Metabolic Profiling and Early Diagnosis of Alcoholic Fatty Liver Disease Using Support Vector Machine Model
Abstract
Alcoholic fatty liver disease (AFLD) is one of the most common pathological changes associated with alcoholic liver disease. This study is aimed at investigating the specific metabolic changes occurring in AFLD and to develop a diagnostic method based on the blood metabolomics of AFLD. Twenty-four rats were randomly divided into an AFLD group and a control group. The AFLD model was established by administering 40% alcohol and verified by pathologic examination. Metabolic changes in the blood were investigated by GC-MS, and both hepatic function and metabolic ability were assessed. Using the metabolic data, a diagnostic model was developed with a support vector machine (SVM). The model was validated using cross-validation techniques and achieved a classification accuracy of 100%. Additionally, there were statistically significant differences in metabolic, hepatic function, and pharmacokinetic changes between the two groups. The level of urea, hydroxysuccinic acid, 2-propenoic acid, total cholesterol, and high-density lipoprotein cholesterol increased, area under the concentration-time curve (AUC) (0 − t), AUC (0 − ∞), and Cmax of phenacetin shorten in the AFLD group (p < 0.05). The classification accuracy of the SVM model based on metabolic data was 100%. In conclusion, the metabolic ability was heightened in the early stage of AFLD, leading to accelerated metabolism of urea and hydroxysuccinic acid. The SVM model can be used to detect early changes in AFLD based on metabolic data.
1. Introduction
Alcoholic liver disease (ALD) is one of the most common liver diseases in the world [1]. Because of changed modern lifestyle and habit, the population of alcoholics has risen sharply in recent years, which directly increased the incidence of ALD. So far, in many industrialized and developing countries, ALD has become a more serious health issue than other liver diseases, such as acute viral hepatitis and hepatapostema [2].
Severe ALD leads to a series of complications, such as high bilirubin concentration, hypoprothrombinemia, and hepatic encephalopathy. Alcoholic fatty liver disease (AFLD) is the most common pathological change in the early stage of ALD [3]. In long-term alcohol consumers, 90%–100% of them will develop AFLD, 10%–35% of them will have alcoholic hepatitis, and 8%–20% of them will suffer liver cirrhosis [4]. AFLD is the main cause of fat metabolic disorders, including decreases in fatty acid oxidation rate and increases in the synthesis of triglycerides [5]. Such changes may lead to fat accumulation in the liver and transform liver cells turn into fat cells in differentiation formation, which affect the detoxification metabolism of the liver [6]. Moreover, excess fat is not without consequences. In fact, hepatic steatosis contributes to the progression towards liver fibrosis [7]. Therefore, early diagnosis and treatment will be helpful to control or delay the progression of AFLD.
So far, there have been few studies focused on the early blood metabonomics of AFLD. Xue et al. investigated the effects of Lactobacillus rhamnosus GG on AFLD based on hepatic and fecal metabolomics; however, they did not focus on the blood metabonomics of AFLD [8]. The liver is rich in cytochrome P450 (CYP) enzymes, which are involved in the metabolism of many endogenous and exogenous substances. Studies have shown that the process of alcoholic liver formation is accompanied by changes in CYP enzyme activities [9–11]. CYP enzymes, particularly subfamilies such as CYP2E1, are known to be induced by chronic alcohol consumption, leading to increased oxidative stress and lipid peroxidation, which contribute to liver injury. In theory, the progression of AFLD, accompanied by changes in CYP enzyme activities, leads to altered metabolism of various substances present in the bloodstream.
Gas chromatography combined with mass spectrometry (GC-MS) is a powerful analytical platform in metabolomics. The analyte eluted from capillary gas chromatography (GC) columns are detected by mass spectrometer which will provide a plenty of information of analytes. The objectives of this study were to investigate the metabonomic profile of AFLD in the blood based on GC-MS and to evaluate the diagnostic value of metabolic information for early AFLD. In order to predict AFLD accurately, support vector machine (SVM), a kind of machine learning method, was used to develop a diagnostic model.
