Comprehensive Analysis of Clean Energy Generation Mechanisms in Microbial Fuel Cells
Abstract
This paper reviews the current state of microbial fuel cell (MFC) technology for energy generation. It begins by exploring clean energy alternatives, focusing on waste-to-energy solutions, and introduces the concept, applications, and advantages of MFCs. The biochemical processes within MFCs are explained, highlighting how microorganisms metabolize substrates through glycolysis, the Krebs cycle, and the electron transport chain to generate electrons. These electrons flow through an external circuit and combine with protons and oxygen at the cathode to produce water or reduced forms of nitrogen and sulfur. This paper also analyzes 10 key parameters affecting MFC performance: coulombic efficiency, pH, temperature, substrates, organic loading rate, electrode potential, open circuit voltage, treatment efficiency, organic removal rate, and hydraulic retention time. Recent advancements in MFC technology are also discussed, including innovations in reactor configuration and scaling, the development of new membrane materials like earthen and ceramic, and improvements in wastewater treatment methods. The advancements also extend to genetic engineering techniques to enhance microbial efficiency and component modifications, such as the use of carbon-based nanomaterials and metal catalysts for improved performance, innovations in proton transfer membranes, and mediator-less MFCs utilizing metal-reducing bacteria. Challenges facing MFC technology, such as cost, scalability, and environmental sensitivity, are mentioned. The paper concludes with future directions, including the use of advanced materials, integration with wastewater treatment infrastructure, and the potential for nutrient recovery and chemical synthesis. This comprehensive review aims to provide knowledge into optimizing MFCs for sustainable energy generation and environmental benefits.
1. Introduction
Clean energy includes renewable and sustainable power sources with minimal environmental impact. Technologies such as solar, wind, hydro, biomass, and geothermal energy harness natural processes to generate electricity without emitting greenhouse gases, making them reliable and environmentally friendly options for the world’s growing energy needs [1]. Clean energy innovations also include hydrogen energy, which uses hydrogen as a clean fuel with water vapor as the only emission, and waste-to-energy systems, which convert municipal, agricultural, and industrial waste into electricity and heat. These systems reduce landfill use, providing versatile solutions for waste management while also advancing renewable energy production. Among waste-to-energy technologies are incineration, gasification, pyrolysis, and fuel cells. Incineration burns waste at high temperatures to generate heat for electricity or heat production. Gasification converts solid waste into syngas for electricity generation, fuel production, or chemical synthesis [2]. Pyrolysis employs thermal decomposition to produce bio-oil, biochar, and syngas from organic materials, usable as fuels or chemical feedstocks [3]. Fuel cells convert the chemical energy of fuels, such as organic waste, hydrogen, or natural gas, directly into electricity through a reaction with an oxidizing agent, typically oxygen [4]. A specific type of fuel cell, the microbial fuel cell (MFC), uses anaerobic digestion to break down organic waste into energy. MFC utilizes microorganisms to convert organic matter directly into electricity through microbial metabolism. The organic waste materials, including wastewater, organic sludge, or agricultural waste, serve as fuel for the microorganisms within the MFC. As these microorganisms metabolize the organic compounds, they release electrons that can be captured as electrical energy [5]. The idea of energy generation from bacteria started with Professor M.C. Potter at the University of Durham botany department in 1911 [6]. Studying the degradation of organic compounds by microorganisms, M.C. Potter discovered that small amounts of electrical energy were produced in the process. For many decades, researchers have employed various strategies and methodologies to optimize the design and operation of MFCs with the goal of capturing as much energy as possible. For example, Farooq et al. [7] conducted a comprehensive study on remodeling MFC design and parameters for sustained electricity production. They identified inefficiencies in electron transfer and energy output. The research introduced novel design improvements, including optimized electrode materials and configurations, which significantly enhanced the MFC’s performance. In another work, Sonawane et al. [8] addressed issues with scalability and practical deployment of MFCs in real-world environments by integrating them with other renewable energy technologies and developing hybrid systems to enhance overall energy output and sustainability. Jadhav et al. [9] also developed advanced modeling algorithms for the prediction of MFC behavior under different environmental conditions to realize higher output efficiencies. This study provided a framework for systematically improving MFC designs, which is crucial for their practical application. Similarly, Lin et al. [10] utilized response surface methodology through chromium (VI) contaminant removal to optimize MFCs for increased power production. In many other studies, researchers improve overall MFC energy outputs by rather integrating with different waste-to-energy sources. Corigliano et al. [11], for instance, analyzed a hybrid energy system combining solid oxide fuel cells (SOFC) and gas turbines by utilizing syngas from biomass gasification, which led to about 63% improved electric efficiency and substantial energy savings. Additionally, they also investigated systems combining anaerobic digesters with high-temperature fuel cells (HTFCs), such as SOFCs and molten carbonate fuel cells. These systems converted urban solid waste into biogas and used in HTFCs for electricity and heat production, thus, providing a dual benefit of waste management and high renewable energy production [12]. Corigliano’s work also includes a numerical simulation model for HTFCs fed by biogas from urban solid waste. This study examined the performance of these fuel cells, addressing challenges like reduced hydrogen production and carbon deposition due to carbon dioxide presence. Indirect internal reforming was incorporated to mitigate these issues, presenting a sustainable approach to waste management and fuel cell energy production [13].
Evidently, optimization of MFCs is critical for enhancing their performance and making them viable for practical applications. In this century of environmental awareness, industries actively explore innovative methods of energy production, such as the MFC technology. MFCs are being applied even beyond energy production. Some of these applications include; wastewater treatment and denitrification of cathodes, production of biosensors, and bioremediation hydrogen production. In wastewater treatment, MFCs are preferred due to their ability to produce 50%–90% less excess sludge, which tends to minimize the cost of sludge disposal [14]. MFCs are increasingly utilized in wastewater treatment due to their scalability and reduced limitations associated with ion-exchange membranes [14]. In line with this, Corbella and Puigagut [15] explored improving domestic wastewater treatment efficiency using constructed wetland MFCs, focusing on the influence of anode material and external resistance and leading to an enhanced wastewater treatment efficiency and electricity generation simultaneously. Pandit et al. [16] discussed recent advancements in scaling up MFCs for wastewater treatment. They identified significant challenges in maintaining the efficiency and stability of the wastewater treatment process at larger scales. Their work introduced innovative strategies, such as modular designs and advanced materials, to ensure consistent performance and reliability in practical settings. In quantifying the efficiency of applying MFC in wastewater treatment, Samer [17] developed a software program to compute the coulombic efficiency (CE) and power densities generated by the treatment process. This study helps researchers to easily conduct comparative analysis for different wastewater treatment scenarios in terms of expected process outputs and efficiencies. For the application of MFCs in denitrification processes, biological nitrates are usually employed while utilizing bacteria such as Geobacter species. Studies demonstrate that Geobacter species efficiently reduce nitrate without relying on hydrogen electron formation or external power sources [18]. Reguera and Kashefi [19] reviewed the electrifying physiology of Geobacter bacteria. The study highlighted the unique ability of Geobacter to transfer electrons directly to electrodes without the need for external mediators in various metabolic pathways, demonstrating their potential in enhancing MFC performance in denitrification processes. MFCs also find applications in biosensor development for pollutant analysis and in situ process monitoring. According to Cui et al. [20], MFC-based biochemical oxygen demand (BOD) sensors offer superior operational stability, reproducibility, and significantly extended lifespan. Perchikov et al. [21] complemented this finding by exploring the features of microbial biofilm formation and their potential use in bioelectrochemical devices such as MFC-based biosensors. These features were enhanced in terms of their sensor stability and sensitivity to increase the operational efficiency of MFC-based biosensors. MFCs have also been applied to the production of alternative fuels or hydrogen through organic matter degradation. Hydrogen generated during this process can be stored for electricity production or as a transportation fuel. Studies show that the use of acetate-substrate for MFCs has achieved up to 53% hydrogen production in bio-electrochemical systems [22]. Florio et al. [23] conducted a preliminary study on biohydrogen production from MFC acetate substrates. Their findings proved that acetate produces more hydrogen when employed in MFCs compared to glucose, sucrose, and other traditional substrates.
Furthermore, MFCs play a crucial role in bioremediation, leveraging specific bacteria, such as Geobacter species, which act as electron acceptors [18]. Effective bioremediation of contaminated environments hinges on the use of electron acceptors or donors to stimulate biodegradation processes [24]. Shabani et al. [25] explored the application of MFC technology in bioremediation. They developed an optimized MFC systems that could simultaneously degrade contaminants and generate electricity, providing a dual benefit for environmental cleanup and energy recovery. Mukherjee et al. [26] also conducted a study on using a novel bacterial consortium for the bioremediation of aromatic hydrocarbons and maximized bioelectricity generation in MFCs to address the challenges associated with degradation rates of complex pollutants.
The advantages of MFCs are mainly by their status as a renewable energy source, characterized by environmentally benign electricity and fuel production processes free from carbon emissions and environmental pollution. As discussed, the versatility of MFCs extends to various applications in contaminant removal and bioremediation, facilitating the recovery of valuable compounds from substrate degradation. However, challenges remain, necessitating ongoing research and development efforts. These include low microbial growth rates, material toxicity concerns, modest power outputs, limited electrode durability and strength, as well as high operational and material costs. In the context of MFC challenges, Ramadan and Purwono [27] studied the development of MFC technology in Indonesia and identified low microbial growth rates as well as material toxicity as the major factors affecting the efficiency and sustainability of MFC systems. Ramadan established the need for localized research and tailored solutions to overcome these obstacles, emphasizing the importance of developing region-specific strategies for MFC implementation. In another study, Chandrasekhar et al. [28] addressed the challenges of low power outputs and limited electrode durability and strength in MFCs. The contributions of their research included recommendations for innovative materials such as graphene-coated electrodes and bio-compatible conductive polymers, as well as design improvements like optimized electrode spacing and enhanced microbial community structuring to boost power generation and extend electrode life, paving the way for more robust and efficient MFC applications. Additionally, challenges related to cost were analyzed by Hassan et al. [29]. Their study established that high operational and material costs are the most significant barriers to the large-scale deployment of MFC technology. Thus, they conducted an evaluation of cost-effective materials, such as stainless-steel mesh anodes and carbon cloth cathodes, and scalable configurations, including modular stacking and integrated biofilm reactors, to reduce overall costs and improve the feasibility of MFC systems for industrial applications. The research also emphasized that integrating MFCs with other renewable energy technologies has the potential of enhancing their economic viability and overall environmental impact. Scientists are focusing more on conducting researches that address each of these discussed drawbacks through further technological advancements to unlock the full potential of MFCs across diverse fields. The scope of this paper includes a comprehensive review of MFC technology, with a particular focus on its biochemical and electrochemical processes, key performance parameters, and recent advancements. The objectives are to provide a detailed understanding of the operational principles and processes in MFCs, analyze the impact of various parameters on the efficiency and performance of MFCs, review the latest research advancements and technological innovations in the field, identify the challenges hindering the practical implementation of MFCs, as well as the future research directions and potential applications of MFCs. The aim is to provide knowledge guiding researchers to optimize MFC performance and its integration into sustainable energy systems.
