Assessing the Efficacy and Safety of Intradermal Injection of Different Doses of Botulinum Toxin Type A: A Randomized, Double-Blind, Placebo-Controlled, Split-Face Pilot Study in Rosacea Patients with Erythematic Telangiectasia
Abstract
Introduction. Rosacea is a common chronic inflammatory skin disease of the central facial skin with unknown origin, significantly impacting quality of patient’s life and causing various psychosocial problems. Erythematotelangiectatic rosacea (ETR) is characterized by paroxysmal flushing that occurs repeatedly and is easily resistant to therapeutic drugs. While microinjection of type A botulinum toxin (BTX) can treat ETR, there is no consensus on the injection dose, and strong evidence to verify its efficacy and safety is lacking. This randomized, double-blind, split-face clinical study aimed to investigate the efficacy, safety, and optimal dose of two different single-point injection doses (0.5 U and 1 U) of BTX in the treatment of rosacea. Methods. Twenty-six patients with ETR were randomly assigned to receive different single-point injections of BTX (0.5 U and 1 U, respectively) every 1 cm on one half of the face. The Clinical Erythema Score (CEA), VISIA red area absolute value, Global Aesthetic Improvement Scale Score (GAIS), and recurrence at 12-week follow-up were evaluated at baseline, 2,4, 8, and 12 weeks after injection. Additionally, the Dermatological Quality of Life Index (DLQI) questionnaire survey and adverse reactions were also recorded. Results. All twenty-six patients completed the follow-up visits and were included in the analysis. Compared to the 0.5 UBTX-treated side, the CEA score showed significantly improvement in erythema and flushing at 2, 4, and 8 weeks after injection on the 1 U BTX-treated side (P < 0.05). The mean absolute value of the red area of VISIA was -19.12 ± 51.91 on 1 U BTX-treated side and 2.5 ± 42.08, on 0.5 UBTX-treated side at 4 weeks after treatment, showing significant improvement on the 1 U side (P < 0.05). GAIS and DLQI were also significantly improved from Week 4 to Week 12 and Week 2 to Week 12, respectively (P < 0.05). There was no recurrence of symptoms with either 0.5 U or 1 U injection by 12 weeks. Apart from one patient who experienced facial tightness and three patients who had temporary aggravation of erythema, all of which resolved without treatment, 22 patients did not report any side effects except for injection pain during the procedure. Conclusions. BTX-A can significantly improves symptoms and quality of life in patients with refractory rosacea with few side effects. A single-point injection of 1 U was more effective. This trial is registered with NCT06282679.
1. Introduction
Rosacea is a common chronic inflammatory skin disease affecting the central facial skin, with an unknown origin. The etiology and pathophysiology of rosacea are poorly understood. It is often characterized by sudden facial eruptions of large coalescing pustules, nodules, and cystic structures with diffuse erythema and edema involving the central face, forehead, and chin [1]. Rosacea is often difficult to control, and significantly impacts quality of life, causing various psychosocial problems [2]. Studies have shown that patients with rosacea have higher rates of embarrassment, social anxiety, depression, and decreased quality of life compared to other populations [3]. Therefore, early treatment and attention to patients’ mental health can improve the quality of life for those with rosacea.
The ideal treatment for rosacea is limited, and most patients experience recurrent episodes for several years, requiring intermittent treatment. Patients with erythema telangiectasia are resistant to treatment due to impaired skin barrier function and skin sensitivity, resulting in recurrent episodes of paroxysmal flushing symptoms [4]. Thus, safe and effective treatments to relieve the suffering of rosacea patients are urgently needed.
Even though the pathologic mechanism of rosacea is largely unknown, existing evidence reveals that the abnormal neuro vascular regulation in rosacea may be related to the release of various neuro peptides such as substance P and vasoactive intestinal peptide (VIP) [5]. Studies have also shown that the mechanism of increased skin blood flow involves sympathetic cholinergic nerves and acetylcholine release. Among them, VIP and pituitary adenylate cyclase-activating peptide (PACAP) are present in the skin as active vasodilators and can be associated with acetylcholine colocalization [6].
In recent years, type A botulinum toxin (BTX) has attracted significant attention due to its ability to inhibit the release of VIP substances and acetylcholine (Ach) [7]. BTX is derived from Clostridium botulinum, and acts as a muscle relaxant by inhibiting the release of Ach from presynaptic vesicles at the neuromuscular junction of peripheral nerve terminals [8]. Additionally, BTX can reduce axonal reflex and neurogenic vasodilation in human skin and inhibits their release. Previous reports have documented the efficacy and safety of the same single dose of intradermal BTX injections for the treatment of facial erythema and flushing [9–13].