2. Material and Methods
2.1. Chemicals
Metroprolol, phenacetin, tolbutamide, diazepam (used as the internal standard (IS)), trimethylchlorosilane (TMCS) and N-methyl-N-(trimethylsilyl) trifluoroacetamide (all >98%) were purchased from Sigma-Aldrich (St. Louis, USA). The ultrapure water was prepared by Milli-Q purification system (Millipore Bedford, USA). Methanol and acetonitrile were HPLC grade (Merck Company, Darmstadt, Germany).
2.2. Animals
A total of 24 male Sprague-Dawley rats (Shanghai SLAC Laboratory Animal Co., Ltd, China), weighted 220 ± 20 g, involved in this study. They were housed at a 22°C and natural light–dark conditions for a week. The experiment was approved by the Wenzhou Medical University Administration Committee of Experimental Animals (approved ethics number: wydw 2023-0011).
2.3. Instruments
The analysis of metroprolol, phenacetin, and tolbutamide was performed with an Agilent 1200 Series liquid chromatograph combined with a Bruker Esquire HCT mass spectrometer (Bruker Technologies, Bremen, Germany), which is equipped with an electrospray ion source and controlled by Agilent ChemStation software (version B.01.03 [204], Technologies, Waldbronn, Germany).
An Agilent 6890N-5975B GC/MS and HP-5MS (0.25 mm × 30 m × 0.25 mm) was used to analyze the compounds (Agilent Company, Santa Clara, California, USA). The temperature of GC oven was set at 80°C, then kept for 5 min, it was increased to 260°C at a rate of 10°C/min, and then kept for 10 min at 260°C. MS detection was set at EI mode with electron energy of 70 eV, and then in full-scan mode with an m/z of 50–550, at last, by splitless mode injection.
2.4. Experiments Design
The 24 rats (220 ± 20 g) were randomly divided into the AFLD group and control group each with 12 rats. The rats in the AFLD group were given alcohol (40%) (alcohol in water, v/v) by oral administration (4 g/kg/day) every morning, which lasted for 8 weeks. The control group was given saline. In 8 weeks, the blood sample was collected for examination of the liver and kidney function, metabonomics, and pharmacokinetic study. After the collecting the blood sample, the rats of the two groups were anesthetized with 200 mg/kg pentobarbital sodium and the liver tissues were rapidly isolated for pathological observation by routine hematoxylin-eosin (HE) method.
2.5. Blood Metabonomic Study
At the morning after 8 weeks, 0.3 mL of the blood samples was obtained from the caudal vein and centrifuged at 10,000g for 5 min. Then, 100 μL serum was separated and added with 250 μL acetonitrile in an ice bath. A total of 150 μL supernatant was transferred into a tube and evaporated by nitrogen gas. After that, 50 μL of methylhydroxylamine hydrochloride and 50 μL catalyst (MSTFA with 1% TMCS) were added and the methoximation carried out at 70°C for another hour. The QC sample was collected by equally mixing the rat serum of the AFLD and control groups and processed by using the same method as the samples.
The metabonomic data was processed by using SIMCA 13 software. Firstly, the GC-MS peaks of the metabolites of the AFLD and control groups were analyzed by principal component analysis (PCA) and partial least squares discriminate analysis (PLS-DA). The high values of variable importance for project (VIP) (>1) scores were further analyzed and identified in the NIST (National Institute of Standards and Technology) 2005 mass spectral library (NIST, Gaithersburg, MD, USA). For more information, please refer to our previous work [12].
2.6. Hepatic Function and Metabolic Ability
After completing the metabonomic study, 0.3 mL of blood was drawn from the tail vein for liver function tests. The liver function was evaluated using a clinical biochemistry test conducted by a fully automated biochemical analyzer.
