An Observational Study of Early Treatment Response and Predictors of Dupilumab in the Treatment of Moderate-to-Severe Atopic Dermatitis
Abstract
Background. Dupilumab has shown good effectiveness and safety in patients with moderate-to-severe atopic dermatitis. However, there is a lack of clinical data that focuses solely on the treatment response for the endpoint to observe the short-term goal (12 weeks) in the treatment-to-target (T2T) concept. Study on predictors of early treatment response is also limited. Objective. To evaluate the early effectiveness and safety of dupilumab in the treatment of moderate-to-severe atopic dermatitis and to identify possible predictors of response. Methods. Using a retrospective study method, patients with moderate-to-severe atopic dermatitis who received dupilumab for ≥12 weeks at the Southwest Hospital between September 2019 and April 2022 were included. Results. Totally 16.25% of patients achieved EASI75 after 4 weeks and 53.75% achieved EASI75 after 12 weeks. SCORAD, EASI, and NRS were 50.17 ± 17.35, 13.51 ± 12.33 and 7.10 ± 1.82 in turn at baseline, and decreased to 29.94 ± 15.01, 6.97 ± 7.92, 3.64 ± 1.39 and 14.96 ± 10.31, 3.05 ± 4.16, 2.19 ± 1.09 after 4 and 12 weeks, respectively, with statistically significant differences (p < 0.01). After 12 weeks, the changes in peripheral blood eosinophil count (decreased from (0.60 ± 0.43) ∗ 10^9/L to (0.30 ± 0.21) ∗ 10^9/L), total IgE level (decreased from 547.00 (179.00, 2167.50) IU/ml to 216.50 (106.00, 825.00) IU/ml), and LDH (decreased from (166.11 ± 171.59) IU/L to (67.54 ± 70.68) IU/L) from baseline were significant (p < 0.01). Elevated peripheral blood eosinophil counts might be associated with an inadequate response to dupilumab (SCORAD12w: p = 0.007; EASI12w: p = 0.003; NRS12w: p = 0.030). The most common adverse events were reactions at the injection site (6/80) and conjunctivitis (4/80). Conclusion. Dupilumab showed good early effectiveness and safety in real-world practice in Chinese patients with moderate-to-severe atopic dermatitis.
1. Introduction
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease that seriously affects the quality of life of patients. Diagnosis of atypical AD phenotypes in adults represents a challenge for the dermatologist. What should be of concern is that moderate-to-severe AD with extensive skin lesions and treatment resistance has always been a challenge for the dermatologist too, and it requires systemic treatment. Traditional systemic drugs include immunosuppressive agents, and glucocorticoids. However, with many adverse reactions, patients must be closely monitored, above all in special populations. During clinical application, doctors and patients have many concerns. In this context, dupilumab came into being. As a fully human monoclonal antibody that inhibits the IL-4/IL-13 signaling pathway by blocking the alpha subunit of the IL-4 receptor [1], dupilumab was approved by the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and National Medical Products Administration (NMPA) for the treatment of moderate-to-severe AD patients who were poorly controlled with topical medication or not recommended to use topical medication.
AD is considered a heterogeneous disease with different clinical manifestations, severities, and different or mixed inflammatory infiltration patterns [2, 3], which brings great challenges to the treatment [4]. It has been speculated that ethnicity may have an impact on the effectiveness of dupilumab, as AD in Asians has been reported to be dominated by mixed Th2/Th17 inflammation [5]. Compared with the data in Caucasians, the data on the use of dupilumab in Asians, especially in Chinese, are still very limited. The main focus is on the long-term efficacy and safety at 16 weeks and above [6–8], and there is a lack of clinical data focusing solely on early treatment responses, while the response rate of early treatment will affect the patient’s subsequent compliance. Understanding the early treatment response and its possible influencing factors is not only helpful for objectively evaluating treatment efficacy and screening clinical characteristics of patients more suitable to receive dupilumab but also of great significance in improving patient compliance, adjusting treatment regimens, and alleviating the condition. The treatment-to-target (T2T) concept for AD was proposed recently, in which 12 weeks is the endpoint to observe the short-term goal (remission-induction therapy) [9].
