Synthesis, Characterization, and Applications of ZnO, Ag2O, and ZnO/Ag2O Nanocomposites: A Review
Abstract
Nanoscale dimensional materials represent a captivating area of research in modern technology, owing to their unique properties and vast potential applications. The distinct advantage of nanosized materials lies in their large surface area to volume ratio, which endows them with enhanced stability and significant functional characteristics. Among these, metal oxide nanomaterials have garnered significant attention due to their substantial energy bandgaps and high exciton binding energies, making them suitable for a wide range of applications, including antimicrobial activity and optoelectronics. This review delves into the synthesis methods of various metal oxides, emphasizing the advantages and drawbacks of physical, chemical, and biological approaches. Specifically, it focuses on the synthesis, characterization, and applications of single metal oxide nanoparticles (NPs), such as zinc oxide (ZnO) and silver oxide (Ag2O), as well as binary or composite metal oxides like ZnO/Ag2O nanocomposites. Through this comprehensive literature review, readers will gain insights into the fabrication techniques and explore the intrinsic properties of both single and composite metal oxide NPs, thereby broadening their understanding of these innovative materials and their potential technological impacts.
1. Introduction
Nanotechnology is well-defined as the study of deploying matter on the atomic as well as molecular scales. Generally, nanotechnology deals with structures whose sizes diverge to between 1 and 100 nm in one dimension, at least that comprise emerging materials with one dimension in size ranges [1–3]. It covers numerous areas that are fluctuating from conservative devices to totally novel methodologies grounded on molecular self-assembly, from emerging materials with dimension of the nanoscales to researching out whether they can control matters on small scales. It can create a number of new materials within a huge range of claims, like in medication, biomaterials (materials which are used to replace damaged body parts in sick people), optoelectronics, as well as production of energy types of energy [4, 5]. Conversely, nanotechnology raises numerous concerns about poisonousness the impacts of the nanomaterials on the atmosphere, as well as their influences on the universal financial side [6]. Nanomaterials have extended consideration in research due to their notable performances in electricity, nanophotonics, and optics [7–9]. Researchers have recognized the significant influence that crystal size has on the physical, biological, and chemical properties of nanomaterials [10–12]. The shapes of nanoscaled materials might be one-dimensional (1D), two-dimensional, as well as three-dimensional (3D) [13].
Through the establishment of the literature review being described here, science direct database is used. After inserting the keywords in advance search, the authors filtered through the very recent 5 years of reported papers, 2019–2024, review articles and research articles, including subject areas (material science, chemical engineering, physics and astronomy, engineering, biochemistry, genetics and biomolecules, pharmacology, toxicology, and pharmaceutical) with open access 133 papers existed; from these 11 of them are very close to present study. According to this literature, this search can be classified into three. These are the physical, chemical, and green synthesis methods used for the preparation of zinc oxide-silver oxide (ZnO–Ag2O) nanocomposite materials, which were identified after searching for each nanoparticle (NP) using these specific keywords. Generally, NPs can be synthesized through two techniques that have been recommended for NPs preparation: bottom-up and top-down approaches. The top-down approach comprises milling or abrasion of huge macroscopic particles. It includes preparing large-scale forms primarily and dropping them to nanosized levels via plastic deformations. This method cannot be labored for bulky scale manufacture of NPs due to its expensiveness and dawdling procedure [14–16]. Interferometry lithographic is the furthermost collective method which pays plays the role of top to down approaches for nanomaterial synthesization [17]. This method comprises the preparation of NP from already reduced atomic constituents via self-assembly. This includes formation via physical as well as chemical methods. It is a moderately cost-effective method [18].
2. Various Techniques in NP Synthesization
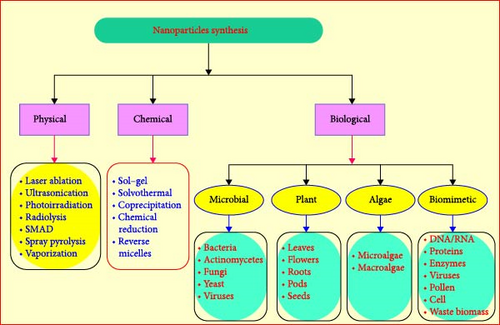
In physical approaches, physical powers are intricate in the fascination of nanoscale particles and formations of huge, firm, and well-defined nanostructures. An example comprises NPs preparations through colloidal dispersal techniques. It also comprises basic methods like vapor condensation, amorphous crystallizations, physical fragmentations, etc. [19–21]. NP preparations are facilitated by physical, chemical, and green techniques [22]. These physical approaches involve the use of expensive apparatus, high temperatures, and pressure on large spaces for location machinery. The chemical technique also involves the use of poisonous chemicals which can be proven to be dangerous for the environment and the person treatment it [23]. The literature explains that some of the poisonous chemicals that we use in physical and chemical approaches may exist in NPs designed which may prove dangerous in the arena of their applications in the medicinal fields [24]. Therefore, we wanted an environmentally approachable and cheaper technique for NP preparation [25]. Physical procedures include the use of high emptiness in procedures like pulsed laser evaporation, thermal evaporation, etc. Physical procedures include the use of high emptiness in procedures like pulsed laser evaporation, thermal evaporation, etc. [26], as well as chemical approaches, including chemical microemulsion, wet-chemical, spray pyrolysis, electrodeposition [27], chemical, as well as direct precipitation and microwave-assisted combustion [28]. Supplementary coatings and calming agents are wanted in green synthesis methods [29, 30]. Figure 1 displays the synthesis methods of NPs.
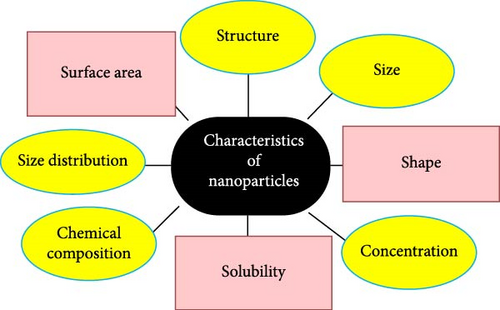
In order to tune the optical absorption of ZnO into the visible region and an improvement of charge carrier separation, it is coupled with narrowband gap semiconductors such as Nb2O5/ZnO [31], Bi2O/ZnO, Ag2O/ZnO [31], and CuO/ZnO [32]. Thus, the joined semiconductors have been vigorously considered to improve their photocatalytic activities in visible light [23]. Joined Ag2O is a chocolate colored p-type semiconductor that retains a simple cubic structure and has a bandgap stated to be 1.2 eV with the energy levels of the C–B edge at +0.2 eV (vs. SHE) [33]. It is also extensively used in several industrial uses as washing agents, preservers, dyes, conductor materials, photocatalysts for ecological remediation, as well as organic transformations [34]. Ag2O also originated to be a steady and extremely well-organized photocatalyst under visible lights [35]. In the next review phases, the researchers discussed the reports of recent works [36]. Figure 2 displays characterization techniques and their properties.