Subsequently, the liver’s metabolic ability was assessed using a cocktail method, which was conducted as follows. Three probe drugs mixed in corn oil were orally given to rat at a single dosage, 10 mg/kg for metroprolol and phenacetin, and 0.1 mg/kg for tolbutamide. After that, 0.3 mL of blood samples was collected at 0.0833, 0.5, 1, 2, 3, 4, 6, 8, 12, 24, and 36 h from the tail vein. The blood samples were centrifuged for 10 min at 14000g/min, after that 100 μL plasma was drawn into a 1.5-mL centrifuge tube and then followed by adding 200 μL of acetonitrile (containing 50 ng/mL IS). The mixture was centrifuged at 13000g/min for 15 min. A total of 2 μL supernatant was used for analysis in the LC-MS system.
The LC-MS analytical conditions, calibration curves, and validation of method including accuracy, precision, recovery, and stability were all conducted according to our previous works. The main pharmacokinetic parameters of the two groups were analyzed by pharmacokinetics DAS V 3.0.
2.7. Data Analysis and SVM Model
The statistic difference between the AFLD group and control group was analyzed by independent samples t-test. The diagnostic values of metabolic and pharmacokinetic data of AFLD were evaluated by Fisher’s discrimination. The SVM model of AFLD was developed as follows. The target data of classification task was set as {xi, yi}, i = 1, ⋯l, yi ∈ {−1, 1}, xi ∈ Rd. In the formula, xi means the data points of input vector, and yi means the corresponding output labels, l means the number of training, and d means the dimensions of input data.
The RBF Gaussian kernel was adopted as decision function in this study. The parameter γ defines the nonlinear mapping from the input space to some high-dimensional feature space. The SVM model was developed in MATLAB 2011a by using LibSVM package. For further details regarding SVM, please refer to our previous references [13, 14].
3. Results
3.1. Development of AFLD Model
According to the routine HE staining method of the liver, there was morphologic change observed in rats of the AFLD group. As a whole, the fatty degeneration of hepatic cell was not serious. Only some of the hepatic cells showed fat vacuoles in the cytoplasm but no ballooning degeneration or alcoholic hyaline was observed. The structure of hepatic lobules maintained integrity; the portal area and central vein can be recognized clearly. Therefore, the early AFLD model was successfully developed in the AFLD group, the pathological examination is shown in Figure S1.
3.2. Metabolic Changes in Serum
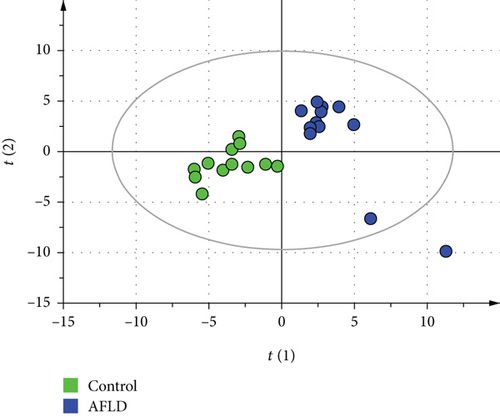
The representative GC-MS spectra of the AFLD group and control group are shown in Figure 1. There was a total of 327 metabolites assigned according to retention time (RT) and identified using the NIST 2008 MS database. The GC-MS metabolic data of the AFLD group and control group were analyzed by PLS-DA. Although some rats of the AFLD group were mixed with the rats of the control group in the PCA score chart, they were separated well in the PLS-DA score chart. In the PLS-DA model, the R2Y and Q2 values were 0.855 and 0.721, respectively; their score charts are shown in Figure 2.
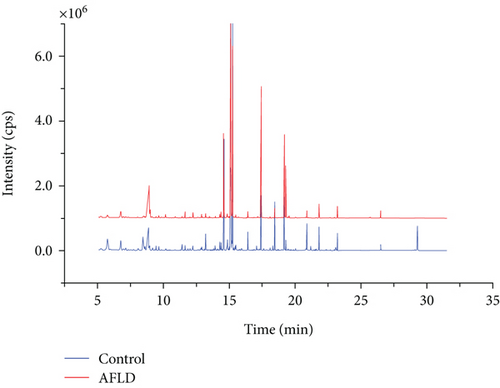
According to the PLS-DA model, there were 15 metabolites that had VIP > 1.0 which are listed in Table 1. The most important compound was urea, followed by hydroxysuccinic acid, propanoic acid, and ethanedioic acid. Among these metabolites, most of metabolites were increased in the AFLD group, except for butanedioic acid and xylitol which had no statistical difference.
