Therapeutic and Prophylactic Effects of Fulvic Acid on a Breast Cancer Model Established by MCF-7 Cell Line in SCID Mice
Abstract
Introduction. There is still minimal scientific understanding of effects of fulvic acid (FA) on breast cancer. We investigated the prophylactic, therapeutic, and combined effects of FA in a breast cancer model created using MCF-7 cell line in severe combined immunodeficiency disease (SCID) mice. Results. Four experimental groups were established as the control group (Group C), prophylaxis group (Group P), therapeutic group (Group T), and prophylaxis + therapeutic group (Group P + T). Tumor growth was observed by the in vivo imaging system and macroscopically in mammary glands of all mice (100%) of Group C, microscopically in only one mouse of Group P (12.5%), in four mice in Group T (50%), but only one animal (12.5%) in Group P + T. Immunohistochemistry (IHC) showed that p53 staining was significantly higher in tissues of Group C compared to other groups (P < 0.05). No difference was found in IHC scores for p53 between Group P and P + T (P > 0.05). Bcl-2 staining was significantly higher in Group C compared to Group P + T (P = 0.015) and higher in Group P + T compared to Group T (P = 0.021) but no significant difference was found between Group P and others (P > 0.05). Bax staining was significantly higher in Group C compared to others (P < 0.05) but no significant difference was found between FA groups (P > 0.05). Conclusion. Prophylactic FA treatment can prevent tumor formation by inducing variations in the expression of p53, BcL-2, and Bax proteins in mammary glands of SCID mice before tumor formation. This suggests that FA may be a powerful inhibitory candidate for the prevention of tumorigenesis in breast cancer.
1. Introduction
Breast cancer is the most common cancer among women and the leading cause of death following the lung cancer [1]. It accounts for 24% of all female cancers and 14% of cancer deaths [2]. Having replaced lung cancer as the most commonly diagnosed cancer globally, breast cancer today accounts for 1 in 8 cancer diagnoses and a total of 2.3 million new cases in both sexes combined [3]. Although this cancer can be detected at any age, it is most common in the postmenopausal period [4].
Breast cancer is a multifactorial disease such as lifestyle, genetic factors, and environment. The genetic variations and transmission are crucial in the development of breast cancer; therefore, the genetic factors and positive family history are unchangeable factors [5]. An estimated 7%–35% of breast cancer cases are heritable due to underlying genetic transmission, but the genetic alterations accounting for these breast cancers are not fully defined [1, 6, 7]. In a study from Turkey, 33.9% of 20,000 patients had a family history of any cancer and 15.8% of them had a family history of breast cancer [1]. Mutations in dominant high and moderate penetrance breast cancer susceptibility genes, such as BRCA1, BRCA2, PALB2, ATM, and CHEK2, have been identified in 5% of the breast cancer cases in the general population and only 30–40% of the cases associated with a family history of breast cancer [8, 9]. 40% of the hereditary breast cancer cases are due to BRCA1 and BRCA2 gene mutations. BRCA1 and BRCA2 mutations were reported in 55–65% and 45% of breast cancer cases in the 70s, respectively [10].
Although the incidence of breast cancer has increased, the mortality rate has decreased significantly over the last 20 years. This change is due to advances in screening programs, diagnosis, and treatment. Genetic and biological advances and endocrine and systemic treatments have improved the clinical outcomes of early and advanced stage disease [11]. Today, radical mastectomy has been replaced by breast-conserving surgeries, and axillary lymph node dissection has been replaced by sentinel lymph node dissection [12]. The aim of breast cancer treatment is to largely protect the breast, which is important for women. For this, screening programs, genetic tests, and early diagnosis methods are important. Early diagnosis and preventive treatment methods are even more important for the prevention of breast cancer, especially for women in the high-risk group [13].
Today, plant compounds and substances of natural origin as bioproducts are strongly recommended for the prevention and treatment of cancer [14]. In biomedicine, humus and its derivatives, including fulvic acid—a water-soluble organic acid mixture—are recognized for their antiviral, anti-inflammatory, and antioxidant properties [15]. It is an organic acid with anti-inflammatory, analgesic, hyperemic, antimicrobial, antifungal, antiviral, antioxidant, and anticarcinogenic properties [16]. When taken into the body, it is metabolized in the liver. Fulvic acid is known to promote electrochemical balance as a donor or a receptor possessing many biomedical functions. A number of studies have demonstrated the anticancer properties of fulvic acid [17]. An in vitro study showed that fulvic acid induced apoptosis and reduced cell viability and gene expression in the cured breast cancer cells (MCF-7) [15]. However, there is still minimal scientific understanding of the claims of therapeutic properties of fulvic acid on breast cancer. The present study investigated the therapeutic, prophylactic, and therapeutic + prophylactic effects of fulvic acid in a breast cancer model created using MCF-7 cell lines in mice with severe combined immunodeficiency disease (SCID).
