. Ethical approval and consent to participate are not applicable. We have registered our study on PROSPERO with the registration number CRD42023479805.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Conception and design of the study were performed by Fen Li. Administrative support was given by Fen Li. Provision of study materials or patients was done by Fen Li and Yan Zeng. Collection and assembly of data were done by Haonan Feng. Data analysis and interpretation were done by Fen Li and Haonan Feng. Manuscript writing was done by Fen Li. Final approval of the manuscript was done by all the authors.
Acknowledgments
This study was supported by Health Commission of Chengdu (2022620) to Fen Li.
Open Research
Data Availability
All data generated or analyzed during this study are included in this article and supplementary information files.