Our goal is to share the experience of using dupilumab in China’s tertiary hospitals, evaluate early effectiveness and safety, identify possible predictors of response, and provide reference for clinical treatment. This project has passed the ethical review, and the batch number was (B)KY202277.
2. Materials and Methods
2.1. Patients
We retrospectively screened moderate-to-severe AD patients treated with dupilumab at the Southwest Hospital in Chongqing between September 2019 and April 2022. Inclusion criteria: (1) score of scoring atopic dermatitis (SCORAD) at baseline ≥25 points; (2) duration of treatment with dupilumab ≥12 weeks; and (3) complete data for each score at 4 weeks and 12 weeks. All included patients had discontinued systemic glucocorticoids and immunosuppressive agents at least 2 weeks before the start of using dupilumab and could receive concurrent topical glucocorticoids or calcineurin inhibitors.
2.2. Prescription Method
Adults started with a loading dose of 600 mg, followed by a maintenance dose of 300 mg once every 2 weeks. The first dose and maintenance dose for adolescents and children were determined based on the body weight and age: body weight ≥60 kg: started with a loading dose of 600 mg, followed by a maintenance dose of 300 mg once every 2 weeks; body weight ranging from 30 kg to 60 kg: started with a loading dose of 600 mg, followed by a maintenance of 300 mg once every 2 or 4 weeks if only the strength of 300 mg was available in the hospital, or started with a loading dose of 400 mg, followed by a maintenance dose of 200 mg once every 2 weeks if both the strength of 300 mg and 200 mg were available in the hospital; body weight ranging from 15 kg to less than 30 kg: patients aged 6–17 years old started with a loading dose of 600 mg, followed by 300 mg once every 4 weeks, and those aged 6 months to 5 years started with 300 mg, followed by 300 mg once every 4 weeks; body weight ranging from 5 kg to less than 15 kg: started with 200 mg, followed by 200 mg once every 4 weeks.
2.3. Data Collection
The following data were collected at baseline: sex, age, age at onset of AD, concurrent personal and/or familial history of atopic diseases, peripheral blood eosinophil count, total serum IgE, allergen-specific IgE, lactate dehydrogenase (LDH), concomitant use of topical glucocorticoids and/or calcineurin inhibitors, concomitant use of dust mite desensitization therapy, previous treatment regimen and scores of SCORAD, eczema area and severity index (EASI), and numeric rating scale of itching (NRS). The following data were also collected at 4 weeks and 12 weeks (if any): peripheral blood eosinophil count, serum total IgE, allergen-specific IgE, LDH, and scores of SCORAD, EASI, and NRS. Adverse events (AEs) were also collected and were defined as occurrences of any untoward medical condition during the treatment period.
2.4. Statistical Analysis
Statistical analysis was performed using SPSS 21.0 software. Categorical variables were reported as frequencies and percentages. For continuous variables, the normally distributed measurement data were expressed as x ± s, using t test for intergroup comparison; the non-normally distributed measurement data were expressed as M (P25, P75), using the rank sum test. The chi-square test was performed for intergroup comparison of enumeration data. The influencing factors of effectiveness were analyzed by multiple linear regression analysis and correlation analysis. Independent variables with relatively complete data, such as gender and age, were tested with multiple linear regression analysis, and independent variables with plenty of missing data (LDH level at baseline and concurrent use of dust mite desensitization therapy) were tested with correlation analysis. p < 0.05 indicated that the difference was statistically significant.