2. Materials and Methods
2.1. Tumor Cell Lines
MCF-7 cells were cultured in Dulbecco’s modified Eagle medium (DMEM) (10% fetal bovine serum (FBS), 1X penicillin/streptomycin). Cells that reached 80% confluency were first washed with 1X FBS, and 1 ml 0.25% trypsin was added and incubated for 3 minutes in a 30°C incubator and removed. Trypsin was inactivated by adding 5 ml DMEM and the cells were transferred to a 15 ml centrifuge tube. To determine the number of cells, 100 μl of the cell suspension was taken and counted using a hemocytometer. Cells were transferred to 6-well plates (200 thousand cells/well) and transfected the next day with 4 μg of mCherry N1 plasmid (Addgene #54517) per well with TurboFect (Thermo Scientific #R0532) following the manufacturer’s instructions. To express the antibiotic resistance gene, the cells were grown in DMEM (10% FBS, 1X penicillin/streptomycin). After 72 hours, the selection was initiated by adding G418 (500 μg/ml final concentration, Roche Applied Science G418 Solution). The cells were exposed to G418 selection for a total of 4 weeks, with the medium changed every 4 days. During this process, as the cells reached 80% confluency, they were passaged and transferred to new plates. Whether more than 90% of the cells produced mCherry was examined under a fluorescence microscope. At the end of the fourth week, it was decided that the selection was successful and the G418 concentration was reduced to 200 μg/ml. For xenograft, cells were removed and counted as described above. For each injection, 1 million cells were suspended in 100 μl 1X FBS. For stimulation of tumor growth, matrigel (100 μl) was added to make it ready for injection.
2.2. Animals and Experimental Groups
All experimental animal procedures were carried out at Experimental Animal Production and Care Unit of Bogazici University, after obtaining an approval from the Local Ethics Committees for Animal Experiments of Bagcilar Training and Research Hospital (no: 2015/101, date: 6th August 2015) and Institutional Local Ethics Committee for Animal Experiments of Bogazici University (no: 17.08.2015, date: 26th August 2015). Thirty two of the severe combined immunodeficient (SCID) female mice, 6–8 weeks old and weighing 15–20 g, were used for the study. All mice were housed in HEPA-filtered rooms and individually ventilated cages (IVCs) and were fed ad libitum with sterile feed that met their physiological needs. Room conditions were arranged as 40–70% humidity, 20°C temperature, and 15 cycles of ventilation per hour. The mice were housed on a lighting cycle of 12 hours of light and 12 hours of darkness. The humidity, ventilation, and temperature values of the rooms were checked every day with a special automation system, and the cages, litter, and equipment were autoclaved.
Four experimental groups were established according to Table 1. Control group (Group C) represents the breast cancer model injected by MCF-7 cell lines. After injection (including 1 × 107) of MCF-7 cell lines into the flank area (subcutaneously), tumor formation was observed at 3rd week after injection and all mice of the control group were sacrificed at 13th week. Flowchart of the experimental design is shown in Figure 1.
| Groups | Number of animals | Injection of tumor cells (week) | Application of fulvic acid | Tumor formation (week) | End of experiments (week) |
|---|---|---|---|---|---|
| Control group | 8 | 2nd | — | 5th | 13th |
| Prophylaxis group | 8 | 2nd | 5 weeks before tumor formation | 5th | 13th |
| Therapeutic group | 8 | 2nd | 8 weeks after tumor formation | 5th | 13th |
| Prophylaxis + therapeutic group | 8 | 2nd | 5 weeks before + 8 weeks after tumor formation | 5th | 13th |
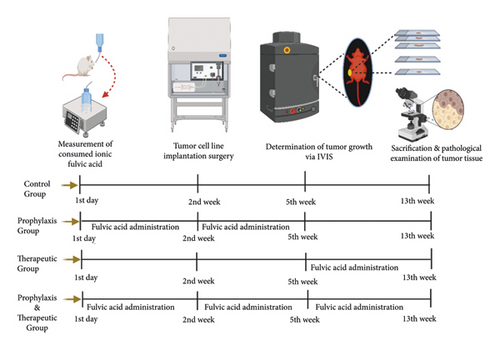
The prophylaxis group (Group P) was treated with 50 ml of ionic fulvic acid solution (TraceMinerals Research Liguimins Dietary Supplement) at 30 mg/L concentration for 5 weeks before the tumor cell line injection. The dose of fulvic acid was determined according to the safe dose determined in the literature [17–22]. Fulvic acid solution was added to 1-liter distilled water bottles and water was changed every two days, and the amount of drinking water was measured in “ml.” After the fulvic acid solution was administered, injection of tumor cells was performed at 2nd week and the tumor formation was observed at 5th week. Tumor development was monitored without fulvic acid treatment until the sacrification at 13th week.