Currently, microinjection therapy is the primary clinical approach for utilizing BTX type A in the treatment of rosacea. Nevertheless, the recommended injection dose of BTX for the treatment of rosacea varies per injection site in previous studies, and there is still a lack of clear dosage recommendations and specific suggestion for Chinese patients. Moreover, to date, no published study has assessed the effects of intradermal BTX injections using different doses in split-face studies.
This study aimed to prospectively evaluate the efficacy and safety of different dose of intradermal BTX for facial erythema and improvements in DLQI in subjects designed for split-face studies.
2. Materials and Methods
2.1. Ethics
This study was approved by the Ethics Committee of Nanjing Hospital Affiliated to Nanjing Medical University (Nanjing First Hospital) (KY20220701-03), and was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from each participates, and photographs were published with the consent of the patients.
2.2. Patients and Study Design
This study was a single-center, prospective, randomized, double-blind, split-face clinical study. Each cheek was randomly assigned to receive intradermal 0.5 U BTX or 1 U BTX using a computer-generated randomization method. Other treatments for rosacea were restricted during the 12-week clinical period. A total of 26 individuals with facial flushing were recruited from Department of Dermatology, Nanjing First Hospital Affiliated to Nanjing Medical University from July 2021 to March 2024. The inclusion criteria were as follows:
(1) Participants with mild to moderate erythematotelangiectatic- (ETR-) type and facial erythema rosacea, meeting the standard guidelines of the National Rosacea Society Expert Committee by a dermatologist [14]; (2) Age ranging from 18 to 60 years; (3) Total follow-up period of 12 weeks.
The exclusion criteria for this trial were as follows:
(1) Participants with facial surgery or BTX treatment within 6 months prior to this treatment; (2) facial flushing due to systemic diseases such as auto immune diseases or menopause; (3) A history of BTX allergy; (4) pregnant or breast feeding; (5) treatment for other facial dermatologic or oral conditions, including rosacea 4 weeks before the study and other treatments; (6) neuromuscular underlying diseases such as myasthenia gravis, and amyotrophic lateralizing sclerosis; and (7) use of any oral aminoglycosides, benzodiazepines, or muscle relaxants in the 4 weeks prior to the study.
2.3. Treatment Procedure
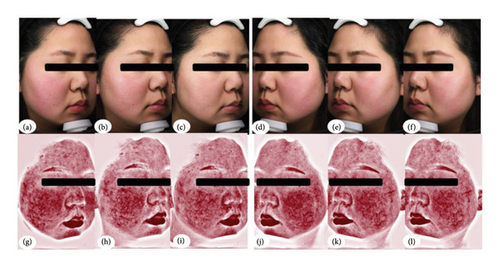
Subjects first received an appropriate amount of lidocaine cream on the face and waited about 30–45 minutes for the anesthetic to take effect. Subsequently, the lidocaine was cleaned off and the injection points were marked on the erythema area of each participator’s face with 25–30 points on one cheek, at intervals of 1 cm. BTX type A, reconstituted from a 50-unit Botox Vial of on a botulinum toxin A (Allergan Pharmaceuticals Ireland Limited, Import drug registration number: S20171003), was diluted to a concentration of 4 U per 0.1 mL with 1.25 mL saline for injection and administered using a 30-gauge needle. A total of 25–30 units of BTX were used for one cheek. The remaining BTX diluted again with the same volume of saline, was used on the other cheek. Using a randomized Split-Face Pilot Study, 0.5 U (a volume of 0.025 mL) of BTX type A was injected at each point on one cheek, and 1 U (a volume of 0.025 mL) was injected on the other cheek. The total amount injected on the opposite side of the face treated with 0.5 U/0.025 mL was between 12.5 and 15 U (Figure 1).

2.4. Postoperative Precautions
- (1)
After 24 hours of treatment, the face should be properly cleaned to avoid high-temperature environments
- (2)
Avoid taking aminoglycosides, benzodiazepines, or muscle relaxants within 2 weeks of treatment
2.5. Assessment Criteria
The Clinical Erythema Score (CEA) was evaluated by a nontreating investigator. We evaluated patients using the Global Aesthetic Improvement Scale Score (GAIS), Dermatology Life Quality Index (DLQI) [15], red area absolute value of VISIA, and adverse reactions at 0, 2, 4, 8, and 12 weeks after treatment. Additionally, we assessed recurrence at 12-week follow-up in patients on both sides using the CEA score. Among these parameters, CEA (Table 1) and GAIS [16, 17] indicate the severity of rosacea and were evaluated by nontreating physicians. The Physician Global Aesthetic Improvement Scale (PGAIS) was determined using side-by-side comparisons with baseline photographs (1 = very much improved, 2 = marked improvement, 3 = improved, 4 = no change, and 5 = worse).
| CEA | |
|---|---|
| 0 = clear | Clear skin with no signs of erythema |
| 1 = almost clear | Almost clear; slight redness |
| 2 = mild | Mild erythema, definite redness |
| 3 = moderate | Moderate erythema; marked redness |
| 4 = severe | Severe erythema; fiery redness |
- CEA, clinician erythema assessment.