3. Results
3.1. Demographic and Clinical Characteristics
A total of 80 patients met the inclusion criteria. The majority were male, with a total of 54 cases (67.5%). The youngest was 2 years old (2 cases whose parents had signed the informed consent), all the rest were ≥6 years old, and the oldest was 80 years old. There were 19 cases (23.8%) in the child and adolescent group aged 2 to 18 years, and 61 cases (76.2%) in the adult group aged ≥18 years. The cut-off value between early-onset AD and adult-onset AD was 18 years, and there were 45 cases (65.3%) of early-onset AD. The most common comorbid individual atopic disease was allergic rhinitis, with a total of 24 cases (30.0%). Eosinophilia was defined as an eosinophil count >500 × 10^6/L, and a total of 39 patients (48.8%) had eosinophilia at baseline. High IgE level was defined as serum total IgE >1,000 IU/ml, and a total of 23 patients (31.9%) had high IgE level at baseline. Extrinsic AD (EAD) was defined as total IgE >150 IU/ml and/or positive allergen-specific IgE (sIgE) (grade 2 or above), whereas intrinsic AD (IAD), and a total of 72 cases (93.5%) had EAD. All patients received oral antihistamines and topical glucocorticoids or calcineurin inhibitors, 34 patients (42.5%) received compound glycyrrhizin, 6 patients (7.5%) received tripterygium glycosides, 8 patients (10.0%) received systemic glucocorticoids, 18 patients (22.5%) received immunosuppressive therapy (including 16 patients (20.0%) with cyclosporine and 2 patients (2.5%) with methotrexate), 2 patients (2.5%) received phototherapy (NB-UVB), and 18 patients (22.5%) received traditional Chinese medicine bath therapy. Moreover, a total of 56 patients (70.0%) were treated with topical glucocorticoids or calcineurin inhibitors, and 5 patients (6.3%) received dust mite desensitization therapy, as shown in Table 1.
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Sex, male | 54/80 (67.5) |
| Age, years old | 30.6 ± 19.8 |
| Children and adolescents | 19/80 (23.8) |
| Adults | 61/80 (76.2) |
| Early onset | 45/80 (65.3) |
| Family history of allergy | 13/80 (16.3) |
| Allergic comorbidities | |
| Allergic rhinitis | 24/80 (30.0) |
| Asthma | 8/80 (10.0) |
| Allergic conjunctivitis | 3/80 (3.8) |
| Food allergy | 5/80 (6.3) |
| Comorbidities | |
| Hypertension | 4/80 (5.0) |
| Type 2 diabetes | 1/80 (1.3) |
| Hyperthyroidism | 1/80 (1.3) |
| Chronic hepatitis B infection | 2/80 (2.5) |
| Polycystic ovarian syndrome | 1/80 (1.3) |
| Amyopathic dermatomyositis | 1/80 (1.3) |
| Baseline eosinophilia | 39/80 (48.8) |
| Baseline high IgE levels | 23/72 (31.9) |
| Dust mite grade 5 or above | 29/62 (46.8) |
| Baseline elevated LDH | 18/36 (50.0) |
| EAD | 72/77 (93.5) |
| Previous treatment | |
| Topical therapies | 80 (100.0) |
| Antihistamine | 80 (100.0) |
| Compound glycyrrhizin | 34 (42.5) |
| Tripterygium glycosides | 6 (7.5) |
| Systemic corticosteroids | 8 (10.0) |
| Ciclosporin | 16 (20.0) |
| Methotrexate | 2 (2.5) |
| Phototherapy(NB-UVB) | 2 (2.5) |
| Traditional Chinese medicine bath | 18 (22.5) |
| Topical therapies simultaneously | 56/80 (70.0) |
| Desensitization therapy | 5/80 (6.3) |
- AD, atopic dermatitis; SD, standard deviation; LDH, lactate dehydrogenase; EAD, exogenous atopic dermatitis; NB-UVB, narrow-band ultraviolet B.
3.2. Treatment Outcome and Clinical Findings
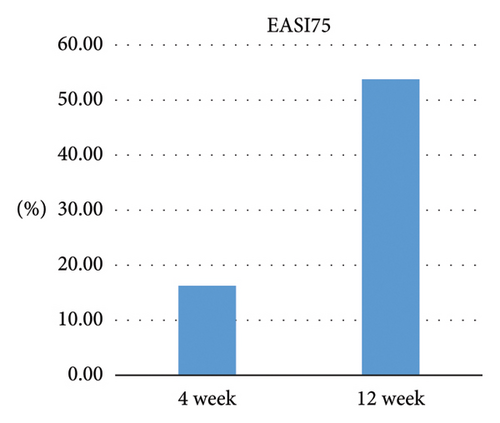
After 4 weeks of treatment with dupilumab, 16.25% (13/80) of all patients achieved EASI75, 15.79% (3/19) of children and adolescents achieved EASI75, and 16.39% (10/61) of adults achieved EASI75, indicating no significant difference between the two age groups (Z = −0.062, p = 0.950). After 12 weeks of treatment with dupilumab, 53.75% (43/80) of all patients achieved EASI75, 47.37% (9/19) of children and adolescents achieved EASI75, and 55.74% (34/61) of adults achieved EASI75, indicating no significant difference between the two age groups (Z = −0.639, p = 0.523), as shown in Figure 1.