The mice of the therapeutic group (Group T) were injected with tumor cells at 2nd week and the tumor formation was observed at 5th week. Thereafter, 50 ml of fulvic acid solution at 30 mg/L concentration was administered for 8 weeks until the sacrification at 13th week. Fulvic acid solution was added to 1-liter distilled water bottles and water was changed every two days, and the amount of drinking water was measured in “ml.”
The mice of the prophylaxis + therapeutic group (Group P + T) were treated with 50 ml of fulvic acid solution at 30 mg/L concentration for 13 weeks from the beginning of study until sacrification at 13th week. Fulvic acid solution was added to 1-liter distilled water bottles and water was changed every two days, and the amount of drinking water was measured in “ml.” The injection of tumor cells was performed at 2nd week and the tumor formation was observed at 5th week.
2.3. Injection of MCF-7 Cell Lines in Experimental Animals
All surgical procedures were performed under a laminar hood. 1–3% isofluorane gas anesthesia was used as an anesthesia method. After the MCF-7 cells were prepared and checked for the viability, they were transferred to the operating room. First of all, 0.36 mg cell pellets with a diameter of ∼3 mm 17β-estradiol (Cat# SE-121; 0.36 mg/pellet; Innovative Research of America, FL, USA) that release estrogen for 60 days were placed under the skin of the necks of each mouse. A skin incision (∼0.5 cm) was made from the skin to the subcutaneous area of the neck and the pellets were placed in this space. The skin was stitched with 4/0 PGA suture. Thereafter, the cells were injected into the “flank” region. For the postoperative care, all mice were placed in single cages until they recovered.
2.4. Imaging of Tumor Formation in Experimental Animals
The tumor formation was observed (approximately 3 weeks after the injection) via using an in vivo imaging system (IVIS Lumina Series III). During the imaging, the animals were undergone an isoflurane anesthesia and placed inside the device on a heating pad to prevent the hypothermia. Since the fluorescent material used in the imaging was MCherry, and the spectral filter ranges selected were in the 520–845 nm band. In the acquired image, region of interest (ROI) icons were placed in the regions where tumor injection was performed and intensity analysis was performed. The significance of these densities was evaluated using GraphPad (Prism9) statistical program.
2.5. Histopathological Evaluation
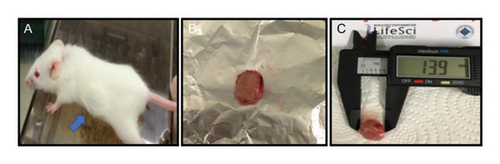
According to the fulvic acid treatment protocol, after tumor formations were observed, the animals were necropsied and the tumor tissues were removed from the mammary glands in a sterile environment, and their large and small diameters were measured with a caliper (Life Sciences) (Figure 2). The tissues were weighted and then were fixed in 10% buffered formalin. The fixed tissues were routinely processed for macroscopic and microscopic examinations, shortly, embedded in paraffin, sectioned at 5 μm thickness, and finally stained with haematoxylin and eosin (H&E) to be evaluated under a light microscope (Olympus BX50).
For immunohistochemistry (IHC), the primary antibodies against p53, Bcl-2, and Bax proteins were used. Tissue sections were initially collected on positively charged slides, deparaffinized in xylene, hydrated gradually through graded alcohols (100%, 96%, 80%, and 70%, respectively), and finally in distilled water. For antigen retrieval, slides were treated with citrate buffer (pH 6.0) for 20 min in a microwave oven at high temperature (750 W) and then left to cooling. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in methanol for 10 min at room temperature. Then, the slides were placed onto a humid chamber and covered with blocking solution for 10 min in order to prevent nonspecific binding. Subsequently, slides were incubated with the following primary antibodies for 90 min at room temperature: polyclonal anti-p53 (Sc-6243, 1 : 100 dilution, Santa Cruz), polyclonal anti-bcl2 (NB100–92142, 1 : 200 dilution, Novus Biologicals), and monoclonal anti-bax (Sc-526, 1 : 200 dilution, Santa Cruz). After the incubation period, the protocol proceeded with a commercially available detection kit (Expose Mouse and Rabbit Specific HRP/DAB Detection IHC Kit, Kat no. ab80436, Abcam) according to the manufacturer’s instructions. Immunolabelling was visualized by 3,3-diaminobenzidine (DAB) as chromogen. Finally, the slides were counterstained with Mayer’s haematoxylin. Tween 20 added buffered phosphate solution (pH 7.4) was applied as the rinsing solution between each step starting from antigen retrieval to counterstaining throughout the whole staining protocol except prior to the step of incubation with primary antibodies, which was substituted with antibody diluents for negative controls.