ZnO is a semiconductor inanimate nanomaterial with a bandgap ranging between 3.1 and 3.3 eV. It can have three crystal structures, like zinc blends, wurtzite, and rock salts. Its wurtzite structures are thermodynamically steady with every lone zinc atom tetrahedrally synchronized with four oxygen atoms [37]. ZnO has huge applications for medication, antibacterial activity, photocatalytic, and antibacterial progressive spiral gauzes. Numerous kinds of mineral metallic oxides have been prepared and persisted in current investigation, like TiO2, CdO, CuO, and ZnO. The energy bandgap (1.2 eV) of Ag2O is close to an ultimate value which is appropriate for photocatalytic applications in the visible region [38]. All these features of Ag2O play vital role in character formation of nanocomposite with ZnO nanorods for better photocatalytic performance under visible light. Hence, the present study of combining Ag2O with ZnO is being undertaken. Now, the ZnO NP is chosen as the main material because of its 1D nanostructure, which retains numerous benefits over NPs. The improved visible light smattering and absorptions, fast diffusion transport, free electron transport sideways, longer directions, and small numbers of grain boundaries are the benefits of such composite materials [39, 40]. All of these metallic oxides, ZnO NPs are of supreme attention because they are low cost to yield, harmless, and can be synthesized simply [37]. Because of its UV-clarifying characteristics, it has been widely used in makeup like sunscreen creams [35]. It has an extensive range of biological medication applications, such as drug delivery, anticancer, antidiabetic, antibacterial, antifungal, as well as agricultural characteristics [41, 42]. Though ZnO NPs are applied to battery drug deliveries, it still has the limitations of cytotoxicity which is yet to be needed research [43, 44]. ZnO NPs have very sturdy antibacterial effects on very small concentrations of Gram-negative and Gram-positive bacterial strains as inverted by the research, they have revealed. They have antibacterial effects like the ZnO NPs prepared chemical approach [45–49].
ZnO NPs have been described in various morphological shapes, such as nanoflake, nanoflower, nanobelt, nanorod, nanostar, nanosheet, and nanowire [50–53].
The green approach is attractive root due to this numerous efforts have been reported to prepare ZnO NPs on various bases, such as bacterial mediated, fungal, algae, and parts of plants (Figure 1). Some physical and chemical approaches are also conveyed. Based on literature review in a direct science database, directly reported manuscripts are filtered according to years (since 5 years), including both research papers and review papers and subject areas (material science, chemical engineering, energy, chemistry, engineering, physics, and astronomy) and all filtered papers have energy open access. Through this research, the researchers perceived only 15 related works prepared by physical and chemical methods of synthesis of ZnO NPs and 37 green synthesized materials through different body plant extracts [54]. Table 1 has been placed to review the treasured works in physical and chemical techniques of ZnO NPs.
| Methods | Precursors/reagents | Properties | Applications | Limitations | References |
|---|---|---|---|---|---|
| Laser ablation | Zinc metal, chloroauric acid | Average grain sizes were 50.31 nm and 36.73 nm spherical shape | Catalytic |
|
[55] |
| Laser ablation | Zn plate of 5 mm thickness and 99.99% purity, Ag plate 99.99% | The bandgap of ZnO NPs to 3.40 ± 10 eV while the bandgap for the ZnO–Ag bimetallic increased to 5.80 ± 5 eV, The particle sizes of the synthesized bimetallic NPs ranged from 30 to 130 nm | Anticancer activity | The bandgap observed is very wide, expensive, and toxic chemicals were used; large particle size is observed | [56] |
| Pulsed laser ablation | Zinc plate (Fello Co., Inc.; 99.9%), ethanol | The blue shift indicated through UV–visible absorption spectra test, larger particle sizes, spherical, and homogeneous | — | Costly chemicals and toxic | [57] |
| Laser assistant method | Zn metal, 4-nitrophenol (4-NP), and sodium borohydride (NaBH4) | Average crystal size 24 nm and 23 nm, hexagonal wurtzites crystal structure | Catalytic activity | The reagents used were toxic to humans and environment | [58] |
| Hydrothermal method | Zn(NO3)2·6H2O, HAuCl4·3H2O, 1,8 diamino, 3,6 dioxaoctan, and graphene oxide | Hexagonal crystal system, bandgap value is around 3.05 eV | Photodegradation | The particle size is not estimated | [59] |
| Hydrothermal | Bis-(triethoxysilylpropyl) amine | The bandgap varied between 3.21 and 2.98 eV, hexagonal wurtzites phase. The crystal size was 22.32–40.17 nm | Photocatalytic degradation | Very expensive autoclave is utilized in these syntheses of NP | [60] |
| Thermal shock method | Zn(CH3COO)2·2H2O/K2C2O·H2O, AgNO3, and KF 99% | Their average particle size is 50–100 nm, hexagonal structure | Photocatalytic | The instruments used are not cost-effective | [61] |
| Coprecipitation method | Chloride (ZnCl2) and sodium hydroxide (NaOH), nickel chloride (NiCl2), and cobalt chloride (CoCl2·6H2O) | Crystal size varies between 5 and 7 nm, hexagonal wurtzites phase, and bandgap 5.25 eV | Optoelectronic devices | Harmful to human health since the size is less than 10 nm | [62] |
| Coprecipitation method | Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), copper (II) nitrate trihydrate (Cu(NO3)2·3H2O), and NaOH | Bandgap energy from 3.27 to 3.44 eV, average crystallite sizes of 25–22 nm | Photocatalytic activities | — | [63] |
| Chemical precipitation | Zinc acetate (Zn(CH3COO)2, 2H2O) and (NaOH) | An average size 62 nm. UV–vis spectra revealed a decrease in energy bandgap (3.15–3.05 eV), wurtzite structure | Photocatalyst may be used to remediate synthetic leather dye-contaminated wastewater | Uses very high temperature | [64] |
| Coprecipitation | Zinc acetate and silver nitrate | The estimated crystalline size ranges from 30.79, 23.72, 36.97, 21.49, and 25.66 nm | Water contaminant treatment | — | [65] |
| Ultrasonic assisted | Zn(NO3)·6H2O, NaOH | Hexagonal phase, crystal size varies between 17 and 66 nm | Photocatalytic activity | The instrument used is very expensive | [66] |
| Microwave-assisted chemical synthesis technique | Zinc acetate dihydrate (Zn[CH3(COO)]2·2H2O), (NaOH), (AgNO3), and (AlNO3) | The optical bandgap values showed a blue shift (3.54–3.89 eV), hexagonal wurtzite structure, and crystal size ~20 nm | Photocatalytic activity | Toxic by-products released to environment | [67] |
| Microwave plasma treatment | Zinc acetate dehydrates (Zn(CH3COO)2·2H2O) and NaOH | Crystallite size of ZnO NPs from 3.81 nm up to 4.68 nm, wurtzites hexagonal | Electronic devices | The particle prepared is very toxic to human health | [68] |
| Sol–gel mediated ultrasonic hydrothermal | Zinc nitrate hexahydrate ethanol (99%), isopropanol, and potassium hydroxide | Energy bandgap varied in 3.26−3.23 eV, Hexagonal size ranged from 21.1 to 32.41 nm | Photocatalytic treatment | Not cost-effective | [69] |
- Abbreviations: NPs, nanoparticles; ZnO, zinc oxide.
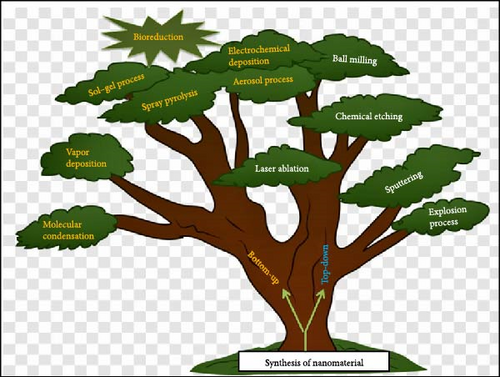
As discussed in Table 1, the synthesis of ZnO NPs has the aforementioned limitations. In addition, the physical and chemical methods took time to prepare NPs and had low production rates, even though very expensive toxic chemicals [70]. The various techniques used for the synthesis of NPs are shown in Figure 3.