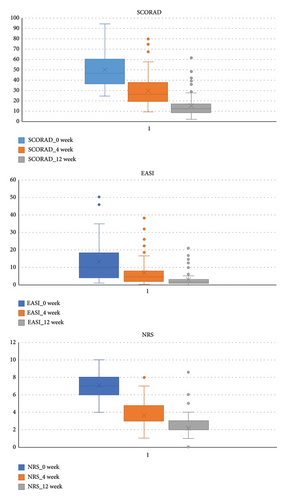
At baseline, the SCORAD score was 50.17 ± 17.35, the EASI score was 13.51 ± 12.33, and the NRS score was 7.10 ± 1.82. After treatment with dupilumab, all the three scores were significantly decreased, with 29.94 ± 15.01, 6.97 ± 7.92, 3.64 ± 1.39, and 14.96 ± 10.31, 3.05 ± 4.16, 2.19 ± 1.09 at 4 weeks and 12 weeks, respectively, and all the differences were statistically significant (p < 0.01). There were no significant differences in the changes of the three scores between the two age groups (children and adolescents vs. adults) (p > 0.05). Laboratory test results at baseline and 12 weeks showed significant changes in peripheral blood eosinophil count (decreased from (0.60 ± 0.43) ∗ 10^9/L to (0.30 ± 0.21) ∗ 10^9/L), total IgE level (decreased from 547.00 (179.00, 2167.50) IU/ml to 216.50 (106.00, 825.00) IU/ml), and LDH (decreased from (166.11 ± 171.59) IU/L to (67.54 ± 70.68) IU/L) (p < 0.01), as shown in Table 2 and Figure 2.
| SCORAD | EASI | NRS | Eosinophils count (10^9/L) | IgE (IU/ml) | LDH (IU/L) | |
|---|---|---|---|---|---|---|
| Baseline | 50.17 ± 17.35 | 13.51 ± 12.33 | 7.10 ± 1.82 | 0.60 ± 0.43 | 547.00 (179.00, 2167.50) | 166.11 ± 171.59 |
| 4 weeks | 29.94 ± 15.01 | 6.97 ± 7.92 | 3.64 ± 1.39 | |||
| 12 weeks | 14.96 ± 10.31 | 3.05 ± 4.16 | 2.19 ± 1.09 | 0.30 ± 0.21 | 216.50 (106.00, 825.00) | 67.54 ± 70.68 |
| p | 0.000 | 0.000 | 0.001 | 0.000 | 0.006 | 0.000 |
- SCORAD, scoring atopic dermatitis; EASI, eczema area and severity index; NRS, numeric rating scale; IgE, immunoglobulin E; LDH, lactate dehydrogenase.
To analyze whether changes in the peripheral blood eosinophil count and the total IgE levels were associated with SCORAD, EASI, and NRS scores, we divided 80 patients into groups based on changes (elevation or decrease) in peripheral blood eosinophil counts and total IgE levels at 12 weeks from baseline and evaluated the difference in the effectiveness. The results showed that at 12 weeks, each score in the group with peripheral blood eosinophil count decreased were improved more significantly than the group with peripheral blood eosinophil count elevated, with a statistically significant difference (p < 0.05), and that the change in total IgE level was unrelated to the three scores, as shown in Tables 3 and 4.