Immunolabelling was assessed by light microscopy and then scored according to the distribution and intensity of the immunoreactions [23] of Bcl-2 and Bax antibodies as follows: A: % of IHC positive labeled cells (0 = 0%, 1 = <30%, 2 = 30–60%, and 3 = <60%), B: intensity of IHC reaction (0 = no reaction, 1 = weak, 2 = mild, and 3 = strong); final score: A × B range from 0 to 9 (0 = negative, 1–3 = mild; 4–6 = moderate, and 7–9 = strongly positive). By microscopic evaluation, p53 distribution was assessed in each section. P53-positive cells and total cells were counted in 10 random areas, under 40x magnification objective. P53 intensity was calculated by the following formula: 100 × (mean number of p53-positive cells in 10 random fields)/(mean number of total cells in 10 random fields). All slides were analyzed at different times by two researchers that were blinded to the histopathological information [24].
2.6. Statistical Analysis
A nonparametric test, Kruskal–Wallis, was performed to statistically analyze IHC scoring data on the software program SPSS version 25.0 (SPSS, Inc., Chicago, IL). A further test, Mann–Whitney U test, was conducted to analyze the differences among groups. A P value of P < 0.05 was established as the significance level.
3. Results
3.1. In Vivo Imaging Findings
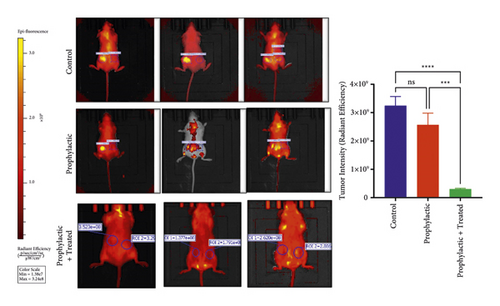
IVIS imaging showed the tumor formation in all mice of the mammary glands of Group C, whereas the intensity of tumor formation was recorded as low in the Group P and also lower in Group P + T. Statistical significance was observed between the Group C and the Group P, with a higher significance between the Group C and the Group P + T (Figure 3).
3.2. Histopathological Findings
Three SCID mice in the Group P, two in the Group T, and one in the Group P + T died due to the immunodeficiency which resulted in a low rate of survival in SCID mice. Therefore, their mammary tissues were not evaluated. The tumor growth was observed macroscopically in the mammary glands of all SCID mice (100%) in Group C (Table 2). The mean weight of tumor tissues of Group C was 2.62 ± 0.92 g and the mean size was 275.93 ± 63.83 mm. There is no palpable tumor in the other groups; therefore, the size of tumors could not be measured in these groups. However, a low-density tumor formation with necrosis was detected in only one of the mice of Group P (12.5%) and it was detected microscopically, hence its size could not be measured. Four mice in the Group T (50%) were positive for tumor formation and necrosis which were detected microscopically, hence its size could not be measured. None of the mice in Group P + T showed a macroscopic tumor formation and necrosis although only one animal (12.5%) had a low-density tumor formation which was detected only microscopically (Table 2).
| Groups | Animal number | Histopathologic findings | IHC staining scores | ||
|---|---|---|---|---|---|
| p53 | Bcl-2 | Bax | |||
| Control (C) group | 1 | Positive for tumor and necrosis | 2.12 | 2 | 2 |
| 2 | Positive for tumor and necrosis | 1.39 | 2 | 3 | |
| 3 | Positive for tumor and necrosis | 0.69 | 2 | 2 | |
| 4 | Positive for tumor and necrosis | 1.26 | 2 | 3 | |
| 5 | Positive for tumor and necrosis | 1.83 | 2 | 3 | |
| 6 | Positive for tumor and necrosis | 1.05 | 2 | 2 | |
| 7 | Positive for tumor and necrosis | 1.23 | 1 | 2 | |
| 8 | Positive for tumor and necrosis | 0.78 | 2 | 3 | |
| Mean ± SD | 1.29 ± 0.49 | 1.88 ± 0.35 | 2.50 ± 0.53 | ||
| Prophylaxis (P) group | 1 | Not evaluated/ex | 0 | 0 | 0 |
| 2 | Not evaluated/ex | 0 | 0 | 0 | |
| 3 | Normal mammary gland tissue | 0 | 1 | 1 | |
| 4 | Very small tumor with necrosis | 0 | 2 | 1 | |
| 5 | Active secretory mammary gland | 0 | 3 | 1 | |