Green (biosynthesis) of NPs is the method of manufacturing NPs through micro-organisms and parts of plants having biomedical claims. This method is an eco-friendly, cheaper, biologically well-matched, nontoxic, and green method [71–73]. Green (biodegradable) synthesis contains synthesis via parts of plants, bacterial, fungus, algae, etc. They agree on huge scales productions of ZnO NPs permitted of extra impurities [48, 74, 75]. NPs prepared from biomimetic method show more catalytic activities and limit the usage of affluent and poisonous compounds.
The biological synthesis comprises the biological as well as green preparation technique which is the choice for physical as well as chemical approaches, it is ecologically benefits and has naturally covering mediator which constrains the agglomerations of NP; these types of preparation do not necessity an artificial covering agents [76]. The biological methods of ZnO NPs are bottom-up approaches that mostly involve decrease and oxidation reactions [77, 78]. The synthesis reactions take in one step, and so the molecules that have double properties such as dropping as well as covering agents are favored [79]. Ecofriendly foundations are measured as assets for biologically synthesis of NPs and they are parts of plants extracts, bacterial, fungus is notable bases [80, 81]. The NPs are instantaneously covered with a protein that molecules manufacture a natural capes and so stopping formations of aggregate. Capping NPs with biological constituents raises lengthier shelf-life and constancy [82, 83].
Parts of plant such as roots, leaves, pods, seeds, and fruits have also been used for NPs preparations as their extracts are richest in phytochemicals which act as both reducing and stabilization agents [84–86]. ZnO synthesized from leaf extract of Ailanthus altissima shows that superficial plasmon peaks were verified at 327 nm, showing the existence of ZnO NPs in the reaction solutions of leaf extract and zinc sulfate hex hydrate salt. These reveal NPs mostly with a spherical shape. Moreover, the ZnO NP displayed a dosage reliant on antibacterial and antioxidant activities [87, 88]. Likewise, ZnO NPs prepared from fruit extracts of Rosa canina act as both dropping and steadying agents for manufactured ZnO NPs, established by Fourier-transform infrared spectroscopy (FTIR) characterization. Biologically, covering is done by carboxylic and phenolic acids existing in fruit extracts. Spherical structures ZnO NPs were designed by aloe-Vera leaf extracts plants that were free of carboxylic as well as the amino groups of the plant performing both dropping and capping agents [89, 90]. Parts of plants such as leaves, stems, roots, fruits, and seeds have been utilized to prepare ZnO NPs due to the exclusive phytochemicals that they yield. Using natural extracts of parts of plants is a very biodegradable, inexpensive procedure, and it does not contain usage from any usage group. It is not a time-consuming method—it does not involve the usage of expensive apparatus and precursors and the bounces a spotless and amount of supplemented product free of impurities [91, 92]. Plant life is the furthermost favored source of preparation due to their large-scale production and manufacture of steady, diverse shapes, and sizes of NPs [93]. Biological decrease includes dropping metallic ions or metallic oxides to zero valence metallic NPs with the help of phytochemicals such as polysaccharides, polyphenolic compounds, vitamins, amino acids, alkaloids, and terpenoids concealed from the plants [94–96]. Furthermore, the most generally useful technique for simple preparation of ZnO NPs from leaves or flowers is that the plant body is cleaned carefully in tap water consecutive as well as pasteurized through double distilled water (some use Tween-20 to sterilize it). Then, it is reserved for dehydrating at room temperature by deliberating and then overwhelming it via mortar and pestle. Milli-Q water is added to the part of plant affording to the anticipated concentrations and then the solution is heated under continuous rousing using a magnetic stirrer [97–99].
The mixture was clarified through Whatman paper and the obtained clear solution was utilized as a plant extract (sample). Some volumes of plant extracts are dissolved with 0.5 Mm of hydrated—zinc nitrate or ZnO or zinc-sulfate and the mixtures are heated at desired temperatures as well as time to achieve efficient fraternization [100–102]. Some achieve optimizations at this point through different temperatures, pH values, extract concentrations, and deposition times. Maturation period marks the alteration of the color of the mixtures to creamy, which is a visual validation of the prepared NPs [103, 104]. Then, characterizations such as UV–visible (UV–vis) spectrophotometry is engaged to approve the preparation of NPs followed by centrifugation of mixtures and dehydrating the pellets in an oven to get the crystal NPs [105]. X-ray diffractometer (XRD), energy dispersion of X-ray (EDX), FTIR, scanning electron microscopy (SEM), transmission-electron microscopy (TEM), field emission-scanning electron microscopy (FE-SEM), atomic-force microscopy (AFM), photoluminescence analysis (PL), X-ray photoelectron microscopy (XPS), Raman-spectroscopy, UV–vis diffuse reflectance spectroscopy (UV-DRS), and dynamic light scattering [106]. Table 2 is widely displayed the study of various parts of plants used for the preparations of ZnO NPs till date.