| SCORAD4 (w) | EASI4 (w) | NRS4 (w) | SCORAD12 (w) | EASI12 (w) | NRS12 (w) | |
|---|---|---|---|---|---|---|
| Elevated Eos group (N = 22) | 0.32 ± 0.19 | 0.36 ± 0.26 | 0.40 ± 0.21 | 0.62 ± 0.15 | 0.66 ± 0.18 | 0.61 ± 0.16 |
| Decreased Eos group (N = 58) | 0.43 ± 0.18 | 0.53 ± 0.23 | 0.48 ± 0.21 | 0.73 ± 0.14 | 0.78 ± 0.15 | 0.70 ± 0.16 |
| df | 78 | 78 | 78 | 78 | 78 | 78 |
| t | 2.415 | 3.364 | 1.529 | 2.746 | 3.077 | 2.209 |
| p | 0.018 | 0.001 | 0.130 | 0.007 | 0.003 | 0.030 |
- SCORAD, scoring atopic dermatitis; EASI, eczema area and severity index; NRS, numeric rating scale; Eos, eosinophil count; w, weeks.
| SCORAD4 (w) | EASI4 (w) | NRS4 (w) | SCORAD12 (w) | EASI12 (w) | NRS12 (w) | |
|---|---|---|---|---|---|---|
| Elevated IgE group (N = 21) | 0.41 ± 0.20 | 0.46 ± 0.25 | 0.40 ± 0.22 | 0.72 ± 0.16 | 0.75 ± 0.17 | 0.69 ± 0.21 |
| Decreased IgE group (N = 51) | 0.40 ± 0.18 | 0.50 ± 0.25 | 0.50 ± 0.20 | 0.69 ± 0.15 | 0.76 ± 0.16 | 0.68 ± 0.15 |
| df | 70 | 70 | 70 | 70 | 70 | 70 |
| t | −0.066 | 0.603 | 1.705 | −0.806 | 0.292 | −0.198 |
| p | 0.947 | 0.548 | 0.093 | 0.423 | 0.771 | 0.844 |
- SCORAD, scoring atopic dermatitis; EASI, eczema area and severity index; NRS, numeric rating scale; IgE, immunoglobulin E; w, weeks.
3.3. Predictors of Response
In order to analyze whether relevant factors can predict early treatment response, we used multiple linear regression analysis and correlation analysis to evaluate the influencing factors of effectiveness. Independent variables included sex, age, age of onset, course of disease, family history of atopic disease, individual history of atopic disease, peripheral blood eosinophil count at baseline, total IgE level at baseline, LDH level at baseline, the type of AD (EAD or not), previous use of immunosuppressive agents, concurrent use of topical glucocorticoids or calcineurin inhibitors, dust mite grade 5 or above at baseline, and concurrent use of dust mite desensitization therapy, and the dependent variable was the improvement in each score, that is, the difference at 4 weeks and 12 weeks from baseline, respectively. Improvements in SCORAD, EASI, and NRS scores at weeks 4 and 12 were not associated with any of the above factors, as shown in Tables 5 and 6.
| SCORAD4 (w) | EASI4 (w) | NRS4 (w) | SCORAD12 (w) | EASI12 (w) | NRS12 (w) | |
|---|---|---|---|---|---|---|
| Sex | 0.005 (0.102) | −0.015 (−0.222) | 0.107 (1.898) | −0.025 (−0.608) | −0.026 (−0.583) | 0.022 (0.466) |
| Age | 0.010 (0.703) | 0.016 (0.830) | 0.018 (1.110) | −0.002 (−0.139) | 0.007 (0.597) | 0.014 (1.055) |
| Age of onset | −0.011 (−0.758) | −0.016 (−0.841) | −0.017 (−1.056) | 0.001 (0.087) | −0.008 (−0.645) | −0.014 (−1.040) |
| Course of the disease | −0.008 (−0.548) | −0.012 (−0.586) | −0.018 (−1.089) | 0.004 (0.304) | −0.002 (−0.141) | −0.011 (−0.811) |
| Familial atopic disease | −0.059 (−0.820) | −0.172 (−1.824) | −0.021 (−0.276) | 0.005 (0.087) | −0.028 (−0.461) | −0.031 (−0.481) |
| Individual atopic disease | −0.039 (−0.756) | −0.017 (−0.252) | −0.011 (−0.192) | −0.000 (−0.002) | 0.003 (0.078) | 0.028 (0.602) |
| Eosinophil count at baseline | −0.005 (−0.081) | 0.044 (0.593) | −0.098 (−1.596) | 0.032 (0.706) | 0.053 (1.103) | −0.012 (−0.230) |
| Total IgE level at baseline | −0.000 (−0.276) | 0.000 (0.567) | 0.000 (0.827) | 0.000 (1.225) | 0.000 (1.235) | 0.000 (0.375) |