| No. | Metabolite | VIP | Change | p |
|---|---|---|---|---|
| 1 | Urea | 1.84153 | ↑ | <0.001 |
| 2 | Propanedioic acid | 1.68595 | ↑ | <0.001 |
| 3 | Propanoic acid | 1.68009 | ↑ | <0.001 |
| 4 | Ethanedioic acid | 1.63644 | ↑ | 0.001 |
| 5 | Butanedioic acid | 1.60443 | ↑ | <0.001 |
| 6 | Acetamide | 1.4671 | ↑ | 0.002 |
| 7 | Butanoic acid | 1.44953 | ↓ | 0.002 |
| 8 | Hydroxysuccinic acid | 1.36056 | ↑ | 0.012 |
| 9 | Tyrosine | 1.35835 | ↑ | 0.007 |
| 10 | dl-Xylitol | 1.30823 | ↑ | 0.008 |
| 11 | Ethanol | 1.30654 | ↑ | 0.007 |
| 12 | Disiloxane | 1.30624 | ↓ | 0.011 |
| 13 | 2-Propenoic acid | 1.26800 | ↑ | 0.011 |
| 14 | 2,4,6-Trifluoroaniline | 1.24516 | ↑ | 0.016 |
| 15 | Benzoic acid | 1.20894 | ↑ | <0.001 |
- Note: Variable importance for project (VIP) scores obtained from PLS-DA analysis; p value obtained from independent samples t-test.
3.3. Hepatic Indexes and Pharmacokinetic Parameters
The indexes of liver function are shown in Table 2. Alanine aminotransferase, aspartate aminotransferase, total cholesterol, and high -density lipoprotein cholesterol values were increased in AFLD; total cholesterol and high-density lipoprotein cholesterol were significantly increased (p < 0.05).
| Indexes | Control group | AFLD group |
|---|---|---|
| Total bilirubin | 2.38 ± 2.46 | 2.73 ± 3.55 |
| Indirect bilirubin | 1.12 ± 2.41 | 0.44 ± 0.44 |
| Direct bilirubin | 1.27 ± 0.46 | 2.29 ± 3.17 |
| Alanine aminotransferase | 56.42 ± 17.55 | 71.82 ± 34.12 |
| Total protein | 70.98 ± 5.22 | 71.84 ± 8.11 |
| Albumin | 28.79 ± 3.67 | 29.97 ± 3.34 |
| Globulin | 42.18 ± 2.80 | 41.86 ± 6.21 |
| Albumin–globulin ratio | 0.67 ± 0.10 | 0.72 ± 0.10 |
| Aspartate aminotransferase | 241.50 ± 35.11 | 275.55 ± 12.03 |
| Total cholesterol | 1.55 ± 0.22 | 2.02 ± 0.23* |
| Triglyceride | 1.38 ± 0.25 | 1.39 ± 0.19 |
| High-density lipoprotein cholesterol | 0.93 ± 0.15 | 1.22 ± 0.16* |
| Low-density lipoprotein cholesterol | 0.38 ± 0.04 | 0.39 ± 0.07 |
- Note: Total cholesterol and high-density lipoprotein cholesterol were significantly increased in the AFLD group.
- *p < 0.05.