| 6 | Active secretory mammary gland | 0 | 3 | 2 | |
| 7 | Active secretory mammary gland | 0 | 1 | 1 | |
| 8 | Not evaluated/ex | 0 | 0 | 0 | |
| Mean ± SD | 0 ± 0 | 1.25 ± 1.28 | 0.75 ± 0.71 | ||
| Therapeutic (T) group | 1 | Positive for tumor and necrosis | 0.3 | 2 | 1 |
| 2 | Positive for tumor and necrosis | 0.32 | 1 | 2 | |
| 3 | Active secretory mammary gland | 0 | 2 | 1 | |
| 4 | Active secretory mammary gland | 0 | 2 | 1 | |
| 5 | Positive for tumor and necrosis | 0.25 | 2 | 1 | |
| 6 | Not evaluated/ex | 0 | 0 | 0 | |
| 7 | Positive for tumor and necrosis | 0.018 | 1 | 2 | |
| 8 | Not evaluated/ex | 0 | 0 | 0 | |
| Mean ± SD | 0.11 ± 0.15 | 1.25 ± 0.89 | 1.0 ± 0.76 | ||
| Prophylaxis + therapeutic (P + T) group | 1 | Active secretory mammary gland | 0 | 3 | 1 |
| 2 | Not evaluated/Ex | 0 | 0 | 0 | |
| 3 | Active secretory mammary gland | 0 | 3 | 1 | |
| 4 | Active secretory mammary gland | 0 | 2 | 1 | |
| 5 | Active secretory mammary gland | 0 | 1 | 2 | |
| 6 | Active secretory mammary gland | 0 | 1 | 2 | |
| 7 | Active secretory mammary gland + tiny tumor | 0 | 1 | 1 | |
| 8 | Active secretory mammary gland | 0 | 2 | 2 | |
| Mean ± SD | 0 ± 0 | 1.63 ± 1.06 | 1.25 ± 0.71 | ||
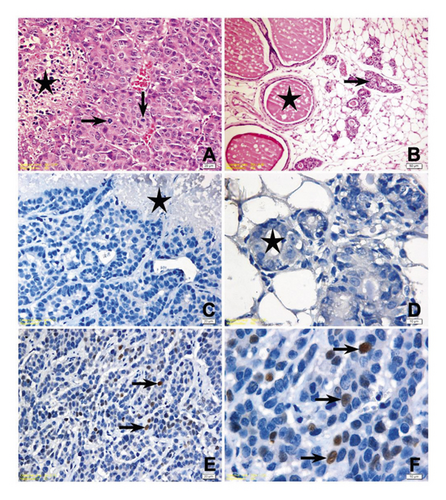
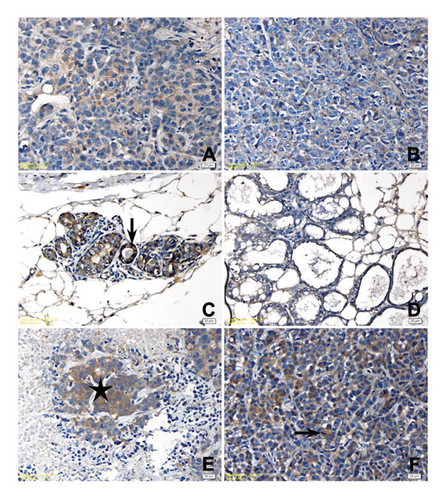
Histopathological examinations revealed the tumor growth in the mammary glands of all animals in Group C. The cells with high pleomorphism, round to ovoid nuclear structure, marked nucleolus, hyperchromasia, or diffuse mitotic figures showed adenoid alignment; almost in all, neoplastic foci central tumor necrosis was determined (Figure 4(A)). In sections of mammary gland of only one animal in Group P, they had a flank tumor development in a very small area (Figure 4(B)), while the secretory changes were determined in the glands of other animals. Similarly, in Group P + T, no pathological changes were observed except for a very small tumoral focus in the tissue of one animal. A secretory activity in the glands of all animals was determined in other sections of Group P + T. The histopathological examination of 4 animals in the Group T showed tumor necrosis with central foci. Histopathological examination could not be performed to a few animals due to sudden death of animals before the tumor development in the experimental groups and these animals were not included in statistical analysis.
3.3. Immunohistochemical Findings
Histopathologic and immunohistochemical (IHC) findings of the study groups are presented in Table 2, and the significance levels of two-pair and multiple comparison of immunohistochemical scores among study groups are presented in Table 3. IHC staining scores for P53 protein were significantly higher in the mammary tissues of the control group compared to other groups (P < 0.05). No significant difference was found in IHC scores for P53 protein between the Group P and Group P + T (P > 0.05). Different intensities of p53 positivity were observed in all microscopical images of tissue samples with tumor development, while no p53 positivity was determined in mammary tissues without tumor development in Group P (Figures 4(D)–4(F)). Bcl-2 staining was significantly higher in the tissues of Group C compared to the Group P + T (P = 0.015) and higher in the tissues of Group P + T compared to the Group T (P = 0.021) but no significant difference was found between the tissues of Group P compared to the other groups (Figures 5(A)–5(C)). Bax staining was significantly higher in the tissues of Group C compared to the other groups (P < 0.05), but no significant difference was found between the tissues of mice treated with fulvic acid (P > 0.05) (Figures 5(D)–5(F)).