| Species | Reagents used | Parts of plants | Physical properties | Application | References |
|---|---|---|---|---|---|
| A. altissima | Zinc sulfate hexahydrate salt, NaOH | Leaves | Spherical in shape, crystalline size of 13.27 nm | Antibacterial and antioxidant activity | [107] |
| Ocimum basilicum | Ethanol (C2H5OH), (NaOH), (Zn(CH3COO)2·H2O) | Leaves | Crystallite size 25 nm which and wurtzite hexagonal structure | Antibacterial, antidiabetic activity photocatalytic | [108] |
| Ficus palmate | Zinc acetate, sodium hydroxide | Leaves | Hexagonal, average diameter 35 nm | Diabetic | [109] |
| Soursop (Annona muricata L.) | Zinc nitrate hexahydrate Zn(NO3)2·6H2O | Leaves | Average diameter of 37 nm, quasispherical shape | Antibacterial activity | [110] |
| Cocos nucifera | Zn(NO3)2·6H2O, NaOH | Leaves | Hexagonal wurtzite, average crystal size 16.6 nm | Biomedical, medicinal, and pharmaceutical | [111] |
| Mesua ferrea | Zinc nitrate | Leaves | Spherical shape, crystallite size of around 20 nm | Antibacterial activity | [112] |
| Lavandula angustifolia | Zinc acetate dehydrate, sodium hydroxide | — | Hexagonal wurtzite, average size 61.52 nm | Antibacterial | [34] |
| Ginger (Z. officinale) and garlic (Allium sativum) | Zinc acetate, ethanol, (NaOH), dimethyl sulfoxide | Root and bulb | Wurtzite structure, the average particle size was found to be 19.8, 21.94, and 23.86 nm | Antimicrobial | [113] |
| Bush tea (Athrixia phylicoides DC) | Zn(NO3)2·6H2O, cerium nitrate hexahydrate, and silver nitrate | Leaf | Crystallite size ranges between 24.53 and 63.02 nm. The bandgap energies were 2.75 and 3.17 eV | Bioreduction | [114] |
| Hagenia abyssinica | Zinc acetate dihydrate, sodium hydroxide, methyl orange, and ethanol | Leaf | Hexagonal, particle size 27.833 nm | Antibacterial | [115] |
| Origanum vulgare | Zinc nitrate hexahydrate (Zn(NO3)2·6H2O) | Leaf | The crystalline size varying sizes of crystallites from 19.67 to 28.78 nm, hexagonal wurtzite | Antimicrobial | [116] |
| Solanum melongena | Zinc acetate | Leaf | Spherical, particle sizes ranging from 5 to 12 nm | Antifungal | [117] |
| Dovyalis caffra | Zinc acetate dihydrate, (NaOH), and 1,1-diphenyl-2-picrylhydrazyl (DPPH) | Fruit | Hexagonal ZnO–NP wurtzite, crystallite size of the synthesized ZnO–NPs was found to be 19.27 | Antioxidant activity | [118] |
| Thyme plant | Zinc nitrate hexahydrate salt [Zn(NO3)2·6H2O], sodium hydroxide (NaOH) | Leaf | Wurtzite–hexagonal, the measured average particle size was 35.202, 35.203, 42.249, and 43.308 nm | Photocatalysis, water pollution removal, (antibacterial) applications, solar cells | [119] |
| Psidium guajava | Zinc acetate dehydrate, NaOH | Leaf | Hexagonal, the crystalline size of ZnO NPs is 15.8 nm | Antibacterial | [120] |
| Scoparia dulcis | (Zn(NO3)2·6H2O), 2,2-diphenyl1-picrylhydrazyl (DPPH), ascorbic acid, methanol, and KOH | Leaf | Hexagonal, average particle size ~20.2 nm | Antimicrobial and antioxidant | [121] |
| Tetradenia riperia | (AgNO3), (Zn(NO3)2·6H2O), NaOH | Leaf | Hexagonal wurtzite, average particle size decreased from 23.6 to 14.8 nm | Antimicrobial | [122] |
| Phoenix roebelenii | Zn(NO3)2·6H2O | Leaf | Wurtzite, crystal size 15.6 nm, energy bandgap is 3.24 eV | Gram-positive and Gram-negative pathogenic bacterial | [79] |
| Mangrove leaves (Avicenna marina) | Zn(OAc)2·2H2O, NaOH | Leaf | Hexagonal having sizes range from 23.7 to 39.2 nm with an average of 29.1 nm | Removal of toxic metal Ions (Cd2+ and Pb2+) | [123] |
| Peltophorum pterocarpum | Zinc acetate dihydrate, NaOH | Pod | Hexagonal wurtzite, bandgap was calculated as 3.43 eV, fower-like morphology, size of 17.79 nm | Removal of harmful dyes | [124] |
| Moringa | (Zn(NO3)2·6H2O) | Leaf | Crystal size 50 nm, flower-like shapes | Antibacterial | [78] |
| Punica granatum | Zinc nitrate sodium hydroxide and ethanol | Peel | Hexagonal phase of wurtzite, crystalline size was found to be 22 nm | Antimicrobial | [105] |
| Tulsi (Ocimum tenuflorum) | Zinc nitrate | Leaf | Average crystallographic size 52.24 nm, hexagonal | Antibacterial and antifungal activities | [125] |
| Melia azedarach | Zn(ac)2·2H2O and (NaOH) | Seed | The average crystallite size was 57.74 nm, hexagonal | Antibacterial | [126] |
| Bryophyllum pinnatum | Zinc acetate | Leafs | Wurtzite hexagonal, crystallite size to be 19.83 nm | Antibacterial | [77] |
| Vitex negundo Linn | Zinc nitrate | Leafs | Average particle size of ZnO NPs was around 33.4 nm | Malaria, flariasis vectors, antioxidant, HeLa cells, and MB blue dye | [127] |
| Cassia auriculata | Zinc acetate, sodium hydroxide | Leaf | Hexagonal, the average crystallite size was calculated from 24 to 30 nm | Antibacterial | [126] |
| Rubus fairholmianus | zinc nitrate | Root | The bandgap energy was calculated at 3.47 eV | Antibacterial | [128] |
| Cayratia pedata | Zinc nitrate (Zn(NO3)2·6H2O) | Leaf | Hexagonal, average crystalline size found to be 52.24 nm | — | [54] |
| Pelargonium odoratissimum (L.) | zinc acetate dihydrate (Zn(CH3COO)2·2H2O) | Leaf | Hexagonal wurtzite, the average crystalline size 14 nm | Antioxidant, antibacterial, and anti-inflammatory | [129] |
| Actinidia deliciosa | Zinc nitrate hexahydrate (Zn(NO3)2·6H2O) | Fruit peel | Hexagonal, the crystallite size was found to be 18.40 nm, optical energy gap value (Eg) and it was found to be 3.06 eV | Antimicrobial agent, anticancer agent | [2] |
| Withania somnifera | Zinc acetate | Root | Hexagonal wurtzite, bandgap of 3.28 eV, the average particle size 32 nm | Antibreast cancer | [3] |
| Cassia alata | Zinc acetate | Leaf | Spherical in shape formed within diameter range of 80–130 nm | Fungicidal | [130] |
| Barrintonia acutangula | Zinc nitrate (Zn(NO3)2·6H2O) | Leaf | spherical structures with an average grain size 5−20 nm | Antibacterial | [5] |
| Crocus sativus (Safron) | Copper (II) acetate zinc nitrate hexahydrate and sodium hydroxide | Flower | The average crystallite size was found to be 23 nm, hexagonal | — | [6] |
| Lantana camara | Zinc nitrate [Zn(NO3)2·6H2O] | Flower | Hexagonal, average crystallite size 25 nm | Anti-inflammatory | [7, 24] |
| Salvia officials | Zinc nitrate dehydrate, polyvinyl alcohol, and hexamethylenetetramine | Leaf | The mean size ranged from 14.0767 to 14.1650 nm, hexagonal wurtzite | Optoelectronic | [8] |
- Abbreviations: NPs, nanoparticles; ZnO, zinc oxide.
NPs synthesis through bacterial strains is a green approach, but it has numerous drawbacks, as transmission of microorganisms is a time-consuming procedure, watchful monitoring of culture broth, and the whole procedure is essential to escape contamination, the absence of controls on NPs sizes, shapes, and cost supplementary with the culture media utilized to cultivate bacterial strain is also very extraordinary. Photocatalysis produces vigorous species by absorptions of light which destroys the organic waste materials and thus can be applied as current bioremediation tools [131]. An alga is a photosynthetic organism which ranges from unicellular arrangements (e.g., Chlorella) to multicellular ones (e.g., brown algae). Alga has nonexistent basic plant structures like roots as well as leaves. Aquatic algae are characterized depending on the colorant current in them, like Rhodophyta consuming red pigments, Phaeophytas with chocolate pigments, and chlorophytes with lime pigments. Algae have been utilized widely for the preparation of Au and Ag NPs but its applications for ZnO NPs preparations are partial, as well as described in very few reports [132]. Microalgae draw special consideration due to their capacity to destroy poisonous metals and change them to less poisonous methods [133, 134]. The extracellular preparations of NPs from the fungus are extremely advantageous due to their great scale production, suitable downstream dispensation, and financial feasibility [135]. Fungiform anxieties are preferred over bacterial due to their improved acceptance and metallic bioaccumulation bacteria [136]. ZnO is a versatile compound utilized in a broad spectrum of products ranging from sunscreens to electronic devices. While it is generally considered safe for use in many consumer goods, certain forms, and exposures to ZnO can pose potential health risks. One primary concern is inhalation, particularly relevant for workers in industries where ZnO dust or NPs are prevalent. When inhaled, ZnO can cause respiratory issues such as metal fume fever, characterized by flu-like symptoms including fever, chills, and muscle aches. Chronic inhalation exposure could potentially lead to more severe respiratory conditions and lung damage, highlighting the importance of proper protective equipment and ventilation in occupational settings [137].