The pharmacokinetic parameters of the three probe drugs were analyzed at a noncompartment model (Table 3). There was no difference in pharmacokinetic parameters of metroprolol and tolbutamide between the AFLD group and control group. While for phenacetin, compared with the control group, area under the concentration-time curve (AUC) (0 − t) decreased (p < 0.05), CL increased (p > 0.05), and Cmax decreased (p < 0.05). Therefore, the metabolism of phenacetin was increased in the AFLD group, which indicated that the catabolism abilities of CYP enzymes were enhanced.
| Parameters | AUC (0 − t) | AUC (0 − ∞) | t1/2z | CLz/F | Vz/F | Cmax | |
|---|---|---|---|---|---|---|---|
| (ng/mL ∗ h) | (ng/mL ∗ h) | h | (L/h/kg) | (L/kg) | (ng/mL) | ||
| Phenacetin (CYP1A2) | Control | 3318.9 ± 1998.2 | 3325.7 ± 2000.5 | 0.6 ± 0.1 | 6.6 ± 9.9 | 6.0 ± 10.0 | 1601.7 ± 764.7 |
| AFLD | 1958.0 ± 1147.8* | 1967.0 ± 1148.5* | 0.6 ± 0.2 | 7.5 ± 5.5 | 7.4 ± 7.0 | 991.0 ± 464.4* | |
| Tolbutamide (CYP2C11) | Control | 73877.9 ± 9591.0 | 88637.0 ± 18457.4 | 13.3 ± 6.4 | 0.001 | 0.021 ± 0.008 | 3709.4 ± 646.2 |
| AFLD | 71296.3 ± 23490.3 | 93425.8 ± 46449.9 | 11.8 ± 5.0 | 0.001 ± 0.001 | 0.021 ± 0.010 | 3925.1 ± 1614.4 | |
| Metroprolol (CYP2D1) | Control | 1464.8 ± 910.3 | 1521.6 ± 940.5 | 1.9 ± 2.6 | 9.6 ± 7.3 | 47.9 ± 115.9 | 618.1 ± 350.4 |
| AFLD | 1199.5 ± 600.1 | 1206.5 ± 599.2 | 1.1 ± 0.8 | 12.5 ± 13.4 | 33.6 ± 84.3 | 485.3 ± 216.9 | |
- Note: For phenacetin (CYP1A2), AUC (0 − t) and Cmax show statistically significant differences in the AFLD group, the AUC and Cmax values are lower than in the control group.
- *p < 0.05.
3.4. SVM Model
The metabolic data was normalized by “premnmx” into the interval of [-1, 1] and trained separately. After training, when the penalty coefficient (c) and Kernel function (g) was 1024, g was 2, the classification accuracy reached 100%, and the mean squared error of regression was 3.7693. The classification accuracy of metabolic data was improved obviously between the control group and AFLD group, which was better than that in Fisher’s classification. Using the stepwise statistical method and Fisher’s classification, the results indicated that 90.6% of the original grouped cases were correctly classified, as well as 90.6% of the cross-validated grouped cases. The two-dimensional classification models of metabolic data are shown in Figure 3.
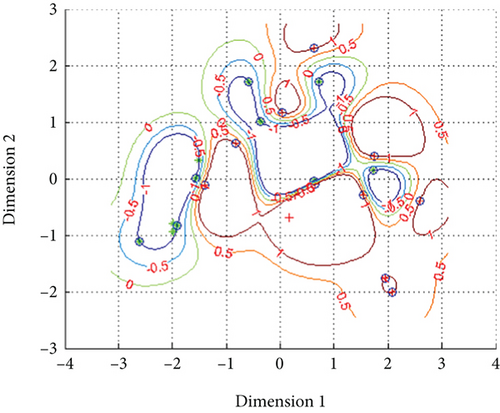
4. Discussion
AFLD model is specifically designed to study liver damage due to chronic alcohol consumption, capturing the direct effects of alcohol metabolism on the liver tissue. It is directly translatable to alcohol-related liver diseases observed in humans, making it valuable for studying potential therapeutic interventions targeting alcoholic liver conditions. Tan et al. [15] reported that administration of 15% (v/v) alcohol in the drinking water caused AFLD in mice, but it needs over 8–9 months. In this study, we selected high-purity edible alcohol to develop the AFLD model within 8 weeks, which was diluted to 40% alcohol by pure water. The high-purity edible alcohol contains alcohol of >96% and little impurity which guarantees the development of the AFLD model. During the modeling, there was a rat in the AFLD group that died at 7 weeks. Rats in the AFLD group were more easily irritated than those in the control group, which made intragastric administration difficult. We speculated that the rat may have died of injury of intragastric administration.