2. Biochemical and Electro-Chemical Processes in MFCs
2.1. Description of MFCs
The main components of a typical MFC include anode and cathode chamber, microbes, exchange membrane, electrode connection, and an electric circuit for energy generation. The anode is a conductor material normally made of stainless-steel mesh with graphite rods to generate higher current density. The anodic chamber houses microorganisms responsible for the determination of electron generation. Similarly, the cathode chamber is commonly designed with platinum (Pt), which serves as a catalyst for the recombination of protons and electrons. Both the anode and cathode chambers may be housed by a made-up glass, plexiglass, or polycarbonate material. Researchers have made several other discoveries with the use of traditional electrode materials in terms of MFC power outputs. Mateo et al. [30] suggested using novel nanomaterials for cathode construction, as they could potentially address efficiency issues while reducing costs. In a different study by Boas et al. [31], they emphasize that there is a high cost associated with platinum-based catalysts and propose alternative materials, such as carbon-based catalysts and bio-based materials, to reduce costs. In another MFC project by Sivasankar et al. [32], they targeted correcting stability issues by constructing microbial anodes using conductive polymers and biofilms, which enhanced stability and performance over extended periods. As the name suggests, “microbial” fuel cells operate with a wide range of microbes. Such microorganisms or bacteria are classified into two based on their culture: axenic bacteria and mixed bacterial fuel. Axenic bacteria may include; Shewanella putrefaciens, Geobacter species, Rhodoferax ferrireducens, and clostridium beijerinckii. Mixed bacterial fuel may include Pseudomonas aeruginosa, Desulfuromonas, Clostridium butyricum, and nitrogen-fixing bacteria such as Azospirillum and Azoarcus. In most MFC technologies, S. putrefaciens are used for their advantage of modified cell membranes that allow them to fully oxidize organic matter and generate higher amounts of electricity [33]. Wu et al. [34] confirmed the effectiveness of S. putrefaciens in MFCs by demonstrating that a hierarchical porous carbon–silica composite anode significantly enhances interfacial bioelectrocatalysis. The study revealed that the macropores and mesopores of the composite provide ample surface area for bacterial adhesion and active sites for flavin-mediated electron transfer. This structure enabled S. putrefaciens CN32 to form a uniform biofilm, leading to a maximum power density of 580.7 mW/m2, which is 4.5 times higher than conventional carbon cloth anodes. Ali et al. [35] further supported these findings by exploring the modified cell membranes of S. putrefaciens that facilitate efficient electron transfer in MFCs. Their research highlighted the ability of S. putrefaciens to fully oxidize organic matter, resulting in higher electricity generation compared to other microbial strains. This advantage is attributed to the bacterium’s enhanced extracellular electron transfer mechanisms, which are crucial for optimizing MFC performance under various operational conditions. The organic matters undergoing biochemical oxidation by these microbes for electricity are called substrates. In most MFCs, the substrates used are acetate ion, glucose, butyrate, glycerol, malate, citrate, and acetic acid [36]. MFCs also possess an exchange membrane usually made of Nafion and may serve as either a cation exchange membrane or a proton exchange membrane. This component allows protons to flow through from anode to cathode. In order to generate energy using MFCs, the electrode (anode and cathode) is connected via a conductor network such as copper wire. An external circuit or load is added to the electric network chain to evaluate the amount of energy produced using a multimeter. This electric circuit contains a resistor that results in high voltages and low currents and controls power production. MFCs are of two types: single chamber and double chamber. In single-chamber type MFCs, the anode and cathode are typically placed within the same chamber, separated by a membrane or separator [37]. Double-chamber MFCs have separate compartments for the anode and cathode, typically with an ion-permeable membrane separating them to allow for higher efficiency of operation [37]. Other classifications of MFC technology include mediator-free and mediator-type. The mediator-free type uses electrochemical bacteria such as S. putrefaciens with cytochromes in their outer membranes to aid the transfer of electrons to an electrode, while in the mediator type, the bacteria require support from humic acids that capture the electrons to reduce the oxidation state for easy transfer to the anode for reoxidation [4]. These types are uncommon because of their cost and toxicity, and they are usually applied in laboratories. The components discussed in this section are expected to work together for optimal MFC energy output. However, accurately predicting the performance behavior of MFCs under varying environmental conditions remains challenging. Xia et al. [38] address these challenges by employing computational approaches such as finite element analysis and computational fluid dynamics to simulate MFC behavior. They identify a significant gap in the predictive accuracy of these models, particularly under varying environmental conditions like pH levels, temperature fluctuations, and substrate concentration variations. Xia et al. propose the integration of advanced machine learning techniques, including neural networks and support vector machines, to improve model accuracy and robustness. Figure 1 is a layout of an MFC.
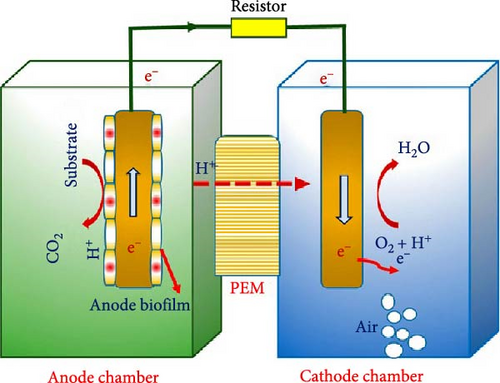
2.2. Microbial Metabolism and Electron Transfer
Electrical energy in MFCs is primarily generated through redox reactions facilitated by microbes. These microbes degrade the organic substrate into carbon dioxide, producing protons and electrons in the anodic chamber. The electrons then travel through an external circuit to the cathode chamber, separated by a Nafion semipermeable membrane or salt bridge, creating a potential difference that generates electricity. This electrical energy can be monitored using a load and read with a multimeter. At the cathode, electron acceptors such as oxygen react with the electrons to form water. Although hydrogen peroxide and ferricyanide can also serve as acceptors, oxygen is mostly preferred for its abundance and high reduction potential. In single-chamber MFCs, air replaces the cathode chamber to facilitate electron and proton transfer [40]. Microorganisms metabolize substrates via biochemical pathways, primarily respiration and fermentation, to generate electrons. During respiration, microbes convert substrates like glucose into energy through glycolysis, the Krebs cycle, and the electron transport chain, producing ATP and releasing electrons [41]. Glycolysis breaks down glucose into pyruvate, which then enters the Krebs cycle, generating electron carriers (NADH and FADH2) [42]. These carriers transfer electrons to the electron transport chain, ultimately leading to the production of water and the release of energy. According to Uria et al. [43], optimizing microbial community composition through selective enrichment, such as using high-throughput sequencing to profile microbial communities and correlate specific microbial species with enhanced electron transfer capabilities, can improve MFC performance. This observation was affirmed by Aiyer [44], whose study focused on optimizing the concentration and type of redox mediators to maximize electron transfer efficiency and overall power output of MFCs. Aiyer’s study highlighted the potential of redox mediators, such as riboflavin and humic substances, to bridge the gap between microbes and the electrode. In MFCs, bacteria take glucose through their cell membranes, where enzymes oxidize the substrate via a cycle called the TCA cycle to pyruvic acid [45]. When pyruvic acid is fully oxidized, it produces carbon dioxide, water, and electrons. In eukaryotes, these electrons are captured in the mitochondrial inner membrane, while in prokaryotes, they accumulate in the cell membrane via NADH and FADH2 [46]. Protein complexes in these membranes maintain the flow of electrons, leading to ATP generation and synthesis by membrane enzymes [46]. A study by Fuhrmann [41] further explained that modifying the operating conditions, such as maintaining a neutral pH and optimal temperature range (20−30°C), can enhance microbial activity and electron transfer efficiency. Exoelectrogenic bacteria are crucial in MFCs as they transfer electrons generated during substrate oxidation directly to the anode through direct electron transfer using conductive pili (nanowires) or outer membrane cytochromes or mediated electron transfer via secreted electron shuttles like flavins and quinones [47]. Zheng et al. [48] discussed this process in detail and suggested that the genetic engineering of specific microorganisms to express conductive pili can significantly improve electron transfer rates. They also explored engineered microbial strains and conductive materials, such as graphene and carbon nanotubes (CNTs), to enhance the electron transfer efficiency. In MFCs, electrons are directly extracted from NADH or from the electron flow generated by substrate decomposition, and some of these electrons are carried to the cathode through an external circuit. One glucose molecule, for example, yields 24 electrons, while the complete oxidation to carbon dioxide and water involves 10 NADH and 2 FADH2 molecules [47]. To illustrate, assuming glucose is used as a substrate to build a MFC fed with Saccharomyces cerevisiae bacteria, Figure 2 shows the chemical process involved in decomposing the glucose molecule to generate the ATP energy required to transfer electrons through the electric circuit from the anode to the cathode.
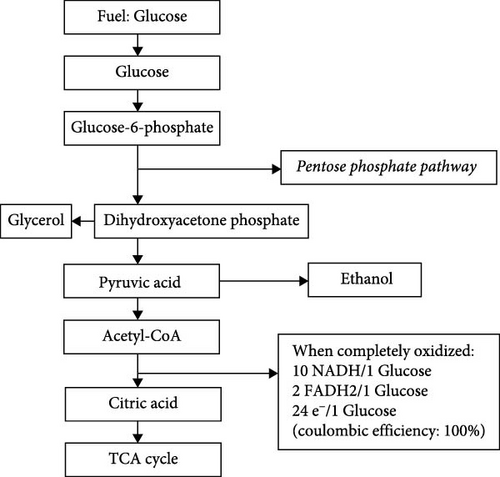
As mentioned earlier, the efficiency of the electron transfer process in MFCs is a critical factor influencing their overall performance. High efficiency in electron transfer is achieved through effective interactions between microbes and electrode surfaces. This can be enhanced by optimizing electrode materials and configurations, as well as improving the microbial communities involved. Efficient electron transfer results in higher power outputs and better energy conversion, making it a key area of focus for advancing MFC technology. To illustrate this, Zhou et al. [50] examined the bioenergetics and extracellular electron transfer in both MFCs and microbial corrosion. They identified the long-term stability of electron transfer processes due to biofilm formation and fouling. Zhou et al. [50] proposed the use of dynamic flow conditions and periodic electrode cleaning as novel strategies to mitigate biofilm-associated issues and maintain high electron transfer efficiency over extended periods. In another study, Song et al. [51] explored various biocatalysts, such as laccase and bilirubin oxidase, to enhance cathodic reactions. Their work demonstrated that immobilizing these biocatalysts on conductive supports, like carbon cloth, can significantly boost electron transfer rates and improve the overall efficiency of MFCs.