Skin contact with ZnO is common in products like sunscreens and cosmetics, where it functions as a UV filter due to its ability to block harmful ultraviolet rays. While ZnO is typically safe for topical application, individuals with sensitive skin or allergies might experience irritation or dermatitis. Prolonged or repeated skin contact with high concentrations of ZnO in occupational settings can also lead to skin sensitization and other dermatological issues. Ensuring that ZnO-containing products are formulated to minimize skin irritation and that safety guidelines are followed in workplaces can mitigate these risks [138]. Eye contact with ZnO can be particularly hazardous, as the compound can cause significant irritation and damage to the delicate tissues of the eye. Exposure to ZnO dust or particles can lead to redness, pain, and in severe cases, conjunctivitis, or corneal damage. It is crucial to use protective eyewear in environments where ZnO particles are airborne. Ingestion of ZnO, although less common, can also be harmful, leading to gastrointestinal distress, nausea, vomiting, and more severe systemic toxicity if ingested in large quantities. Additionally, the environmental impact of ZnO should not be overlooked; when released into the environment, ZnO NPs can affect aquatic life and ecosystems, potentially causing toxicity to various organisms and disrupting ecological balances. Thus, responsible manufacturing, usage, and disposal practices are essential to minimize the environmental footprint of ZnO.
3. Characterizations of ZnO NPs
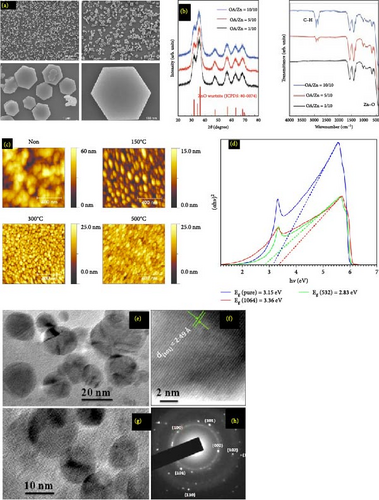
SEM images of ZnO NPs provide critical insights into their morphology, size distribution, and surface characteristics. These images typically reveal that ZnO NPs exhibit a variety of shapes, such as spherical, rod-like, or hexagonal structures, depending on the synthesis method used. The high-resolution capability of SEM allows for detailed observation of particle sizes, often ranging from a few nanometers to several 100 nm. Furthermore, SEM images can illustrate the degree of agglomeration and the uniformity of the NPs, which are crucial for determining their suitability for various applications. Figure 4a–d displays the surface texture, and structural integrity observed in SEM images is essential for understanding the material’s properties, such as its photocatalytic activity, electronic behavior, and potential use in antimicrobial treatments and optoelectronic devices. Through SEM analysis, researchers can optimize synthesis processes and tailor the properties of ZnO NPs to enhance their performance in specific technological applications.
4. Applications of ZnO NPs
ZnO NPs are utilized in a diverse array of applications (Figure 5) owing to their unique properties such as high surface area, chemical stability, and notable optical, electronic, and antimicrobial characteristics. In electronics and optoelectronics, ZnO NPs are employed in transparent conductive films, light-emitting diodes, and piezoelectric devices, enhancing the performance of touchscreens, displays, and sensors. Their photocatalytic properties make them invaluable in environmental remediation, where they degrade organic pollutants in water and air, and in self-cleaning surfaces that break down contaminants upon light exposure. In the biomedical field, ZnO NPs serve as drug delivery carriers, leveraging their biocompatibility for targeted therapy, and as antibacterial agents in medical coatings and wound dressings. They are also explored for cancer treatment through photodynamic therapy and drug delivery systems.
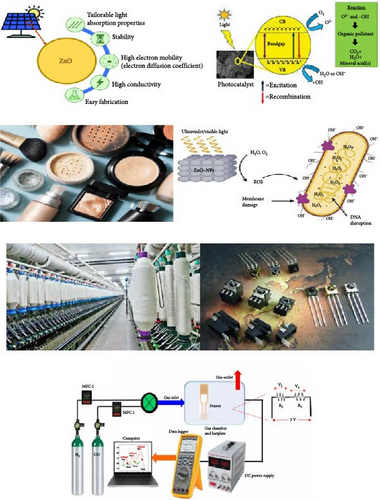
In the cosmetics and personal care industry, ZnO NPs are incorporated into sunscreens and cosmetic products for their UV protection capabilities and antimicrobial effects. Energy applications include their use in solar cells, where they enhance efficiency in dye-sensitized and perovskite solar cells, and in energy storage devices like supercapacitors and batteries. They are also crucial in gas sensors for detecting hazardous gases and in biosensors for monitoring glucose and other biomolecules. Additionally, ZnO NPs find applications in antimicrobial fabrics in the textile industry, air and water purification systems for environmental monitoring, and food packaging to prevent microbial contamination. Their catalytic properties are leveraged in various chemical reactions, while in coatings and paints, they provide UV protection and antimicrobial functionality, extending the durability and effectiveness of these materials [138].
5. Ag2O NPs
Ag2O NPs were newly initiated to have anticipated characteristics for usage in photovoltaic cells, devices, data storage devices, biomedical applications, and photocatalysis [143]. Ag2O NPs have been discovered in numerous investigations aiming at their preparation, characterizations, and photocatalytic enactment. Their worthy optical characteristics and narrow energy bandgap (1.1–1.4 eV), which allow them to captivate the full solar spectrum, make these NPs attractive for visible-light driven photocatalytic removal of carbon-based contaminants. Ag2O NPs photocatalysts were also stated to experience self-stabilizing the procedure throughout photocatalytic degradation of carbon-based compounds, ensuing in high photocatalytic performance [5]. Ag2O NPs have been synthesized through physical and chemical methods of preparation of Ag2O NPs. In Table 3, it is clearly discussed regarding synthesization, characterization, applications, and limitations.
| Methods | Reagents/chemicals | Properties | Application | Limitations | References |
|---|---|---|---|---|---|
| Chemical deposition | Tungsten oxide powder (WO3), silver nitrate (AgNO3), and sodium hydroxide (NaOH) | The average crystallite size for pure WO3 is 35 ± 10 nm, cubic structure | Sensor devices | Chemicals used were expensive | [144] |
| Simple coprecipitation method | Graphene oxide, hydrazine hydrate | Morphology and are well distributed on the crumpled HRG nanosheets as displayed | Industrially applicable for the oxidation of alcohol | Used chemicals were toxic to human health | [145] |
| Coprecipitation methods | (CaCl2·2H2O), (Ag2O), [(NH4)2HPO4], (HCl), and (KMnO4), H2SO4, H2O2, and graphite powder | Hexagonal crystallographic structure | Biomedical applications in general and bone regeneration | Expensive chemicals were used | [146] |
- Abbreviations: Ag2O, silver oxide; NPs, nanoparticles.