| EAD | 0.184 (1.753) | 0.087 (0.629) | 0.138 (1.216) | 0.058 (0.693) | 0.078 (0.873) | 0.004 (0.044) |
| Immunosuppressive agents | −0.020 (−0.363) | −0.031 (−0.419) | −0.012 (−0.194) | −0.074 (−1.652) | −0.008 −0.170) | −0.082 (−1.635) |
| TCS/TCI | 0.063 (1.161) | 0.045 (0.628) | 0.055 (0.934) | −0.016 (−0.376) | −0.034 (−0.737) | 0.056 (1.139) |
| N | 71 | 71 | 71 | 71 | 71 | 71 |
| R2 | 0.120 | 0.111 | 0.159 | 0.120 | 0.176 | 0.108 |
| Adjusted R2 | −0.045 | −0.055 | 0.002 | −0.044 | 0.023 | −0.058 |
| F | 0.728 | 0.671 | 1.014 | 0.731 | 1.147 | 0.648 |
| p | 0.707 | 0.760 | 0.446 | 0.704 | 0.343 | 0.780 |
- SCORAD, scoring atopic dermatitis; EASI, eczema area and severity index; NRS, numeric rating scale; IgE, immunoglobulin E; EAD, extrinsic atopic dermatitis; TCS, topical corticosteroids; TCI, topical calcineurin inhibitors.
| SCORAD4 (w) | EASI4 (w) | NRS4 (w) | SCORAD12 (w) | EASI12 (w) | NRS12 (w) | |
|---|---|---|---|---|---|---|
| LDH level at baseline | −0.196 (0.252) | −0.160 (0.351) | −0.155 (0.368) | −0.016 (0.926) | −0.046 (0.792) | −0.139 (0.418) |
| Desensitization therapy | 0.073 (0.708) | 0.206 (0.283) | 0.028 (0.887) | 0.364 (0.052) | 0.268 (0.160) | 0.306 (0.107) |
- SCORAD, scoring atopic dermatitis; EASI, eczema area and severity index; NRS, numeric rating scale; IgE, immunoglobulin E; LDH, lactate dehydrogenase.
3.4. Safety
Overall, adverse events (AEs) were reported in 17.5% (14/80) of patients. The most common AEs were reactions at the injection site (6/80) and conjunctivitis (4/80). Other AEs included upper respiratory tract infection (1/80), aggravated itching (1/80), facial erythema (1/80), and headache (1/80). All symptoms were relieved or disappeared after symptomatic treatment or observation, and no patient discontinued the administration of dupilumab due to the occurrence of AEs.
4. Discussion
As 12 weeks is the endpoint to observe the short-term goal (remission-induction therapy) in the recently proposed treatment-to-target (T2T) concept for atopic dermatitis [8], we also set the “early” time node here in this study. The study focuses on early treatment response, and the age span of the included patients is large (2–80 years old). Some patients’ medication methods are different from standard drug clinical trials, which better reflects the real-world situation in Southwest China.
Overall, this study showed that dupilumab has good early effectiveness and safety in the treatment in Chinese patients with moderate-to-severe AD. After 12 weeks of treatment, 53.75% of the 80 patients achieved EASI75, which is comparable to the proportions reported in real-world studies in France (49.0%) [10], Germany (57.0%) [11], and Denmark (63.0%) [12] as well as the proportion in a previous meta-analysis of real-world studies (59.0%) [13]. This showed that although AD in Asian was reported to be dominated by mixed Th2/Th17 inflammation, theoretically the effect of clinical use of dupilumab against Th2 inflammation may not be as good as that in European and American populations, and there was no significant difference in the effect in real-world practice. The reason may be that regardless of the immune response mode, the root cause of AD is still associated with Th2 inflammation.