2.3. Electrochemical Reactions at the Anode and Cathode
Table 1 presents a detailed summary of electrochemical processes that occur at both the anodic and cathodic chambers of MFCs for each of the major organic substrates.
| Substrate | Anodic reaction | Cathodic reaction |
|---|---|---|
| Acetate ion | CH3COO− + 4H2O → 2HCO3− + 9 H+ + 8e− | O2 + 8e− + 8 H+→ 4H2O |
| Glucose | C6H12O6 + 6H2O → 6CO2 + 24 H+ + 24e− | 24 H+ + 24e− + 6O2→ 12H2O |
| Butyrate | C4H8O2 + 2H2O → 2C2H4O2 + 4 H+ + 4e− | O2 + 4e− + 4 H+→ 2H2O |
| Glycerol | C3H8O3 + 6H2O → 3HCO3− + 17 H+ + 14e− | 2O2 + 14e− + 14 H+→ 7H2O |
| Malate | C4H5O5− + 7H2O → 4H2CO3 + 11 H+ + 12e− | 3O2 + 12e− + 12 H+→ 6H2O |
| Citrate | C6H5O73− + 11H2O → 6H2CO3 + 15 H+ + 18e− | 3O2 + 18e− + 18 H+→ 9H2O |
| Acetic acid | C2H4O2 + 2H2O → 2CO2 + 8 H+ + 8e− | 2O2 + 8 H+ + 8e−→ 4H2O |
- Source: derived from authors.
According to several practical studies on MFCs, such as the works of Matsena and Chirwa [52], Zhang et al. [53], and Logan et al. [54], reactions in the anode chamber have a potential difference of 0.42 V, while reactions in the cathode chamber have a potential difference of 0.82 V. Therefore, the total potential difference of a typical MFC is 1.24 V [55]. Studies by Capodaglio et al. [56] suggest that, in order to find the total quantity of electrical energy generated in an MFC using glucose as the substrate, for example, one can assume that the 24 electrons are achieved from a single glucose molecule and can be recovered within an hour.
3. Operating Parameters of MFCs
In MFCs, various parameters critically influence the efficiency and effectiveness in generating electrical energy. Understanding these parameters and their impacts allows for the optimization of MFC design and operation. Some of these parameters are summarized in Table 2, along with their definitions and calculations in the context of energy generation in MFCs. This section also includes a comprehensive review of how some of these parameters are key to the performance of MFCs.
| Parameter | Unit | Measurement | Calculation |
|---|---|---|---|
| Substrate concentration | mg/L | Organic matter used by microorganisms as a source of energy and carbon. |
|
| Temperature | °C | Thermal condition within the reactor, which affects metabolic rates of the microorganism. |
|
| pH | pH | A measure of hydrogen ion concentration in the electrolyte or environment where microorganisms are active. |
|
| Electrode potential | V | The potential difference between an electrode and a reference electrode in the MFC, reflecting the ability of the electrode to gain or lose electrons. |
|
| Open circuit voltage (OCV) | V | The maximum voltage available from an MFC when no current is drawn (i.e., when the circuit is open). |
|
| Voltage | V | The electrical potential difference between the anode and cathode when the circuit is closed and current is flowing. |
|
| Current | A | The flow of electric charge per unit time through the external circuit. |
|
| Power | W | The rate at which electrical energy is generated by the MFC. |
|
| Current density | A/m2, A/m3 | The current generated per unit area of the electrode surface or per unit volume of the reactor. |
|
| Power density | W/m2, W/m3 | The power generated per unit area of the electrode surface or per unit volume of the reactor. |
|
| Coulombic efficiency (CE) | % | The ratio of the total charge recovered as current to the theoretical charge available from the substrate. |
|
| Internal resistance | Ω | The resistance within the MFC that impedes the flow of electrons. |
|
| Treatment efficiency | % | The percentage of the substrate (organic matter) removed during the MFC operation. |
|
| Organic loading rate (OLR) | kg/m³/day | The amount of organic substrate fed to the MFC per unit volume per day. |
|
| Organic removal rate (ORR) | kg/m³/day | The rate at which organic matter is removed from the substrate. |
|
| Hydraulic retention time (HRT) | Hour | The average time that the liquid substrate remains in the MFC. |
|
- Source: derived from authors.
3.1. Interpreting Table 2: Impact of Key Parameters on MFC Performance
3.1.1. CE
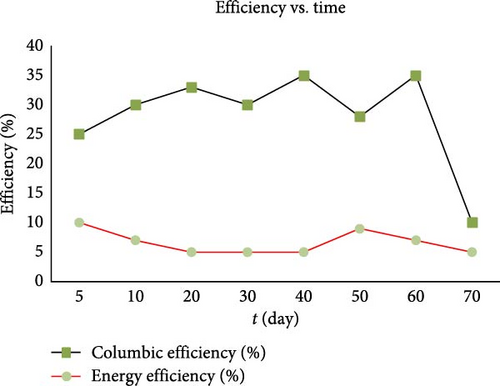
High CE is desirable in MFCs as it signifies efficient electron transfer from the microbial oxidation of organic substrates to the anode, generating usable electrical current. Factors affecting CE include substrate type, microbial activity, electrode materials, and system design. Several studies showcase how the use of specific materials could affect the CE of MFCs. For example, Chen et al. [57] used sodium citrate as an additive to significantly improve the CE by optimizing the microbial community structure and electron transfer processes. The sodium citrate enhanced not only the electricity generation but also the efficiency of electron recovery in MFCs, which is crucial for improving overall system performance. Yang et al. [58] also explored the simultaneous enhancement of power density and CE in MFCs using a hydrophobic Fe–N4/activated carbon air cathode. Their findings indicated that the hydrophobic cathode significantly improved CE by facilitating better oxygen reduction reactions and minimizing electron losses. Maximizing CE enhances the MFC’s energy conversion efficiency, making it more viable for various applications such as wastewater treatment, energy production from renewable sources, and biosensors. Figure 3 is a graph obtained from [59] depicting the changes in CE and energy efficiency of a MFC over a period of 70 days. From the graph, it can be deduced that the variations in CE suggest that the MFC’s ability to convert substrate into electrical current efficiently changes over time, possibly due to factors like microbial community dynamics, substrate concentration, or operational conditions. The relatively stable but low energy efficiency indicates that while the system consistently produces energy, the proportion of energy captured compared to the theoretical maximum remains low. The sharp decline in CE towards the end of the observation period is due to depletion of nutrients, accumulation of inhibitory byproducts, or changes in the microbial community structure. These deductions confirm that CE translates to MFC performance.
3.1.2. Substrates and Microbes
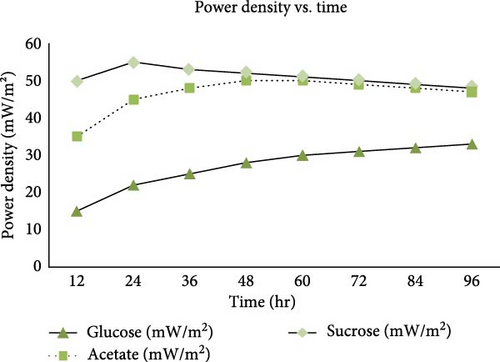
Different types of microbes and substrates from plant and animal sources have been used in the operation and design of MFCs. As concluded by Gezginci and Uysal [60], Prathiba et al. [61], and Ullah and Zeshan [62], the electrochemical activities of these microbes and substrates translate into the overall performance and power output as they constitute the number of bacteria that can be produced to aid in anaerobic digestion. Confirming this, Sarmin et al. [63] investigated the improvement of power generation in MFCs through effective substrate–inoculum interaction mechanisms. They found that optimizing the interaction between substrates and inoculum can significantly enhance power generation. Similarly, Fadzli et al. [64] also reviewed recent developments in the use of organic substrates and bacterial electrode interactions in MFCs and found that different organic substrates affect bacterial-electrode interactions and energy output overall. The choice of substrate is important as it also directly affects operation parameters such as pH, temperature, power density, and CE [65]. Studies show that catalytic microorganisms such as Escherichia coli, Pseudomonas, Fluoscens, Proteus vulgaris, and Pseudomonas methanica are used primarily in MFCs due to their ability to effectively oxidize organic matter to produce electricity [65]. Another study by Tariq et al. [66] tested the three main types of substrates commonly used in MFCs, namely glucose, acetate, and sucrose, to determine their overall impact on energy generation and CE. Tariq et al. [66] found that maximum power densities (corresponding to maximum voltage measurements) of 31, 53.4, and 52.3 mW/m2 were achieved for glucose, acetate, and sucrose, respectively; thus, acetate is more viable for high-performing MFCs. Although this study suggested acetate as the most efficient substrate for MFCs, it is important to recognize that system performance can be affected by many other factors, such as the concentration of substrates, that may change the aforementioned conclusion. For example, David et al. [67] discovered that an MFC system fed a 2,000 mg/L concentration of acetate produces a relatively increased amount of electricity. In a different study by Ullah and Zeshan [62] illustrating the power density (measured in mW/m2) of an MFC over time (hours) using three different substrates: Glucose, Acetate, and Sucrose, as depicted in Figure 4, it was found that all three substrates show a rapid increase in power density within the first 12 hr, indicating that MFCs can quickly metabolize these substrates to generate electricity. Glucose reaches its peak power density later (around 48 hr) and stabilizes at a lower value compared to the other substrates. Acetate peaks early (around 24 hr) and remains fairly stable with slight fluctuations. Sucrose achieves the highest peak power density quickly and remains the most efficient substrate over the observed period. Comparing the efficiency in this study, Sucrose proves to be the most effective substrate for MFC power density, consistently generating the highest values. Therefore, in order to achieve consistent energy production by MFC, one must design an MFC that adjusts to a particular practical substrate concentration stability.