Green synthesis mediated by metal oxide NPs is achieved through extracts from different plants as well as their bodies as a reducing and capping agents. Through numerous metal oxide NPs investigated, the noble metal silver grasps a protuberant residence in nanomaterial research because of its exclusive characteristics that is viable to apply in different fields of study. The characteristics are sensing, optoelectronic, catalysts, and drug delivery. Basic properties of molecular, supramolecular, as well as the atomic level of objects could be agreed by the support of nanotechnology. The green synthesis methods of Ag2O NPs, as presented in Table 4, have been reviewed. However, the production of NPs using chemical methods is low and toxic [157].
| Species | Reagents used | Parts of plants | Physical properties | Application | References |
|---|---|---|---|---|---|
| Diospyrosontana | AgNO3 | Bark | Spherical shaped and 10–50 nm size | Dye degradation, antibacterial, and anticancer | [147] |
| Tea leaves | AgNO3 | Leave | The crystallite size of Ag2O MPs was 7.95 nm | Malachite | [14] |
| Moringa oleifera | AgNO3 | Leaf | Average size ranged from 24 to 40 nm | S. aureus and P. aeruginosa | [148] |
| Fresh garlic | Silver nitrate | Leaf | Average crystal size 30.20 nm, spherical shape | Diabetes | [149] |
| Dunaliella salina | Silver nitrate (AgNO3) | Leaf | Cubic and hexagonal forms size between 10 and 50 nm | Antibacterial | [150] |
| E. adenophorum | Silver nitrate | Leave | Face-centered cubic structure | Antibacteria, antioxidant | [151] |
| Polyalthia longifolia | Silver nitrate | Leaf | Faced-center cubic, crystal size 17–20 | Phytopathogen | [152] |
| Artemisia Herba-Alba | Silver nitrate (AgNO3) | Leaf | Cubic, crystallite size of Ag from 20–25 | Insecticidal and antibactericida | [153] |
| Cynara scolymus | silver nitrate | Leaf | The particle size found 98.47 ± 2.04 nm | Anticancer | [154] |
| Ficus benghalensis prop | AgNO3 | Root | Polycrystalline structure with size is 16.4 nm | Antimicrobial (Staphylococcus aureus, Aspergillus | [155] |
| F. foetida | AgNO3 | Root | Face-centered cubic, the size of Ag NPs was <15 nm | Antifungal | [156] |
- Abbreviations: Ag2O, silver oxide; NPs, nanoparticles.
6. Characterizations of Ag2O NPs
Characterizing Ag2O NPs involves a suite of analytical techniques to thoroughly understand their physical, chemical, and structural attributes (Figure 6). One fundamental method is X-ray diffraction (XRD), which provides insights into the crystalline structure and phase purity of the NPs. Through the analysis of diffraction patterns, XRD can confirm the presence of Ag2O and provide data on lattice parameters and average crystallite size. Complementing XRD, transmission electron microscopy (TEM) offers high-resolution images that reveal the NPs’ morphology and size distribution, allowing researchers to observe their shape and internal structure at the nanometer scale. SEM further assists in examining surface morphology and overall particle size distribution, providing detailed surface images that enhance our understanding of the NPs’ texture and surface features. To assess the elemental composition, energy dispersive X-ray spectroscopy (EDS or EDX) is utilized, identifying and quantifying the elements present in the NPs, and confirming the presence of silver and oxygen. FTIR is another crucial technique, which identifies the functional groups and chemical bonds on the NPs’ surface, offering insights into the molecular vibrations and surface chemistry. UV–vis spectroscopy studies the optical properties by determining the absorption spectrum and bandgap energy, which are indicative of electronic transitions and the NPs’ optical activity.
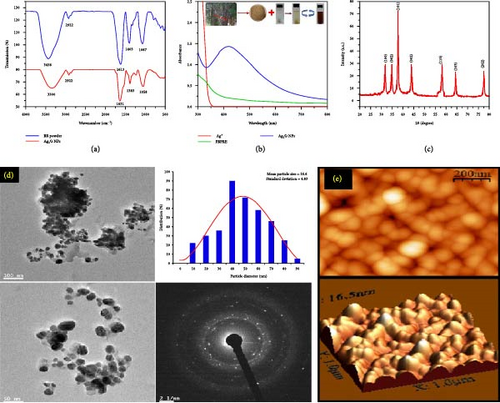
Together, these characterization techniques offer a comprehensive understanding of Ag2O NPs, essential for optimizing their synthesis and enhancing their application performance.
7. Applications of Ag2O NPs
Ag2O NPs possess unique properties that make them highly valuable in a wide range of applications (Figure 7). One of the most significant applications is in the field of antimicrobial agents. Ag2O NPs exhibit strong antibacterial, antifungal, and antiviral properties, making them effective in combating a broad spectrum of pathogens. These NPs are incorporated into medical devices, wound dressings, and coatings for healthcare environments to prevent infections and promote sterile conditions [117]. Their ability to disrupt microbial cell membranes and interfere with essential cellular functions makes them a powerful tool in medical and healthcare settings. In the realm of catalysis, Ag2O NPs are employed as catalysts in various chemical reactions, including organic synthesis and environmental remediation processes. They facilitate the degradation of pollutants in wastewater treatment, acting as efficient photocatalysts under visible light. This capability makes them instrumental in the breakdown of organic contaminants, leading to cleaner and safer water sources. Additionally, Ag2O NPs are used in gas sensors due to their sensitivity to changes in the environment, helping detect harmful gases such as carbon monoxide and hydrogen sulfide, thus contributing to improved air quality monitoring and control [160]. Furthermore, Ag2O NPs find applications in the field of electronics and optoelectronics. Their unique optical properties make them suitable for use in sensors, photovoltaic cells, and other electronic devices. In particular, they are utilized in the development of high-performance batteries and supercapacitors, where their ability to enhance charge storage capacity and stability is highly valued. Additionally, their role in surface-enhanced Raman scattering provides a powerful analytical tool for detecting low-concentration analytes, making them indispensable in chemical and biological sensing applications [161]. The versatility of Ag2O NPs across these diverse domains underscores their significance in advancing technology and improving environmental and healthcare outcomes [160].
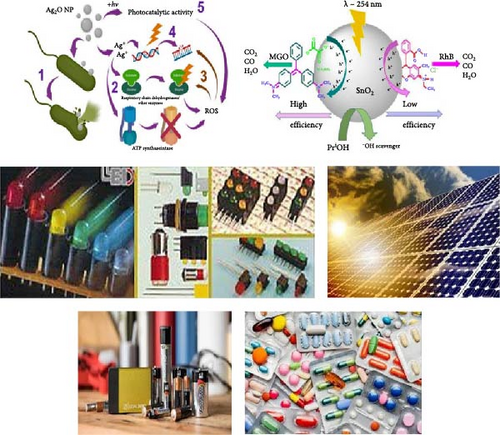
8. ZnO–Ag2O Nanocomposite Materials
Organic preparation is currently attracting a lot of attention in the kingdom of nanomaterial expansion [122]. Biological molecules like proteins, peptides, amino acids, polysaccharides, and vitamins are the main drops and covering proxies in biogenic synthesization. They play a vital role in NP size controls and shape determinations. The hydroxyl groups of tyrosine amino acid and the carboxyl collections of aspartic as well as glutamines amino acids are important reductions in making Ag+ and Zn2+ NPs [38]. Recently, plants have been used as reducing and stabilizing agents in the biosynthesis of NPs, such as Ag2O and ZnO NPs, among others [122]. According to various literary journals, many floras have been engaged in the biological preparation of ZnO NPs with important antifungal actions in contradiction of comprehensive spectrums of bacteria. The manufacturing of Ag2O NPs has been done using extracts from numerous plant species [38]. In manufacturing chocolate crystalline Ag2O NPs with antifungal activities, terpenoids and flavones, and ordinary dropping agents in neem leaf extracts has been engaged as steadying and covering agents [109]. The leaf extract of Lawsonia inermis (Henna) was used in the biosynthesis of ZnO/Ag2O nanocomposite (Bs-ZANc) due to being an ecologically suitable, cost-effective, and nontoxic approach for the preparation of the nanocomposites. Based on literatures reported from 2019 to 2024 in science direct database, researchers reported review and research articles eliminating all short communication, book chapters, and editor comments to enrich relative works 133 papers existed; from these 11 of them are considered in this review paper. Table 5 displays the synthesis of ZnO–Ag2O nanocomposite materials.