In order to identify the AFLD model, we conducted pathological examination of the liver. Only some of the hepatic cells that showed fat vacuoles in the cytoplasm which indicated that the developed AFLD model was in the early stage. The metabolic study showed that the level of urea, hydroxysuccinic acid, and 2-propenoic acid was increased in AFLD, and all had statistical difference (p < 0.05). Urea is a product of urea cycle (also named ornithine cycle); hydroxysuccinic acid is an intermediate in the citric acid cycle. Their increased levels indicated that the metabolic abilities of AFLD strengthened and the urea cycle and citric acid cycle accelerated, which produced more urea and hydroxysuccinic acid in the AFLD group. The study of hepatic function and metabolic ability showed that the total cholesterol and high-density lipoprotein cholesterol increased and the metabolism of phenacetin accelerated in the AFLD group, which indicated that the lipid metabolism and metabolic ability of CYP were induced. Therefore, according to the metabolic ability, we can infer that the metabolic ability of the liver was induced in the early stage of AFLD.
Since the early diagnosis of AFLD is very important, the diagnostic value of metabolic data was evaluated. The Fisher’s discrimination showed the diagnostic accuracy of the metabolic data similar with the hepatic indexes and pharmacokinetic parameters. And in order to develop a more accurate diagnostic model, we introduced SVM. SVM is kind of machine learning method [16, 17], which has been used to solve many nonlinear classification problems in the medical area, including hepatic disease [17]. In this study, although the classification accuracy of metabolic data was improved by the SVM model, which indicated that SVM is a powerful method, we should introduce more machine learning method in the medical area.
Although the study on AFLD presents promising findings, it used a short exposure period (8 weeks) and had a small sample size of rats, which may not reflect the gradual development of AFLD in humans. Besides, AFLD model may not incorporate other factors that affect liver disease progression in humans, such as obesity, viral infections, or genetic predispositions. While the SVM model achieved 100% accuracy in classifying metabolic data, the model was not externally validated using an independent dataset. Additionally, the GC-MS group has certain limitations and cannot fully capture the entire metabolism of AFLD. Therefore, it is necessary to conduct longitudinal studies to track AFLD progression and introduce proteomics and genomics research to reveal AFLD progression in the future.
5. Conclusion
An AFLD model was developed by the administration of 40% alcohol. Pharmacokinetic assessment of the probe drugs suggested that phenacetin metabolism was accelerated in the AFLD group, while metabolism of tolbutamide and metoprolol remained unchanged. The metabolic ability was strengthened in early AFLD stage, which caused the metabolism of urea and hydroxysuccinic acid to be accelerated. According to the metabolic data, a SVM model was developed, which had high accuracy in early diagnosis of AFLD, and better than Fisher’s classification.
Nomenclature
-
- ALD
-
- alcoholic liver disease
-
- AFLD
-
- alcoholic fatty liver disease
-
- AUC
-
- area under the concentration-time curve
-
- Cmax
-
- peak serum concentration
-
- CYP
-
- cytochrome P450
-
- TMCS
-
- trimethylchlorosilane
-
- HE
-
- hematoxylin-eosin
-
- PCA
-
- principal component analysis
-
- PLS-DA
-
- partial least squares discriminate analysis
-
- RT
-
- retention time
-
- SVM
-
- support vector machine
-
- VIP
-
- variable importance for project
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research is supported by the health research project of the National Natural Science Foundation of China (82104283) and Wenzhou Municipal Science and Technology Bureau (Y2020096 and 20220191).
Acknowledgments
This work is supported by the health research project of the Wenzhou Science and Technology Bureau (Y2020096 and Y20220191), Zhejiang Provincial Medical Association Project (YS2022-2-007), and Medical Science and Technology Project of Zhejiang Province (2024KY406).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