Furthermore, Table 3 presents a comprehensive list of different microbes and substrates commonly used in various MFCs studies, as well as the maximum power or currents recorded in each study.
| Microbes | Substrates | Type of inoculum | Type of MFC | Maximum power or current produced | References |
|---|---|---|---|---|---|
| Actinobacillus succinogenes | Glucose | Anaerobic sludge | Dual-chamber | 0.38 mW/m2 | [68] |
| Aeromonas hydrophila | Acetate | Wastewater sludge | Single-chamber | 0.25 mW/m2 | [69] |
| Clostridium beijerinckii | Glucose | Soil or sediment | Single-chamber | 0.6 W/m2 | [70] |
| Clostridium butyricum | Starch, glucose, lactate, molasses | Anaerobic sludge | Dual-chamber | 0.45 W/m2 | [71] |
| Desulfovibrio desulfuricans | Starch, glucose, lactate, molasses | Marine sediment | Dual-chamber | 0.2 W/m2 | [72] |
| Erwinia dissolvens | Sucrose | Soil | Single-chamber | 0.3 W/m2 | [73] |
| E. coli | Glucose | Activated sludge | Single-chamber | 1.1 W/m2 | [74] |
| Geobacter sulfurreducens | Glucose, sucrose | Pure culture | Single-chamber | 2.5 W/m2 | [75] |
| Geobacter metallireducens | Acetate | Pure culture | Dual-chamber | 2.0 W/m2 | [76] |
| Gluconobacter oxydans | Glucose | Mixed culture from compost | Single-chamber | 0.7 W/m2 | [77] |
| Klebsiella pneumoniae | Glucose | Activated sludge | Single-chamber | 1.0 W/m2 | [78] |
| Lactobacillus plantarum | Glucose | Food waste leachate | Single-chamber | 0.4 W/m2 | [79] |
| Proteus mirabilis | Glucose | Wastewater sludge | Single-chamber | 0.5 W/m2 | [80] |
| P. aeruginosa | Glucose | Activated sludge | Single-chamber | 2.2 W/m2 | [81] |
| R. ferrireducens | Glucose, xylose, sucrose, maltose | Pure culture | Dual-chamber | 0.9 W/m2 | [82] |
| Shewanella oneidensis | Lactate | Marine sediment | Dual-chamber | 1.8 W/m2 | [83] |
| S. putrefaciens | Lactate, pyruvate, acetate, glucose | Marine sediment | Single-chamber | 1.9 W/m2 | [84] |
| Streptococcus lactis | Glucose | Dairy wastewater | Single-chamber | 0.3 W/m2 | [85] |
From Table 3, several observations can be made to understand the functionality and efficiency of various microbes and substrates employed in MFCs. Microbes such as G. sulfurreducens and P. aeruginosa are well-known for their high electrogenic capabilities, producing significant power outputs of 2.5 and 2.2 W/m2, respectively. In contrast, microbes like A. succinogenes and P. mirabilis produce lower power outputs of 0.38 mW/m2 and 0.5 W/m2, respectively. The source of the inoculum also plays a critical role. For example, Geobacter and Shewanella species, often derived from sediment, tend to be more effective in MFCs, suggesting that sediment-derived inocula are rich in electrogenic bacteria. Additionally, microbes like C. beijerinckii and C. butyricum can utilize a broad range of substrates (starch, glucose, lactate, molasses), which might contribute to their versatility in different MFC setups, whereas species like E. coli and G. oxydans are more substrate-specific (glucose), potentially limiting their applicability based on available substrates. The type of substrate affects the efficiency of electricity generation. Glucose is a common substrate used by many microbes, according to Table 3, leading to varying levels of power output. The specific metabolic pathways and efficiencies of these microbes dictate the resultant power or current produced. The inoculum type, ranging from activated sludge and pure cultures to soil and sediment, significantly affects MFC performance. For instance, sludge from wastewater treatment plants (WWTPs) provides a diverse microbial community capable of efficient electricity generation, as seen with E. coli and P. aeruginosa. Mixed cultures derived from environments rich in organic matter (e.g., activated sludge) often outperform pure cultures due to the synergistic interactions within microbial communities, enhancing overall electron transfer processes. It is also evident that single-chamber MFCs, as used for G. sulfurreducens and P. aeruginosa, tend to have higher power outputs compared to dual-chamber systems, which are more common for microbes like C. butyricum and R. ferrireducens. This difference may be attributed to reduced internal resistance and more efficient electron transfer in single-chamber designs. Dual-chamber MFCs are often employed for more controlled environments, possibly leading to more stable but lower power outputs, as they can manage separate anodic and cathodic processes more effectively. There is a significant variability in power outputs across different microbes. High performers like G. sulfurreducens achieve up to 2.5 W/m2, whereas others like A. succinogenes produce much lower outputs (0.38 mW/m2). This variability highlights the importance of selecting appropriate microbes based on the desired application and efficiency needs. The performance of each microbe is also dependent on the specific operational conditions, such as temperature, pH, and the presence of electron mediators. Optimizing these conditions can enhance the power output for less efficient microbes. The comparison reveals that while some microbes are inherently more efficient at generating power in MFCs, the choice of substrate, type of inoculum, and MFC design are crucial factors that influence the overall performance. These differences can guide the selection and optimization of MFC systems for various applications, from wastewater treatment to renewable energy production.
3.1.3. Temperature
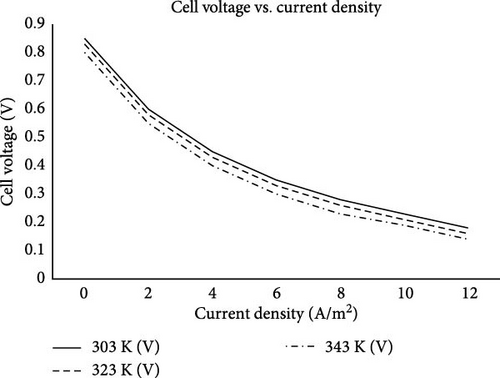
An increase in temperature results in an increase in the reactions of MFC operation; thus, a higher temperature increases hydrogen-ion diffusions to the cathode, which influences microbial metabolic rates and enzyme activities. As concluded by Ren et al. [86], Gadkari et al. [87], as well as Tremouli et al. [88], higher temperatures generally enhance microbial activity and electron production up to an optimal point, beyond which microbial viability may be compromised. Degefa et al. [89] used a different approach employing multi-parameter optimization for advanced MFC systems to arrive at the same result. In their study, the influence of temperature and low-frequency vibrations was discovered through the laminated structure of an MFC piezoelectric energy harvester. A recent study by Li et al. [90] presented a more specific result. They discovered that, at a 20-degree temperature, the internal resistance of the MFC reached its maximum, while at a 40-degree temperature, the internal resistance decreased to a minimum but restored the increase beyond the 40-degree temperature. This implies that energy generation of MFC increases with increasing temperature as microbial metabolism and membrane permeability improves. Singh and Krishnamurthy [91] also confirmed this by conducting research focused on finding the relationship between cell voltage (V) and current density (A/m2) for a MFC at three different temperatures: 303, 323, and 343 K. As shown in Figure 5, the highest temperature (343 K) shows the highest cell voltages across most current densities, followed by 323 K and then 303 K. At lower current densities (0–2 A/m2), the differences in cell voltage between the three temperatures are more pronounced. As current density increases, the curves converge slightly, but the higher temperature still consistently results in higher voltages. This means increasing temperature generally improves cell voltage at a given current density and is likely due to enhanced microbial activity and reaction kinetics at higher temperatures, which can reduce activation losses and internal resistance. As a result, higher temperatures help maintain better performance at higher current densities, which is crucial for applications requiring sustained high-power output. The study also concludes that operating MFCs at higher temperatures could be beneficial for maximizing voltage and overall energy output. However, practical considerations such as thermal management, stability of the microbial community, and operational costs need to be balanced.
3.1.4. pH
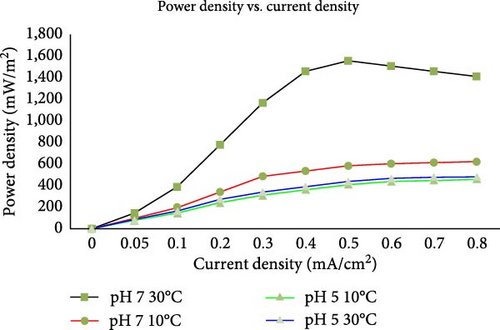
Tang et al. [92] and several other studies have shown that higher voltages are recorded for substrate at neutral pH (pH of 7) than at any other value. A pH of 7 is an indication that the microbes are alive and are actively breaking down the substrate solution under use. Thus, at a neutral pH, higher ATP production is guaranteed and effective electron transfer, translating to higher power output. A study by Li et al. [93] confirmed this by analyzing the effect of pH on bacterial distributions within the cathodic biofilm of MFCs using maltodextrin as the substrate. They found that pH variations significantly influence the composition and activity of the bacterial communities in the cathodic biofilm. Generally, microbes require a pH equal to or close to neutral for optimal growth [88]. This process is known as absorbance. Bagchi and Behera [94] evaluated the effect of anolyte recirculation and anolyte pH on the performance of an MFC employing a ceramic separator. They discovered that maintaining a stable anolyte pH is crucial for sustaining high power output and CE. According to Halim et al. [95], both extremely low and high pH levels can negatively impact power output and microbial viability in MFCs. Acidic conditions disrupt microbial cell membrane integrity, leading to cell lysis and decreased microbial activity, which in turn lowers power output due to the disrupted proton gradient essential for efficient electron transfer. Similarly, alkaline conditions cause denaturation of microbial enzymes and proteins, impairing microbial metabolism and growth and consequently reducing power generation efficiency by affecting the proton gradient and electron transfer processes. As a result, in cases where the pH value does not encourage optimal microbial activity, acid and base dosing or the addition of a chemical buffer could restore the pH to an optimal value. The graph in Figure 6 is an experiment conducted by Puig et al. [96] to assess the impact of pH on MFC performance. Puig et al. [96] studied the result of increasing or decreasing pH and temperature values on power and current densities. They found that a pH 7 at 30°C shows the highest power density among all conditions. At that condition, he records peak values at approximately 1,600 mW/m2, around 0.5 mA/cm2 current density. The power density sharply increases initially and then decreases after reaching the peak. In contrast, a pH of 7 at 10°C shows a lower power density compared to pH 7 at 30°C and peaks around 200–300 mW/m2. The curve is much shorter and lower compared to pH 7 at 30°C, indicating reduced performance at lower temperatures. At a pH of 5 at 10°C, he records the lowest power density, which peaks around 100 mW/m2. The curve indicates a very limited power generation capability under these conditions. The graph demonstrates that a neutral pH (pH 7) is more conducive to higher power densities in MFCs compared to a more acidic environment (pH 5). Again, higher temperatures (30°C) result in significantly higher power densities compared to lower temperatures (10°C), indicating that temperature is a crucial factor for microbial activity and MFC efficiency. The study concludes that the best performance of MFC is only observed at pH 7 and 30°C, suggesting that both neutral pH and higher temperatures are optimal for maximizing power density in MFCs.