| Nanocomposite material | Methods | Properties | Application | References |
|---|---|---|---|---|
| ZnO/Ag2O | Pseudo-SILAR assisted |
|
E. coli and B. subtilis | Ahmad et al. [162] |
| Ag/Ag2O/ZnO | Solution combustion using glycine and Thymus vulgaris extract | — | C. albicans and S. epidermidis | Hezam et al. [163] |
| ZnO/Ag2O | Electrospinning, hydrothermal | Nanorod | Photocatalyst | Warsi et al. [164] |
| ZnO/Ag2O | Thermal decomposition and precipitation technique | Heterostructure | Photocatalyst | Xu et al. [165] |
| ZnO/Ag2O-NDC | Chemical method | Heterostructure | Antibiotic degradation | Alhokbany [166] |
| ZnO–Ag2O | Microwave assisted | Size 10–100 nm | MCF-7 breast cancer cell lines | Gaikwad et al. [167] |
| ZnO/Ag2O | Microwave assisted |
|
Electrochemical sensing and photocatalytic degradation of hazardous 4-NP | Chakraborty et al. [168] |
| Ag2O/ZnO | Simple hydrothermal | Heterostructure | Photocatalytic | Liu [169] |
| Ag/Ag2O/ZnO | Chemical method | 3D-flowerlike | Photocatalytic activity | Ding [170] |
| ZnO/Ag2O | Facile mechanochemical | Heterostucture | Photocatalytic | Yang [171] |
| ZnO/Ag2O | Ionic liquid-assisted photochemical | Flower-like architecture whose size was about 3 μm | Photocatalytic activity than ZnO sample | Zhao [172] |
- Abbreviations: Ag2O, silver oxide; ZnO, zinc oxide.
9. Characterizations of ZnO/Ag2O Nanocomposite Materials
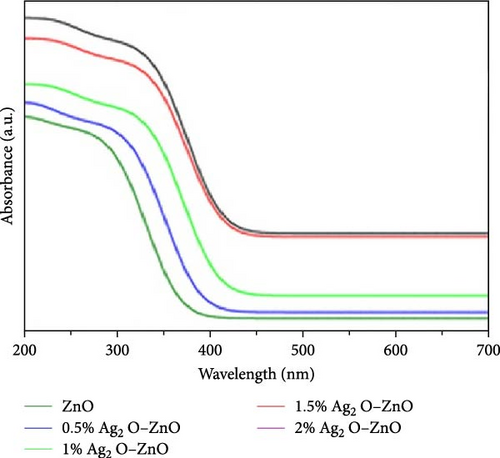
Characterizing ZnO/Ag2O nanocomposite materials involves multiple analytical techniques (Figure 8) to comprehensively understand their structural, morphological, optical, and chemical properties. XRD is crucial for determining the crystalline structure and phase composition of the nanocomposites. XRD patterns provide information on the lattice parameters and crystallite size, helping to confirm the presence of both ZnO and Ag2O phases. The analysis of peak positions and intensities in the XRD spectra can also reveal the degree of crystallinity and any potential interaction between the ZnO and Ag2O components, which is critical for understanding the material’s overall structural integrity and phase distribution [164]. SEM and TEM are essential for examining the morphology and size of the nanocomposites. SEM provides detailed surface images, showcasing the overall shape, size distribution, and surface texture of the particles. This helps in understanding the homogeneity and dispersion of Ag2O within the ZnO matrix. TEM offers even higher resolution images, revealing the internal structure of the nanocomposites at the nanometer scale. TEM analysis can show the interface between ZnO and Ag2O, giving insights into the nanocomposite’s core–shell structures, particle sizes, and possible agglomerations. Together, SEM and TEM provide a comprehensive view of the nanocomposites’ morphological characteristics. UV–vis spectroscopy is employed to study the optical properties of the ZnO/Ag2O nanocomposites. The UV–vis absorption spectra help determine the bandgap energies of the materials, which are critical for applications in photocatalysis and optoelectronics. By analyzing the absorption edge shifts, one can infer the interaction between ZnO and Ag2O and the influence of Ag2O on the optical properties of ZnO. Enhanced light absorption in the visible region often indicates improved photocatalytic activity, which is beneficial for environmental and energy-related applications. This technique provides valuable information on how the nanocomposites interact with light and their potential efficiency in various applications. FTIR and AFM further characterize the chemical and surface properties of the nanocomposites. FTIR spectroscopy identifies functional groups and chemical bonds present in the nanocomposites, revealing how the ZnO and Ag2O components are chemically linked and any changes in surface chemistry due to composite formation. Peaks corresponding to Zn–O and Ag–O bonds can confirm the successful synthesis of the nanocomposite. AFM provides topographical mapping of the nanocomposite surfaces at the nanoscale, allowing for the measurement of surface roughness and particle distribution. AFM analysis helps understand the surface characteristics, which are crucial for applications involving surface interactions, such as catalysis and sensor technology. Together, FTIR and AFM offer a detailed view of the chemical bonding and surface morphology of ZnO/Ag2O nanocomposites, complementing the structural and optical data obtained from other techniques [174].