| Groups | P53 | Bcl-2 | Bax |
|---|---|---|---|
| Group C vs. Group P | 0.003 ∗∗ | 0.130 | 0.005 ∗∗ |
| Group C vs. Group P + T | 0.001 ∗∗∗ | 0.015 ∗∗ | 0.006 ∗∗ |
| Group C vs. Group T | 0.003 ∗∗ | 0.271 | 0.012 ∗∗ |
| Group P vs. Group P + T | 1.000 | 0.853 | 0.428 |
| Group P vs. Group T | 0.054 ∗ | 0.121 | 0.513 |
| Group P + T vs. Group T | 0.025 ∗ | 0.021 ∗ | 0.925 |
| Total | 0.000 ∗∗∗ | 0.035 ∗ | 0.005 ∗∗ |
- ( ∗) = P ≤ 0.05; ( ∗∗) = P ≤ 0.01; ( ∗∗∗) = P ≤ 0.001.
4. Discussion
Breast cancer remains a significant health concern globally, despite advancements in screening, diagnosis, and treatment modalities. While mortality rates have declined due to these advancements, challenges persist, particularly in managing treatment side effects and overcoming drug resistance. Traditional therapies such as surgery, radiation, and chemotherapy, though effective to some extent, can be associated with adverse effects and the development of resistance, underscoring the need for alternative therapeutic approaches [25–27]. In order to treat cancer, selecting appropriate medications and natural products with fewer side effects is crucial. There are limited numbers of in vitro studies examining the effects of fulvic acid, a natural organic acid isolated from humus, in breast cancer [26, 27]. Therefore, in the present study, we investigated the therapeutic, prophylactic, and therapeutic + prophylactic effects of fulvic acid in a breast cancer model created using MCF-7 cell lines in SCID mice and found that the tumor formation was suppressed in the mammary glands of mice especially in the prophylactic group compared to the mice in the therapeutic group. In the light of data obtained by IVIS imaging, our findings provided valuable information about the possible potential on the regression of tumor density in Group P + T. Apart from that, the observation of density reduction in the prophylaxis group compared to the control group is important in terms of providing preliminary information about the efficacy of fulvic acid treatment.
The observation of reduced tumor formation in the prophylaxis group raises intriguing questions about the mechanisms underlying the antitumor effects of fulvic acid. While the involvement of p53 in cancer cells is an essential aspect to consider [17, 28, 29], the absence of tumor formation in the prophylaxis group suggests broader influences on the tumor microenvironment beyond intracellular signaling pathways. The reduced tumor formation in the prophylaxis group hints at potential alterations in the tumor microenvironment mediated by extracellular factors. These extracellular factors may include changes in the tumor immune microenvironment, modulation of angiogenesis, or inhibition of metastatic processes. Such alterations could impede tumor initiation or progression, leading to the observed reduction in tumor formation. Moreover, multifaceted pharmacological properties, such as its antioxidant, anti-inflammatory, and immunomodulatory effects of fulvic acid may contribute to reshaping the tumor microenvironment, creating an inhospitable milieu for tumor growth [17–22, 30]. In addition, FA might exert direct cytotoxic effects on tumor cells or indirectly modulate the activity of stromal cells within the tumor microenvironment. Therefore, while the involvement of p53 in cancer cells is crucial, the absence of tumor formation in the prophylaxis group suggests that FA’s antitumor effects likely involve complex interactions with the tumor microenvironment and extracellular factors.