3.1.5. Organic Loading Rate (OLR)
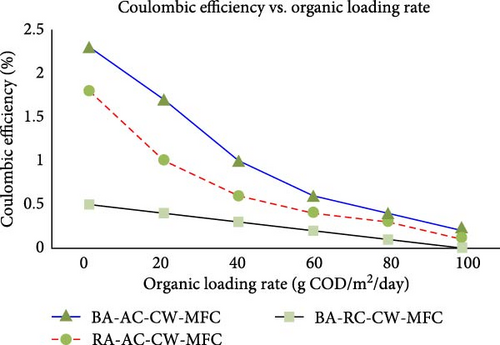
The OLR defines the capacity of the reactor being used per unit volume or the mass of microorganisms available to decompose substrates. Hence, increasing the OLR results in higher substrate degradation and power generation. Don and Babel [97] studied the effects of organic loading on bioelectricity generation and micro-algal biomass production in MFCs using synthetic wastewater. They found that higher OLRs led to increased bioelectricity production up to an optimal point, beyond which performance declined due to substrate inhibition. Similarly, Gurjar and Behera [98] investigated the bio-electrochemical performance of a ceramic MFC treating kitchen waste leachate, focusing on the effects of OLR and anode electrode surface area. They found that increasing OLR improved power density and CE up to a threshold, after which the performance plateaued or decreased. As seen in Figure 7, Xu et al. [99] demonstrated the relationship between CE and OLR for three different types of MFCs and found that increasing OLR negatively impacts CE across all MFC types, indicating that higher organic loads reduce the efficiency with which the MFCs convert organic matter into electrical energy. Xu et al. [99] recommended that MFC systems need to be designed with an expected OLR for optimization and efficiency purposes. Furthermore, in cases of high OLR, strategies such as pretreatment of the organic matter should be adopted to manage or mitigate its corresponding CE impacts.
3.1.6. Electrode Potential
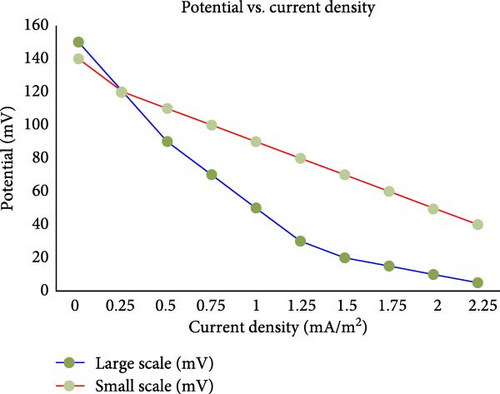
Electrode potential is crucial for determining the efficiency of electron transfer from the microorganisms to the electrode. Higher electrode potential can enhance the oxidation–reduction reactions, leading to better power generation. Ma et al. [100] show that optimizing the anode potential improves the performance of MFCs by facilitating more efficient electron transfer. Shirpay [101] also investigated the relationship between electrode potential (in mV) and current density (in mA/m2) for MFCs on two different scales, large scale and small scale, and concluded that optimizing electrode potential is crucial for maximizing current density and overall power output. Their study revealed that at an optimal electrode potential, MFCs exhibited higher current densities, indicating improved electron transfer efficiency. This relationship was consistent across both large and small scales, suggesting that precise control of electrode potential can enhance the performance of MFCs irrespective of their size. As seen in Figure 8 by Shirpay [101], both the large-scale and small-scale MFCs show a decrease in potential as the current density increases. This inverse relationship suggests that as the MFC generates more current, the voltage drops. The dotted line with the label “Reducing resistance” suggests an ideal path where reducing internal resistance would maintain higher potentials at given current densities. The actual performance of both the large-scale and small-scale cells deviates from this ideal path, indicating higher internal resistance. The graph highlights the challenges in scaling up MFCs, showing that small-scale cells tend to perform better in terms of maintaining higher potential at increasing current densities. Addressing factors such as internal resistance and optimizing conditions for microbial activity are crucial for improving the performance of large-scale MFCs.
3.1.7. Open Circuit Voltage (OCV)
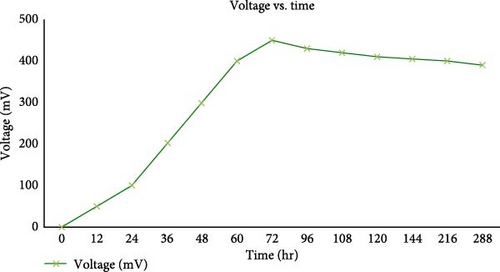
Higher OCV generally correlates with a higher potential for power generation under load. In the study by Littfinski et al. [102], the impact of OCV on MFC performance was examined using various electrochemical methods. The findings indicated that high OCV values were associated with better electron transfer rates, leading to improved power output. However, prolonged high OCV can stress microbial communities, potentially decreasing cell viability and stability over time. Balancing OCV is essential to optimize performance and maintain microbial health within MFC systems. In some cases, OCV is greatly affected by the type of substrate used. For example, Lawal et al. [103] evaluated the OCVs of MFCs using cow and pig dung as substrates. They found that the OCVs generated from these organic wastes varied significantly, with cow dung generally producing higher OCVs compared to pig dung. The use of pig dung in MFCs is notable due to its unique microbial community composition and nutrient profile, which can influence electron transfer processes. The study concludes that one must select a nutrient-rich substrate selection for a higher MFC performance. The graph in Figure 9 obtained from Choudhury et al. [104] demonstrates the OCV (in Mv) versus time (in hours) for a MFC under different resistances: 5,000, 1,000, and 500 Ω. At 0 to ~36 hr, 5,000 Ω, the voltage increases rapidly, reaching a peak of around 400 Mv. At the peak resistance, the MFC builds up to a high voltage as current flow is limited, resulting in less power loss. Reducing resistance to 1,000 Ω increases current flow, causing an initial voltage spike, but the increased current also leads to higher internal losses, resulting in a lower steady-state voltage compared to the initial phase. Further reducing the resistance to 500 Ω increases current flow even more, but the voltage stabilizes around the same level as with 1,000 Ω, indicating that the cell is operating near its optimal power point. The stable voltage at lower resistance indicates the MFC’s optimal operating range, where it can maintain a consistent performance. This data is crucial for optimizing the design and operational parameters of MFCs for better efficiency and power output.
3.1.8. Treatment Efficiency
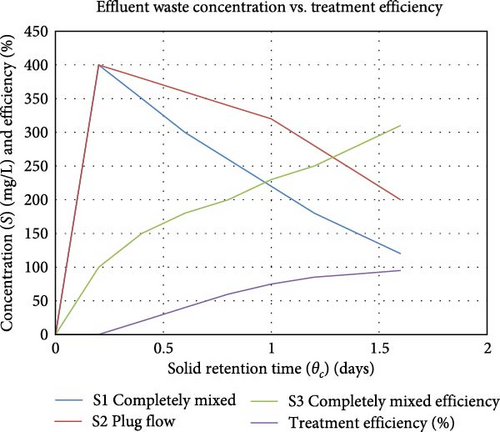
Treatment efficiency reflects the MFC’s ability to remove contaminants from the substrate. Higher treatment efficiencies are indicative of better substrate utilization and cleaner effluents. He et al. [105] showed that the efficient substrate treatment enhances the degradation of organic matter, leading to higher electron availability for electricity generation. This results in improved CE, which measures the proportion of electrons captured as electrical current relative to the total electrons released by microbial metabolism. Additionally, optimizing treatment efficiency reduces biofouling and system resistance, thereby maintaining a stable and higher power density. In a different study, Corbella and Puigagut [15] investigated the improvement of treatment efficiency using constructed wetland MFCs (CW-MFCs). They found that the choice of anode material and external resistance significantly influenced the treatment efficiency and power generation of CW-MFCs. According to the discovery of Huang et al. [106], contaminants like phosphorus and Chlorella vulgaris can be removed by frequently adding small amounts of nitrate. They found that this approach significantly boosted phosphorus removal and supported algal growth, enhancing overall treatment efficiency in MFCs. The graph in Figure 10 is an MFC project conducted by Echiegu [107], depicting the relationship between the solid retention time (θₙ) in days and the effluent waste concentration (S) in mg/L, along with the treatment efficiency (eₛ) in percentage, under different conditions of flow and mixing for a microbial treatment system. The graph includes various parameters and two different system dynamics: plug flow and completely mixed systems. For plug flow, treatment efficiency starts high and increases quickly, achieving near-maximum efficiency at shorter solid retention times. For completely mixed systems, treatment efficiency increases steadily with retention time, reaching higher levels at longer retention times. Increased solid retention time generally improves treatment efficiency as microbes have more time to consume the substrate. Also, the plug flow system reaches higher efficiency more quickly compared to the completely mixed system, indicating better performance at shorter retention times. The inset parameters (k, Kₛ, Y, b, S₀, r) indicate the conditions under which the system operates, affecting microbial growth rates, substrate utilization, and overall treatment performance.
3.1.9. Organic Removal Rate (ORR)
3.1.10. HRT
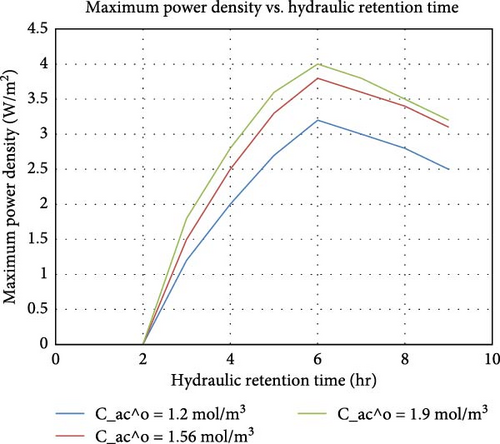
HRT determines the time the substrate stays in the reactor. As established by Xie et al. [113], optimal HRT ensures sufficient time for microbial degradation and electricity generation without overloading the MFC system. This necessitates optimizing HRT for balanced substrate degradation and power output. The impact and application of HRT differ for each MFC type. For continuous flow mode dual-chamber MFC, Ye et al. [114] found that optimizing HRT significantly improved both nutrient recovery rates and electricity generation. Haavisto et al. [115] examined the effect of HRT on continuous electricity production from xylose in an up-flow MFC. They discovered that shorter HRTs enhanced the electricity production rate but reduced the overall xylose removal efficiency. In a different research, Chang et al. [116] explored the effect of HRT on electricity generation using a solid plain-graphite plate MFC in an anoxic/oxic process for treating pharmaceutical sewage. They found that optimal HRTs improved electricity generation while also degrading pharmaceutical compounds. Figure 12 shows the relationship between HRT and Maximum Power Density (in W/m2) for different initial concentrations of acetate used in MFC [117]. It can be observed from the graph that, initially, as HRT increases, the maximum power density also increases for all three initial acetate concentrations. This suggests that at lower HRTs, the contact time between the substrate and the microbial community is insufficient for optimal power generation. Beyond these optimal HRTs, the maximum power density begins to decline. This can be due to several factors, including the depletion of readily available substrates and the accumulation of metabolic byproducts that may inhibit microbial activity. Higher initial concentrations of acetate led to higher maximum power densities. This is because more substrate is available for the microbes to convert into electricity. The optimal HRT increases slightly with higher initial acetate concentrations. This is because the microbes need more time to process the higher substrate levels effectively. Optimal HRTs ensure that microbial interactions with electrodes are maximized (there is a right balance for microbial activity, allowing efficient conversion of organic matter into electricity), improving electron transfer rates and overall power density.