A significant decrease in total IgE and LDH levels after 12 weeks of treatment with dupilumab was observed, which is consistent with the results obtained in previous studies [10–14]. The change in the total IgE level was not associated with any of the three scores. Although the serum total IgE level is used most commonly in clinical trials of AD among indexes currently available, and patients with severe disease also tend to have higher serum total IgE levels, the correlation is not completely linear, and some studies even suggested that the serum total IgE level is only a “bystander” of AD, which is uncorrelated or only weakly correlated with the severity of the disease [15]. The decreased serum total IgE level in patients receiving dupilumab might be caused by the inhibitory effect of dupilumab on the IL-4 and IL-13 signaling pathways that drive the generation of IgE [16]. Since only patients with elevated baseline LDH levels were re-examined after 12 weeks and all the examinations showed a decreased level, the relationship between the change in the LDH level and improvements in the three scores was not explored. We also found that the overall peripheral blood eosinophil count was significantly reduced after treatment and that patients with decreased eosinophil counts had more pronounced improvements in SCORAD, EASI, and NRS scores than those with elevated eosinophil counts. Although current studies generally showed that the peripheral blood eosinophil count was related to the severity of AD [15], and there was similar report [17], no significant change in the eosinophil count after treatment was observed in many real-world studies [18–21], and there was even a report in a real-world study that the proportion of patients with eosinophilia was significantly increased after 16 weeks of treatment with dupilumab relative to that at baseline (43% vs. 31%) [18]. It was speculated that the result might be related to the sample size, race, and individual differences in response to dupilumab.
Scholars at home and abroad have long been committed to finding possible predictors for the effectiveness of dupilumab, and predictors such as sex, age, body weight, peripheral blood eosinophil count, and LDH level have been reported [9, 22–25]. This study only showed that elevated peripheral blood eosinophil counts might be associated with an inadequate response to dupilumab, which is presumably because our attention was limited to the early stage, but there were similar reports [22]. Our study is consistent with the finding in a growing body of studies that the response to dupilumab in AD patients was independent of the total IgE level at baseline [15], and our study also revealed that peripheral blood eosinophil count and LDH, which are biomarkers generally believed to be associated with the severity of AD, were also not associated with the response to dupilumab in AD patients, which is consistent with the finding in some real-world studies [26].
In terms of adverse reactions, a total of 14 patients reported AEs. The most common AEs were reactions at the injection site and conjunctivitis. Other rare AEs included upper respiratory tract infection, herpes simplex, facial erythema, and headache, which are basically consistent with AEs reported in previous clinical studies and real-world studies [12]. All the AEs were mild, and no patient discontinued the administration due to the occurrence of AEs. Conjunctivitis is the AE reported most frequently, with an incidence of 5% to 28% in clinical trials [14–17] and 6% to 62% in real-world studies [13, 27–29]. The incidence of conjunctivitis in this study was 5%, which is at a low level. Currently, the mechanism of dupilumab in inducing conjunctivitis is unclear. The underlying mechanism involves Demodex colonization, which may drive IL-17-mediated inflammatory responses and lead to increased eosinophil counts [30]. Since AD in Asians is dominated by mixed Th2/Th17 inflammation, the increase in IL-17-mediated inflammatory response may not be obvious, which may explain why the incidence of conjunctivitis in real-world studies in China is generally lower than that in Europe and America [10–13, 18, 26–29, 31]. Increased eosinophil counts have resulted from increased levels of eosinophilic factors (such as eotaxin-1 and eotaxin-2) associated with eosinophil recruitment observed in mucus and tear samples from patients with atopic keratoconjunctivitis, as well as increased activity of ligands involved in atopic keratoconjunctivitis, such as OX40 ligands [29].
5. Conclusion
Our study showed that dupilumab had favorable early effectiveness and safety in real-world practice in Chinese patients with moderate-to-severe AD, similar to the results of previous RCTs and real-world studies, and was not affected by the immune reaction mode of AD in Asians. Elevated peripheral blood eosinophil counts might be associated with an inadequate response to dupilumab. Given the importance of early treatment response, studies with a larger sample size are still needed in the future to further explore the application of dupilumab in the early treatment of AD and relevant factors affecting its effectiveness.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Sisi Deng and Huan Wang contributed equally to this study.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81673059 and 82073442).
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.