The catalytic component of DNA protein kinase (DNA-PKcs), a protein complex involved in the physiological response to DNA damage, is encoded by the SCID focus. DNA damage-mediated p53 induction and function was essentially normal in SCID mice which are defective in DNAPK activity [28]. p53 deletion was shown to enhance T-cell growth in SCID mice but has no discernible impact on B lymphopoiesis. Furthermore, in response to ionizing radiation, SCID cells can enter G1 arrest or apoptosis and stimulate p53 protein production, suggesting that DNA-PKcs is not necessary for these responses to DNA damage. These results also indicate that the phosphorylation of p53 by DNAPK seen in vitro is not the essential factor leading to the stabilization of p53 protein or to the downstream cellular responses [29]. A study by Huang et al. investigated the effect of resistin on the endothelial adhesion of colorectal cancer (CRC) and to determine whether fulvic acid elicits an antagonistic mechanism to neutralize this resistin effect. Human HCT-116 (p53 negative) and SW-48 (p53 positive) CRC cells and human umbilical vein endothelial cells (HUVECs) were used in their experiments [17]. While the study mentioned does not explicitly address the effect of FA on p53, it indirectly provides insights into how fulvic acid influences CRC cells, including those with different p53 statuses such as p53 negative and p53 positive. Fulvic acid attenuates resistin-induced effects on HCT-116 cells, a p53-negative cell line. This suggests that fulvic acid might modulate pathways involved in cellular responses irrespective of the p53 status. The study also examined downstream signaling pathways like NF-κB, which are intricately linked with p53 regulation. By inhibiting NF-κB activity, FA might indirectly affect p53 function or downstream targets influenced by p53 [17]. By using SCID animals, we investigated the impact of fulvic acid on the p53 expression in tumor formation in a breast cancer model of mice. The tumor growth was mostly not observed, especially in the prophylaxis and the prophylaxis + therapeutic groups, while p53 expression was also lost in the mammary glands of the mice in these groups. Our findings reveal a hitherto unknown function of p53 as a checkpoint regulator in the early development of tumor growth in mammary gland and show that the fulvic acid treatment caused loss of this element of the cellular defense against DNA damage in the prevention of tumor formation. However, it remains to be determined if the mutation of p53 and its phosphorylation sites have other functional consequences for its transcriptional activity in breast cancer. Also, what are the relevant in vivo downstream cellular responses to DNAPK activation is altered by fulvic acid treatment in SCID mice is not enlightened. To date, there is no evidence that FA may be such an in vivo prophylactic effects on p53 expression in breast cancer. Given that p53 mutations are common in cancers, including breast cancer, understanding how FA influences p53 pathways indirectly could have therapeutic implications. If FA can modulate signaling pathways dysregulated in p53 mutant cells, it might offer therapeutic benefits in breast cancers where p53 function is compromised. While our study provides intriguing insights, direct investigations into FA’s effects on p53, such as its influence on p53 expression, stability, or transcriptional activity, would be valuable for a comprehensive understanding of FA’s mechanisms of action in cancer cells.
The Bcl-2 family of apoptotic mediators is a large family, some of whose members induce apoptosis (Bax, Bad, Bid, and Bcl-Xs), while others inhibit it (Bcl-2 and Bcl-XL). Among the members of this family, the inductive Bax and the inhibitor Bcl-2 are the most researched proteins due to their distinct expression in the apoptotic process, both as good tumor markers and as good control tools in the treatment stages [31]. Bcl-2 is especially found in the outer membrane of mitochondria and regulates ion transport. Bcl-2 has also been found to have an antioxidant effect due to its association with mitochondria and thus can suppress oxidant stress-induced apoptosis [32]. Bax, on the other hand, is found in the cytosol and binds to the mitochondrial membrane upon receiving an apoptotic stimulus, where it induces the formation of small holes “pores,” thus selective ion permeability is lost, resulting in the release of cytochrome c and AIF, known as the apoptosis-inducing factor, from the mitochondria into the cytosol [33]. The Bcl-2 gene was first identified in human B-cell follicular lymphoma [34]. Bcl-2 expression was reported in 70% of human breast carcinomas and that there is a negative correlation between its expression and apoptotic index and prognosis. Previous studies of the expression of the Bcl-2 in breast cancers demonstrated that the presence or absence of Bcl-2 in tumor cells differs from the expression pattern in the respective nontransformed cells [35, 36]. However, the role of the Bcl-2 family in malignant transformation is still controversial [37–45]. Barghou et al. investigated the mRNA expression of Bcl-2 and Bax in 10 human breast cancer samples. All of them were strongly positive for Bcl-2 whereas expression of Bax was mostly low or undetectable [44]. Binder et al. showed that there was a positive association between Bax expression and histological grading and (over) expression of c-erbB-1 and -2 and proliferative activity in primary breast cancer [45]. The correlation was most significant in cases where no concomitant Bcl-2 expression could be detected. However, the Bax expression was not significantly associated with distribution of Bcl-2 expression in tumor tissue [45]. Therefore, we investigated the prophylactic and therapeutic effects of fulvic acid on the SCID mice model for breast cancer in the concept of Bcl-2 family. We found that two of antiapoptotic and proapoptotic proteins Bcl-2 and Bax significantly reduced by prophylactic application of fulvic acid compared to the nontreated mice while no tumor growth was observed in most of the mammary tissue of SCID mice. This finding implies that proproliferative signals may work in concert with antiapoptotic Bcl-2 proteins and proapoptotic Bax to facilitate the onset of breast cancer. We consider that a final assessment must consider the simultaneous expression of various homologues, particularly the primary Bcl-2-antagonist Bax in breast cancer, as the apoptosis-inhibiting function of Bcl-2 is dependent on the interaction with other family members.