4. Current State of MFC Research
4.1. Recent Studies and Advancements in MFC Technology
4.1.1. Reactor Configuration and Scaling Up
Recent studies highlight that stack models or modularized systems in MFCs are pivotal for achieving high power density and scalability. For instance, research by Mukherjee et al. [118] and Rabaey and Verstraete [119] demonstrated that stacking multiple MFC units can significantly increase power output per unit area while maintaining operational stability over extended periods. This approach is crucial for applications requiring higher power densities and larger-scale deployment in practical settings. Several recent studies have focused on improving reactor configurations and scaling up MFCs. For example, Tan et al. [120] investigated the integration of a polypropylene biofilm carrier and a fabricated stainless-steel mesh supporting activated carbon in an MFC configuration. They discovered that this innovative design significantly enhanced the MFC’s performance, resulting in higher power density and improved contaminant removal. The enhancements were attributed to the biofilm carrier’s increased surface area, which facilitated greater microbial growth and activity, and the activated carbon’s effective adsorption properties, which improved electron transfer efficiency within the system. In another research, Song et al. [121] focused on recent advances in MFC reactor configurations and coupling technologies for the removal of antibiotic pollutants. They found that innovative reactor designs and hybrid systems significantly improved pollutant degradation and energy recovery. This improvement was achieved through enhanced contact between pollutants and reactive sites within the reactors, increased microbial activity due to optimized environmental conditions, and the integration of coupling technologies that facilitated more efficient electron transfer and energy conversion processes. Also, Rossi and Logan [122] investigated the impact of reactor configuration on the performance of pilot-scale MFCs. They discovered that certain configurations, such as tubular designs and membrane-less systems, offered superior performance in terms of energy recovery and contaminant removal. These studies emphasize the importance of optimizing reactor configurations to enhance efficiency in MFCs and reduce costs.
4.1.2. Membrane Materials: Earthen and Ceramic
Earthen and ceramic membranes are increasingly favored in MFC technologies due to their sustainability and efficiency. Ceramic membranes, in particular, offer superior proton exchange rates and mechanical strength compared to traditional materials like Teflon [123]. Studies have shown that ceramic membranes can operate effectively over long periods with minimal maintenance, making them suitable for commercial MFC applications [123]. Suransh et al. [124] investigated the modification of clayware ceramic membranes to enhance the performance of MFCs. The modification involved incorporating metal oxides into the clayware ceramic membranes. This alteration aimed to improve the membrane’s electrical conductivity, mechanical strength, and overall stability, thereby enhancing the MFCs’ performance and extending their operational lifespan. They identified the primary research gap as the need for cost-effective, durable membrane materials that improve the efficiency and longevity of MFCs. Other studies like Rao et al. [125] and Suransh and Mungray [126] succeeded at solving this cost issue by employing a novel fly ash blended ceramic membrane and reducing the particle size of vermiculite to produce low-cost earthen membranes, respectively. A summary of advanced ceramic-based MFCs has been summarized in the work of Yousefi et al. [127]. Table 4, obtained from their work, illustrates the promising potential of earthen and ceramic materials as separators in MFC technology. These natural materials, such as porcelain, earthen pots, mullite, and red soil clayware, offer a range of benefits for sustainable and cost-effective MFC designs. The varied performance in COD removal, from as low as 41.5% to as high as 96.5%, confirms the importance of factors such as material composition, separator thickness, and porosity. For instance, the dual-chamber MFC with an earthen pot separator shows remarkable efficiency in treating rice mill wastewater, achieving 96.5% COD removal, which highlights its potential for high-performance wastewater treatment applications. In contrast, the single-chamber MFC with a mullite separator demonstrates lower efficiency, suggesting that the specific combination of anode/cathode materials and the nature of the waste stream significantly influence performance.
| MFC configuration | Separator | Thickness (mm) | Anode/cathode | Anolyte/catholyte | COD removal (%) | Reference |
|---|---|---|---|---|---|---|
| One chamber | Porcelain (100% kaolinite) | 2 | Mn4 + graphite/Fe3 + graphite | Sewage sludge/air-cathode | 787.5 | [128] |
| Dual chamber | Earthen pot | 0.45 | SS mesh/graphite plate | Rice mill wastewater (pH 8.0)/aerated water | 96.5 | [129] |
| Single chamber | Mullite | 4 (27% porosity) | Carbon veil/conductive paint | Urine/air-cathode | 41.5 ± 5.9 | [130] |
| Dual chamber | Red soil clayware | 5 (11.6% porosity) | SS mesh/carbon felt | Acetate medium/aerated water | 78.9 ± 3.9 | [131] |
- Source: [127].
4.1.3. Wastewater Treatment Advancements
Continuous double-chamber MFC systems have been shown to achieve high removal efficiencies for COD in wastewater treatment [110]. Recent studies have optimized the design of these systems to maximize treatment efficiency while minimizing energy input. For example, researchers have explored novel electrode materials and configurations to enhance pollutant degradation rates and overall system performance [132]. Such advancements are crucial for meeting stringent environmental standards and reducing the environmental footprint of wastewater treatment processes. Additionally, in recent projects, researchers are advancing and optimizing MFC systems by combining wastewater treatments with clean energy production. In the study by Arun et al. [133], they integrate microbial electrolysis cells (MEC) and MFCs for simultaneous wastewater treatment and green fuel (hydrogen) generation. The study demonstrated a novel synergistic system where the byproducts of MECs fuel the MFCs, resulting in higher hydrogen yields and more efficient wastewater treatment. Zahran [134] also investigated iron- and carbon-based nanocomposites as anode modifiers in MFCs for both wastewater treatment and power generation. These nanocomposite anodes significantly increased the power density and treatment efficiency of MFCs by enhancing electron transfer rates and microbial adhesion. Similarly, Chaturvedi and Kundu [135] utilized nanostructured graphene for the same purpose of green energy and wastewater treatment. Their novel use of graphene-based electrodes enhanced conductivity and surface area, leading to improved microbial activity and overall MFC efficiency.
4.1.4. Genetic Engineering for Enhanced Performance
Genetic engineering strategies have revolutionized MFC performance by enabling microbes to produce specific electron-shuttling compounds or enhancing their metabolic pathways for electricity generation [136]. Recent developments include the engineering of microbial consortia to optimize electron transfer efficiency and substrate utilization. For instance, synthetic biology approaches have been employed to design microbial communities with tailored functionalities, such as improved tolerance to environmental stresses and enhanced bioelectricity production [137]. These advancements pave the way for more efficient and sustainable bio-electrochemical systems. Surti et al. [138] reviewed genetic engineering strategies for enhancing bioelectrochemical systems, focusing on improving microbial electron transfer capabilities. Their comprehensive analysis highlighted several key strategies and genetic modifications, including the overexpression of electron transfer proteins such as cytochromes, optimizing metabolic pathways for increased electron flow to the anode, and employing synthetic biology approaches to introduce or enhance electron transfer pathways. Additionally, they discussed the use of mutagenesis and directed evolution to develop microbial strains with superior electron transfer properties, as well as heterologous expression of genes from electroactive microorganisms to impart these capabilities to other microbial hosts. Finally, they emphasized the importance of biofilm engineering to create more stable and conductive biofilms on electrode surfaces. These modifications collectively lead to significant improvements in the performance of bioelectrochemical systems, enhancing power generation and efficiency. Philipp et al. [139] also discussed genetic engineering for enhanced productivity in bioelectrochemical systems, highlighting the critical role of metabolic pathway optimization. They emphasized the importance of fine-tuning metabolic pathways to maximize the efficiency of bioelectrochemical reactions. This involves increasing the availability and flow of electrons to the anode, thereby enhancing the overall energy conversion process. By this method, they addressed the gap of integrating complex genetic circuits into microbial genomes, which are designed to regulate and optimize multiple metabolic processes simultaneously. These complex genetic circuits include synthetic promoters, regulatory elements, and engineered enzymes that coordinate the expression and activity of various metabolic pathways resulting in significant improvement in the productivity and efficiency of bioelectrochemical systems such as MFCs.
4.1.5. Component Modifications for Improved Efficiency
Ongoing research focuses on optimizing MFC components such as anodes and cathodes to improve power output and efficiency. Advanced materials, including carbon-based nanomaterials and metal catalysts, are being investigated for their potential to enhance electrochemical performance and durability under harsh operating conditions [140]. Modifications in the anodic chamber of MFCs are critical for improving bacterial affinity towards the anode and enhancing electrical conductivity. Recent advancements include using polymer or carbon materials as molecular wires to connect biofilms to the anode, resulting in increased power densities from 1,479 to 2,355 mW/m2 [141]. Moreover, innovative electrode architectures and fabrication techniques are being explored to achieve higher surface area and better electron transfer kinetics, contributing to overall system efficiency improvements [142]. Hindatu et al. [143], Dessie and Tadesse [144], as well as Savla et al. [145] reviewed various anode modification techniques, such as the use of nanomaterials (nanocomposite-based anode modifiers) and conductive polymers aimed at improving the performance of MFCs. Figure 13 summarizes the results of utilizing nanomaterials for MFC anode modification to aid electron transfer efficiency.