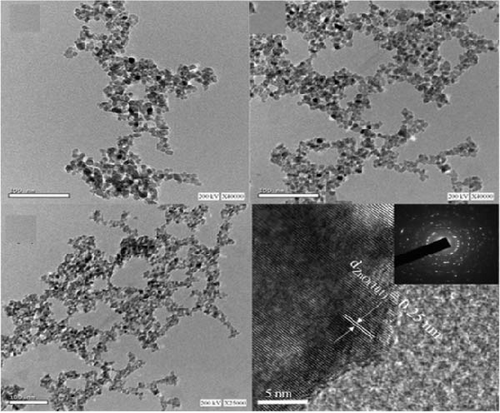
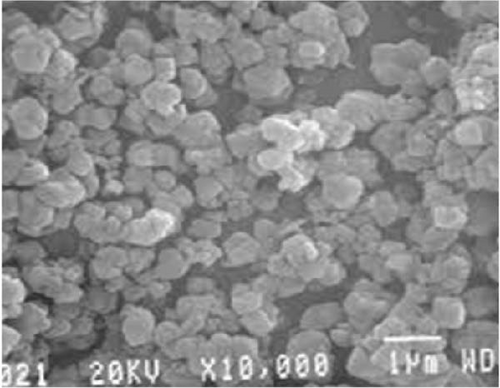
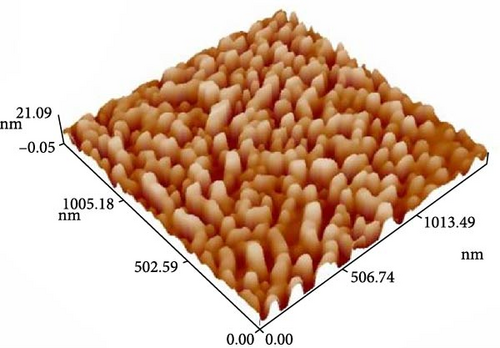
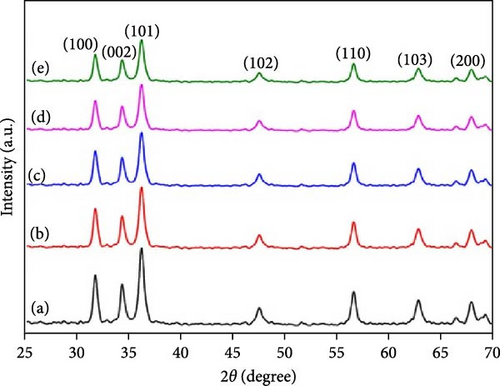
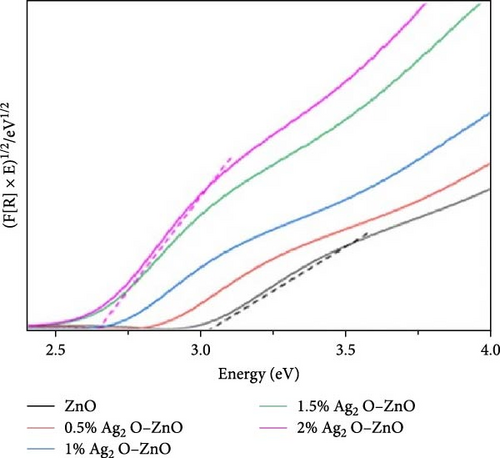
10. Applications of ZnO/Ag2O Nanocomposite Materials
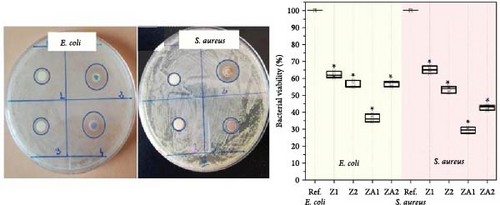
ZnO/Ag2O NPs combine the unique properties of both ZnO and Ag2O, resulting in a composite material with enhanced functionalities and a broad range of applications (Figure 9). One of the primary areas of application is in antimicrobial treatments. The synergistic effect of ZnO and Ag2O provides potent antibacterial, antifungal, and antiviral properties, making these NPs highly effective in medical and healthcare settings. They are incorporated into wound dressings, coatings for medical devices, and even in textiles to prevent the growth of harmful pathogens [175]. The strong antimicrobial activity of ZnO/Ag2O NPs helps reduce the risk of infections, particularly in hospital environments where antibiotic resistance is a significant concern [176].
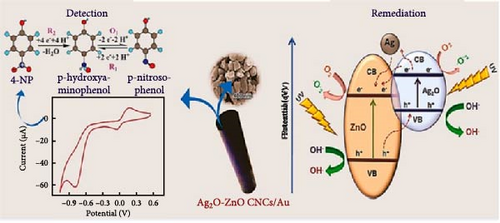




In the field of environmental remediation, ZnO/Ag2O NPs are highly valued for their photocatalytic properties. These NPs can efficiently degrade organic pollutants and dyes in wastewater under light irradiation, making them ideal for purifying contaminated water sources. Their ability to generate reactive oxygen species under UV–vis light exposure enables the breakdown of various environmental pollutants, leading to cleaner water and air. Additionally, ZnO/Ag2O NPs are used in air purification systems to degrade volatile organic compounds and other harmful substances, contributing to improved air quality and environmental sustainability [177].
ZnO/Ag2O NPs also play a significant role in energy applications, particularly in the development of advanced photovoltaic cells and batteries. In solar cells, these NPs enhance light absorption and charge separation, improving the overall efficiency of energy conversion. Their unique optical properties make them suitable for use in dye-sensitized solar cells and perovskite solar cells, where they contribute to higher power conversion efficiencies. In the realm of energy storage, ZnO/Ag2O NPs are used to improve the performance of supercapacitors and batteries by enhancing their charge storage capacity and stability. This makes them valuable for developing next-generation energy storage devices with better efficiency and longevity [178].
Another critical application area for ZnO/Ag2O NPs is in sensor technology. These NPs are utilized in the development of highly sensitive and selective sensors for detecting gases, chemicals, and biological molecules. For instance, gas sensors based on ZnO/Ag2O NPs can detect low concentrations of hazardous gases such as hydrogen sulfide, carbon monoxide, and ammonia, providing early warning and improving safety in industrial environments. In biosensors, the NPs are used to detect biomolecules like glucose, cholesterol, and pathogens, enabling advanced diagnostic tools for healthcare applications. The combination of ZnO’s excellent semiconducting properties and Ag2O’s high sensitivity enhances the overall performance of these sensors, making them crucial for various analytical and monitoring applications [179].
11. Conclusion
The comprehensive review of the synthesis, characterization, and applications of ZnO, Ag2O NPs, and ZnO/Ag2O nanocomposites underscores the immense potential of these materials across various fields. The synthesis methods, including sol–gel, hydrothermal, chemical precipitation, and green synthesis, offer versatile approaches for producing NPs with controlled sizes, shapes, and properties. The combination of ZnO and Ag2O into nanocomposites has demonstrated a significant enhancement in properties due to the synergistic effects of the individual components, resulting in improved photocatalytic, antimicrobial, and optoelectronic performance. Characterization techniques such as XRD, TEM, SEM, AFM, FTIR, UV–vis spectroscopy, EDX, and PL have been pivotal in confirming the structure, morphology, optical properties, and elemental composition of these materials, providing essential insights into their functionality and potential applications.
In terms of applications, ZnO, Ag2O NPs, and ZnO/Ag2O nanocomposites have shown remarkable versatility. Their use in photocatalysis for environmental remediation, antimicrobial agents in medical and consumer products, and active materials in sensors and optoelectronic devices demonstrates their broad impact. The enhanced photocatalytic activity of ZnO/Ag2O nanocomposites, in particular, highlights their promise in addressing environmental challenges, including water purification and pollutant degradation. The antimicrobial properties of these materials make them suitable for a range of applications, from coatings to healthcare products. As research in this area continues to evolve further optimization of synthesis techniques and a deeper understanding of the mechanisms behind their enhanced properties will likely unlock new applications, solidifying the importance of these nanomaterials in advancing technology, and improving quality of life.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors contributed equally to the preparation of this review paper. Conceptualization, data curation, formal analysis, investigation, methodology, and software were conducted by Abel Saka and Leta Tesfaye. Project administration, supervision, and visualization were managed by Leta Tesfaye and Krishnaraj Ramaswamy. The original draft was written by Abel Saka and Leta Tesfaye, with review and editing done by Abel Saka, Leta Tesfaye, and Krishnaraj Ramaswamy.
Funding
There is no funding for this study.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