The limitations of our study were the lack of dose- and time-dependent experiments for fulvic acid treatment in SCID mice and lack of western blot analysis of proteins in tumors. The potential variability in FA response based on dosage and treatment duration would contribute to a more robust interpretation of the results. However, we compared the prophylactic and therapeutic effects of fulvic acid in a breast cancer model and observed the fulvic acid was more efficient in prevention of tumor formation especially when applied before the tumor induction by MCF-7 cells in SCID mice. While previous studies have reported the inhibitory effect of FA on the proliferation of MCF-7 cells [15], our study extends this knowledge by demonstrating the therapeutic and prophylactic effects of FA in an in vivo setting using a SCID mouse model. Our research not only confirms the inhibitory function of FA on MCF-7 proliferation but also provides valuable insights into its efficacy and potential mechanisms of action in a complex biological system. Furthermore, our study goes beyond merely replicating known conclusions by conducting comprehensive organizational analyses, which provide a deeper understanding of the therapeutic mechanisms underlying FA treatment.
Another limitation is using only one breast cancer cell line in this study. To select an appropriate cell line sensitive to estrogen for our research, we opted for MCF-7 due to its widespread utilization and pathological relevance to our breast cancer model. In addition, MCF-7 offered molecular advantages and facilitated cell line production, rendering it a pragmatic choice. By exclusively employing MCF-7, we aimed to gather preliminary data for dosing studies of fulvic acid, assess its initial safety profile, and lay the groundwork for subsequent investigations. This approach enabled us to initiate biodistribution studies and establish foundational data essential for risk analysis, thus informing future research endeavors.
Estrogen receptors are known to play a crucial role in regulating various cellular processes in breast tissue, including cell proliferation, differentiation, and survival. Dysregulation of estrogen receptor signaling is associated with the development and progression of hormone receptor-positive breast cancers. Fulvic acid was shown to have antiestrogenic effects on MVLN (human breast carcinoma) cells stably transfected with luciferase gene under control of ER [45]. In our study, we did not specifically investigate the effect of fulvic acid treatment on estrogen receptor expression. However, it is an intriguing avenue for further exploration of the impact of fulvic acid on estrogen receptor expression which could provide additional insights into its potential mechanisms of action and its utility as a therapeutic agent in breast cancer.
5. Conclusion
In conclusion, we found that the prophylactic fulvic acid treatment can prevent tumor formation by inducing variations in the expression of the p53, Bcl-2, and Bax proteins in mammary glands of SCID mice treated with fulvic acid before tumor formation. This suggests that fulvic acid may be an inhibitory candidate for the prevention of the tumorigenesis in breast cancer. We believe that our findings contribute to the existing literature by elucidating the in vivo efficacy of FA in a relevant breast cancer model, thus laying the groundwork for future translational research and clinical applications. Further studies may include the cell viability assays, apoptotic indices of the mammary glands, and the expression of cell-cell adhesion molecules in mammary glands to understand the underlying reasons of the variations in the expression of tumor formation, progression, and metastasis of the breast cancer treated with the fulvic acid. Future studies incorporating comprehensive molecular analyses and addressing the identified limitations will contribute to a better understanding of FA’s therapeutic efficacy and facilitate its translation into clinical practice.
Acronyms
-
- DAB:
-
- 3,3-diaminobenzidine
-
- DMEM:
-
- Dulbecco’s modified Eagle medium
-
- DNA-PKc:
-
- DNA protein kinase
-
- FA:
-
- Fulvic acid
-
- FBS:
-
- Fetal bovine serum
-
- H&E:
-
- Haematoxylin and eosin
-
- IHC:
-
- Immunohistochemistry
-
- IVC:
-
- Individually ventilated cages
-
- NF-κB:
-
- Nuclear factor kappa B
-
- ROI:
-
- Region of interest
-
- SCID:
-
- Severe combined immunodeficiency disease.
Additional Points
Synopsis for Table of Contents. We investigated the prophylactic, therapeutic, and combined effects of FA in a breast cancer model created using MCF-7 cell line in severe combined immunodeficiency disease (SCID) mice. Prophylactic FA treatment can prevent tumor formation by inducing variations in the expression of p53, Bcl-2, and Bax proteins in mammary glands of SCID mice before tumor formation.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Osman Bilgin Gulcicek conceptualized the study, performed formal analysis, investigation, visualization, and methodology, wrote the original draft, and reviewed and edited the study. Duygu Sultan Oran and Arzu Temizyurek investigated the study, visualized the study, performed the methodology, reviewed and edited the study. Erkan Yavuz, Hakan Yigitbas, Candas Ercetin, and Ali Solmaz investigated and reviewed and edited the study. Funda Yildirim conceptualized, supervised, and reviewed and edited the study. Kivilcim Sonmez investigated and reviewed and edited the study. Atilla Celik conceptualized and supervised the study, performed funding acquisition, wrote the original draft, and reviewed and edited the study.
Open Research
Data Availability
The data used to support the findings of this study are available on request from the corresponding author.