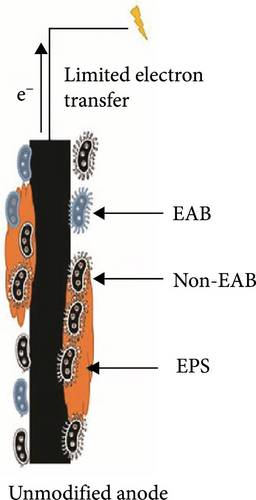
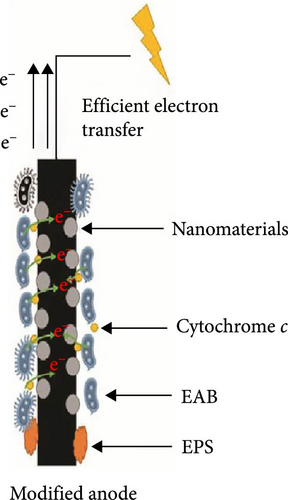
Figure 13(a) illustrates an unmodified anode, where the presence of electroactive bacteria (EAB) is hindered by non-electroactive bacteria (non-EAB) and extracellular polymeric substances, leading to limited electron transfer. In contrast, Figure 13(b) demonstrates a modified anode incorporating nanomaterials, which facilitate the attachment and activity of EAB. These nanomaterials provide a conducive environment for cytochrome c, a key electron transfer protein in EAB, thereby promoting efficient electron transfer. The enhanced electron flow in the modified anode significantly improves the overall performance of the MFC. Though nanomaterials have proven to be better facilitate electron transfer, they are associated with issues related to cost. As a result, some researchers have begun investigating on other materials for MFC component manufacturing. For example, Yaqoob et al. [146] explored alternative materials, such as agricultural waste-derived carbon, which offered a sustainable and economically viable solution for MFC applications while maintaining or enhancing performance. Du et al. [147] also investigated the use of polydopamine as a new modification material to reduce cost and promote anode performance In MFCs. This application of polydopamine coating was found to improve the anode’s hydrophilicity, biocompatibility, and electron transfer properties, leading to faster startup times and enhanced MFC performance while reducing cost. Additionally, techniques such as ammonia gas treatment, heat and acid pretreatments, or anode coating with materials like CNTs or graphene have proven to increase anode surface area and improve performance [148, 149]. As researched by Kadivarian et al. [150] and Zafar et al. [151], cell structure and heat pretreatment of microorganisms impact the performance of MFCs by enhancing electrode conductivity and microbial adhesion, which can boost microbial activity and electron transfer rates. Their study also establishes that the choice and optimization of microorganisms are crucial, as different strains exhibit varying levels of metabolic activity and electron transfer capabilities. Table 5 presents different electrode pretreatment methods currently used in various MFC designs and their respective impacts on power density and CE.
| Types of treatment | MFC fabrications | Methods of treatment | Power density (mW−2) | Coulombic efficiency (%) | Refs. |
|---|---|---|---|---|---|
| NH3 gas treatment of carbon cloth |
|
A thermal gravimetric analyzer—TGA | 1,970 | 60 | [152] |
| NH3 gas treatment of carbon cloth |
|
Using a high-temperature ammonia gas process (700°C for 60 min in 5% ammonia gas) | 988 | 75 | [153] |
| NH3 gas treatment of carbon mesh |
|
Using a high-temperature ammonia gas process (700°C for 60 min in 5% ammonia gas) | 1,015 | 62 | [154] |
| Acetone-cleaned carbon mesh |
|
Using a high-temperature ammonia gas process (700°C for 60 min in 5% ammonia gas) | 893 | — | [155] |
| NH3 gas treatment of carbon brush |
|
Using a high—temperature ammonia gas treatment in a muffle furnace at 450°C for 30 min | 1,280 | 19.5 | [156] |
| Acid treatment: conc. H2SO4 carbon cloth |
|
Acid-soaking with ammonium peroxydisulfate (200 g/L) + concentrated H2SO4 (100 mL/L) in 15 min | 1,100 | 20 | [157] |
From Table 5, it can be observed that NH3 gas treatment of carbon cloth generally achieves the highest power densities (up to 1,970 mW/m2) and moderate to high coulombic efficiencies (up to 75%), indicating this method is highly effective for enhancing MFC performance. Comparatively, acetone cleaning and acid treatment provide decent power densities but lower coulombic efficiencies, highlighting a tradeoff between power output and electron transfer efficiency. The different responses to treatment methods across various materials (carbon cloth, carbon mesh, carbon brush) suggest that the choice of electrode material significantly influences the effectiveness of the treatment. NH3 gas treatment appears more universally effective across different carbon-based materials, while other methods like acetone cleaning and acid treatment show more variability in performance outcomes.
On the other hand, modifications in the cathodic chamber aim to utilize materials with higher redox potentials that can efficiently absorb protons, reducing reduction losses. While platinum (Pt) remains a common cathode material due to its high efficiency, research is increasingly focusing on exploring cost-effective alternatives such as iron-based catalysts, carbon-based materials, and transition metal chalcogenides for their potential to achieve high power outputs at a fraction of the cost of platinum [158]. These alternatives aim to balance performance with affordability, potentially making MFCs more economically viable.
4.1.6. Biosensor Development
MFCs have emerged as promising platforms for biosensor development as a result of their ability to detect various environmental pollutants and biomolecules. Recent advancements include the integration of biorecognition elements with MFC electrodes to create robust and sensitive biosensors for real-time monitoring of water quality parameters, such as BOD and antibiotic residues [21]. These biosensors offer rapid detection capabilities and can be deployed in remote or resource-limited settings, addressing critical challenges in environmental monitoring and public health. Zhou et al. [159] developed an MFC-based biosensor capable of real-time, sensitive toxicity detection by employing advanced anode and cathode materials to enhance electron transfer efficiency and optimizing microbial consortia for increased sensitivity and specificity. They designed the biosensor to monitor changes in output voltage or current correlating with microbial activity inhibition due to toxic substances. Jadhav et al. [160] extended the MFC’s capabilities by integrating sensors for measuring multiple parameters, such as pH and dissolved oxygen, within the MFC setup. This involved modifying electrodes with selective materials, embedding pH and oxygen sensors, and using advanced data acquisition systems to process the MFC’s electrical output along with environmental readings. The integration of MFCs into such biosensors exemplifies significant progress, driven by innovations in bioelectrode design, microbial selection, system miniaturization, and signal processing, resulting in sophisticated, portable biosensors for real-time environmental monitoring. Building on these foundational advancements, further studies have continued to refine and expand the capabilities of MFC biosensors in various environmental applications. For example, Khan et al. [161] addressed the limitations of existing zinc detection methods, which are often complex and costly, by creating a simple, cost-effective, and sensitive MFC-based biosensor for zinc detection. This development showcases the versatility of MFC technology in providing accessible and efficient solutions for heavy metal detection. Similarly, Xiao et al. [162] focused on the hydrodynamic optimization of continuous-flow miniaturized MFC biosensors. They developed an optimized continuous-flow design that improves the efficiency and sensitivity of MFC biosensors, making them more practical for diverse applications. These studies showcase the ongoing advancements in MFC biosensor technology, highlighting innovations in cost reduction, sensitivity enhancement, and practical design improvements.
4.1.7. Innovations beyond Electricity Generation
New MFC designs are driving innovations that enable diverse applications beyond electricity generation, including wastewater treatment, resource recovery, bioremediation, and biohydrogen production. Shabani et al. [25] explored the use of MFC technology for bioremediation. They reviewed various applications of MFCs in treating contaminated environments, explaining their potential for removing pollutants such as heavy metals, hydrocarbons, and dyes while recovering valuable resources like metals and nutrients. The technical innovations included the use of specific electroactive bacterial consortia that can degrade complex pollutants while simultaneously generating electricity. Building on the concept of resource recovery, Ye et al. [163] and Baby and Ahammed [164] conducted feasibility studies on using a double-chamber MFC for nutrient recovery from municipal wastewater. Their systems utilized bioanodes coated with nitrifying bacteria to facilitate the conversion of ammonia to nitrate and biocathodes that supported denitrifying bacteria to convert nitrate to nitrogen gas. The dual functionality of these MFCs was achieved by optimizing the anode and cathode compartments to enhance both electricity generation and nutrient recovery efficiencies, thereby improving the sustainability and economic viability of wastewater management practices. In a different study, Florio et al. [23] investigated biohydrogen production from solid-phase MFC spent substrates. They explored the potential of utilizing byproducts from MFCs, such as organic sludge, for biohydrogen production via dark fermentation and other anaerobic processes. The study involved pretreating the spent substrates to enhance the bioavailability of organic matter, followed by microbial fermentation to produce hydrogen gas. This process demonstrated significant hydrogen yields, presenting a new application for MFC byproducts and contributing to the development of integrated waste-to-energy systems. These advancements are opening up new opportunities for integrating MFCs into biorefinery processes and renewable energy systems, where the efficient conversion of organic substrates into value-added products is paramount. The innovations in microbial selection, electrode materials, system optimization, and multi-functional applications illustrate the significant progress in MFC technology beyond traditional electricity generation.
4.1.8. Mixed Microbial Communities and Biofilm Formation
Research into mixed microbial communities and biofilm formation in MFCs has revealed their crucial role in enhancing electrogenic performance. Biofilms facilitate synergistic interactions between different microbial species, enabling more efficient electron transfer and substrate utilization within MFC electrodes [165]. Recent studies have focused on understanding the dynamics of microbial consortia in biofilms and engineering them for improved stability and performance in diverse environmental conditions. This knowledge is essential for optimizing MFC design and operation to maximize bioelectricity production and wastewater treatment efficiency. Liu et al. [166] investigated how ferric iron affects the microbial community structure of biofilms in MFCs. Their study revealed that ferric iron can significantly influence the composition and function of microbial communities by enhancing the growth of specific electrogenic bacteria, such as Geobacter species, and promoting more robust biofilm formation. Transitioning from the influence of specific elements on biofilm dynamics, Hassan et al. [167] explored the role of microbial consortia in degrading phenol and producing electricity in MFCs. Their research demonstrated that diverse microbial communities, including phenol-degrading bacteria and electrogenic microorganisms, could significantly improve the bioelectrochemical performance of MFCs. With regard to microbial diversity, Cao et al. [168] compared the performance of pure cultures versus mixed microbial communities in the anode of MFCs. Their study showed that mixed communities of electricigens, such as Geobacter and Shewanella species, could enhance MFC performance compared to single-species cultures. This emphasizes the benefits of microbial diversity for effective electricity generation, as different microbes can complement each other’s metabolic capabilities, leading to more efficient substrate utilization and electron transfer. Albarracin-Arias et al. [169] examined the dynamics of microbial communities and their impact on electricity generation in MFCs inoculated with palm oil mill effluent (POME) sludges and pure electrogenic cultures. Their findings highlighted how different microbial inocula affect biofilm formation and electrical output. Specifically, they found that POME sludge inocula promoted the development of diverse and resilient biofilms that enhanced MFC performance. These studies collectively advance the understanding of how mixed microbial communities and biofilm formation contribute to the optimization of MFC technology.
4.1.9. Microbial Catabolic Activities and Biocatalyst Enhancements
Microorganisms like S. oneidensis play crucial roles in enhancing MFC efficiency through their electron transfer capabilities. Recent studies have highlighted that these microorganisms can transport electrons to the electrode surface without the need for mediators, significantly enhancing power output [170]. To further optimize biocatalyst performance, researchers have explored methods such as chemically perforating cell membranes to create pores and channels, which facilitate faster electron transfer and lead to higher power densities in MFCs [147]. Additionally, genetic modifications to increase cell permeability through biosurfactant production have been shown to enhance electron transfer rates by up to 2.5 times, demonstrating significant potential for improving MFC efficiency [171]. Some recent studies on catalyst enhancements include the study by Verma and Mishra [172], who reviewed recent trends in enhancing the performance of yeast as an electrode biocatalyst in MFCs. Their study highlighted various approaches to optimize yeast-based biocatalysts, including genetic and metabolic engineering strategies that improve electron transport chains and cellular respiration rates, thereby boosting MFC efficiency. Also, Chiranjeevi and Patil [136] outlined strategies for enhancing the electroactivity and metabolic functionality of microorganisms in microbial electrochemical technologies. They discussed techniques such as electrode modification with bio-compatible conductive materials and the use of co-cultures to improve the performance of microbial biocatalysts, focusing on both fundamental and applied aspects to enhance MFC efficiency. Similarly, Ummalyma and Bhaskar [173] provided an overview of recent advances in the role of biocatalysts in biofuel cells. Their review covered the latest developments in biocatalyst technology, such as the integration of enzyme cascades and synthetic biology approaches, and emphasized improvements in biocatalyst performance for various biofuel cell technologies. Table 6 summarizes biocatalysts used in MFCs employing novel materials along their respective power densities and substrates.
| Sr. no | Electrode material | Biocatalyst | Power density (mW/m2) |
Substrate | Reference |
|---|---|---|---|---|---|
| 1 | CNT/polyaniline composites | E. coli | 42 | Glucose | [174] |
| 2 | Nitrogen-doped/CNT/rGO |
|
1,137 | — | [175] |
| 3 | 3D CNT/chitosan microchannel nanocomposites | G. sulfurreducens | 2.87 | Acetate | [176] |
| 4 |
|
E. coli | 170 | Glucose | [177] |
| 5 | Graphene oxide/nanofibers modified carbon paper | Shewanella MR−1 | 34.2 | Lactate | [178] |
| 6 | Polypyrrole/graphite oxide | S. oneidensis | 1,326 | — | [179] |
| 7 | Graphene-modified stainless steel mesh | E. coli | 2,668 | Lactate | [178] |
- Source: [145].
Table 6 exemplifies significant advancements in MFC technology, particularly highlighting the enhancements in biocatalysts and electrode materials. The use of CNTs combined with various materials like polyaniline, nitrogen-doped CNT, and reduced graphene oxide (rGO) showcases how these modifications lead to substantial increases in power density. For instance, the incorporation of nitrogen-doped CNT/rGO with dual biocatalysts such as E. coli and S. putrefaciens achieves a power density of 1,137 mW/m2, emphasizing the potential of mixed microbial cultures to optimize performance. Further, the integration of three-dimensional CNT structures with chitosan microchannels, nano-molybdenum carbide, and graphene oxide-modified carbon paper demonstrates the role of advanced nanomaterials in enhancing microbial adhesion and electron transfer. Notably, the highest power density of 2,668 mW/m2 is achieved using graphene-modified stainless-steel mesh with E. coli, highlighting the exceptional conductive properties of graphene and its capacity to form efficient bioelectrochemical interfaces. These innovations indicate a shift towards more efficient, cost-effective, and sustainable MFC systems, driven by the synergistic effects of advanced materials and optimized biocatalyst configurations.
4.1.10. Proton Transfer Membrane (PTM) Innovations
Recent advancements in PTMs for MFCs have focused on enhancing power density and efficiency. Traditional polymeric materials like Nafion, known for their high ionic conductivity and uniform porosity, are costly and have performance limitations [179]. Emerging alternatives include non-Nafion membranes such as polyester cloth and bipolar membranes, which utilize a bilayer cation-exchange layer attached to an anion-exchange layer for improved pH neutrality and performance [180]. Additionally, the development of mediator-less MFCs using metal-reducing bacteria like Shewanella promises cheaper and environmentally friendly energy generation without the need for exogenous electron carriers [181]. Studies by Sirajudeen et al. [182] explored the innovative use of biopolymer composites as proton exchange membranes in MFCs. Their work demonstrated the effectiveness of these biopolymer membranes in generating electricity from real wastewater, showcasing a significant improvement in power density and efficiency compared to traditional materials. Building on this, Neethu et al. [183] developed a novel proton exchange membrane using clay and activated carbon derived from coconut shells. This innovative approach not only enhanced the membrane’s performance but also contributed to sustainability by utilizing low-cost, readily available materials. Their findings showed marked improvements in the overall performance of MFCs, demonstrating the viability of alternative natural materials in MFC applications. Further advancements were made by Saniei et al. [184], who employed a new sulfonated poly (ether ether ketone)-based proton exchange membrane. This membrane was modified with goethite nanoparticles functionalized with tannic acid and sulfanilic acid to enhance performance. The transition from traditional polymeric materials to biopolymers, natural materials, and advanced nanocomposites marks a significant shift towards more efficient, cost-effective, and sustainable PTM technologies for MFCs.
4.2. Summary of Challenges and Future Directions in MFC Technology
4.2.1. Summary of Challenges in MFC Technology
As earlier discussed, cost remains a major barrier to large-scale deployment of MFC technology. Key cost drivers include expensive materials like platinum for catalysts, ion exchange membranes such as Nafion, and manufacturing processes. Researchers are actively exploring cost-effective alternatives, such as non-precious metal catalysts and polymer-based membranes, to reduce initial investment and operational costs [185]. Scaling up MFCs from laboratory prototypes to practical applications also poses significant challenges. Issues such as maintaining consistent performance across larger systems, optimizing electrode designs for efficient mass transfer, and managing biofilm formation and stability at industrial scales need to be addressed. Advances in reactor design and engineering are critical to achieving scalability without compromising performance [186]. Furthermore, MFC performance can be sensitive to environmental factors such as temperature, pH, and substrate composition. Variations in these parameters can affect microbial activity, electrode performance, and overall energy output. Research focuses on developing robust MFC systems that operate reliably under varying environmental conditions, including extreme temperatures and fluctuating organic loads in wastewater streams [187].
4.2.2. Future Directions in MFC Research
Continued exploration of novel materials, including nanomaterials, conductive polymers, and bio-derived materials, holds promise for improving MFC performance and reducing costs. Advanced manufacturing techniques such as 3D printing and roll-to-roll processing are being investigated to enhance electrode fabrication and assembly processes, enabling scalable production of MFC components [188]. Efforts are underway to integrate MFCs into existing WWTPs and decentralized treatment systems [189]. This integration not only enhances organic pollutant removal efficiency but also generates renewable energy from wastewater streams. Research focuses on optimizing MFC design and operation to maximize energy recovery while meeting effluent quality standards set by regulatory agencies. Beyond electricity generation, MFCs show potential for nutrient recovery and chemical synthesis. Nutrient recovery technologies using MFCs aim to capture and recycle valuable nutrients such as phosphorus and nitrogen from wastewater, contributing to sustainable agricultural practices and reducing nutrient pollution in water bodies. Additionally, MFCs can be tailored for biochemical production through microbial synthesis of value-added chemicals and fuels, expanding their role in biorefinery applications [190].
5. Conclusion
MFCs offer promising potential for sustainable energy generation and environmental applications. The double-chamber configuration of MFCs is generally preferred due to its enhanced performance controllability, enabling better pH regulation, increased flow rates, and the addition of electron mediators at the cathode. These factors collectively facilitate efficient energy generation. Microorganisms within MFCs degrade substrates to produce electrons and protons, which traverse an external circuit to generate electricity, quantifiable by a load and measured with a multimeter. The chemical reactions in the anode and cathode chambers, coupled with substrate decomposition by microorganisms, yield ATP and electrons. Advancements in MFC technology have led to significant improvements in energy efficiency and power output. For example, a hybrid system utilizing SOFCs and gas turbines, driven by syngas from biomass gasification, achieved approximately 63% improved electric efficiency and substantial energy savings. In wastewater treatment, MFCs produce 50%–90% less excess sludge, presenting a cost-effective solution. The use of acetate as a substrate in bio-electrochemical systems has been shown to achieve up to 53% hydrogen production. Further enhancements in MFC performance include treating microorganisms with pancreatic enzyme replacement therapy to create pores and channels on cell membranes, facilitating microbial attachment to the anode surface, and improving substrate degradation. Additionally, the use of advanced materials such as CNTs and graphene for anodic surface pretreatment or coating significantly boosts surface charge density. For instance, S. putrefaciens, using a hierarchical porous carbon–silica composite anode, achieved a maximum power density of 580.7 mW/m2, 4.5 times higher than conventional anodes. The total potential difference in typical MFCs is confirmed to be 1.24 V. Substrate selection is crucial, as evidenced by acetate achieving a maximum power density of 53.4 mW/m2, while sucrose exhibited the highest peak power density, indicating substrate-specific performance variability. Electrogenic microbes like G. sulfurreducens have been observed to produce up to 2.5 W/m2, significantly outperforming other microbes. Optimal conditions, such as a neutral pH of 7 and temperatures around 30°C, yield the highest power densities, peaking at approximately 1,600 mW/m2. Innovative approaches, such as modifying electrode materials and optimizing operational conditions, have further enhanced MFC performance. For example, NH3 gas-treated carbon cloth has achieved power densities of up to 1,970 mW/m2. The integration of advanced materials, including nitrogen-doped CNT and graphene oxide, has led to the highest recorded power density reaching 2,668 mW/m2. These innovations highlight the critical importance of optimizing microbial, material, and operational parameters to fully realize the potential of MFCs in sustainable energy generation and environmental applications. Despite the promising advancements, challenges such as the high cost of MFC technology persist. However, continued research and development, particularly in nanomaterial modifications, offer avenues to mitigate these challenges and enhance the overall efficiency and feasibility of MFCs.
Conflicts of Interest
The authors declare that there are no conflicts of interest with respect to the publication of this manuscript.
Open Research
Data Availability
Data supporting the findings of this study are available on request from the authors. Contact Matthew Kwofie at [email protected] for access to the data used in this investigation.