2.6. Statistical Analysis
The SPSS 22.0 software (IBM Co., Ltd., NY, USA) was used for statistical analysis. Dynamic changes in each parameter were calculated as the posttreatment value minus the pretreatment value of each visit. Paired nonparametric Wilcoxon tests were used to compare weekly changes in cheek parameters (i.e., 0.5 U BTX vs 1 UBTX). Measurement data conformed to the normal distribution and were expressed as mean ± SEM (x ± s). The scores of the two groups were compared using an independent sample t-test. Count data were tested by χ2 test. Statistical significance was set as P < 0.05.
3. Results
3.1. Patients
A total of twenty-six patients, including two males and twenty-four females, diagnosed with telangiectasia rosacea were enrolled in the study. None of the patients discontinued the study. The age of the participants were ranged from 23 to 51 years, with a mean age of 33.2 ± 7.3 years. The duration of the disease varied from 7 to 240 months, with an average duration of 52.4 ± 51.7 months.
3.2. Alterations of CEA and GAIS Scores with BTX treatment
As shown in Table 2, the mean CEA scores were −0.77 ± 0.75, −1.23 ± 1.05, −1.35 ± 0.94, and −1.23 ± 0.95 on the 1 U BTX-treated cheek and −0.19 ± 0.8, −0.77 ± 0.71, −0.73 ± 1.04 and −0.5 ± 0.71 on the 0.5 U BTX-treated cheek at 2, 4, 8, and 12 weeks after treatment, respectively. The mean CEA scores between patients treated with 1 U BTX and 0.5 U BTX were statistically different from weeks 2 to 8, with the 1 U BTX injection being more effective than the 0.5 U BTX-treated side. Besides, the mean GAIS was 3.31 ± 1.06.2 ± 0.57.1.69 ± 0.97, and 1.62 ± 1.17 on 1 U BTX-treated cheek and 1.27 ± 1.22.1.58 ± 0.86, 0.96 ± 1.08, and 1.23 ± 1.11 on 0.5 U BTX-treated cheek at 2, 4, 8, and 12 weeks after treatment, respectively. The mean GAIS scores of 1 U BTX-treated side were significantly higher at weeks 4, 8, and 12 (all with P < 0.05; Table 3).
| Group | W2-0 | W4-0 | W8-0 | W12−0 |
|---|---|---|---|---|
| 1 U BTX-treated side (n = 26) | −0.77 ± 0.75 | −1.23 ± 1.05 | −1.35 ± 0.94 | −1.23 ± −0.95 |
| 0.5 U BTX-treated side (n = 26) | −0.19 ± 0.8 | −0.77 ± 0.71 | −0.73 ± 1.04 | −0.5 ± 0.71 |
| w/t | 78 | 76 | 103 | 64 |
| p | 0.0232 | 0.0458 | 0.0195 | 0.0684 |
| Group | W2 | W4 | W8 | W12 |
|---|---|---|---|---|
| 1 U BTX-treated side (n = 26) | 3.31 ± 1.06 | 2 ± 0.57 | 1.69 ± 0.97 | 1.62 ± 1.17 |
| 0.5 U BTX-treated side (n = 26) | 1.27 ± 1.22 | 1.58 ± 0.86 | 0.96 ± 1.08 | 1.23 ± 1.11 |
| w/t | −50 | −99 | 87 | −85 |
| p | 0.106 | 0.0257 | 0.019 | 0.0213 |
3.3. Red Area Absolute Value of VISIA
The mean red area absolute values were −2.65 ± 56.04, −19.12 ± 51.91, −17.62 ± 50.58, and −1.92 ± 49.02 on the 1 U BTX-treated cheek, and 4.77 ± 55.87, 2.5 ± 42.08, −0.92 ± 57.67, and −7.19 ± 45.92 on 0.5 U BTX-treated cheek at 2, 4, 8, and 12 weeks after treatment, respectively. Compared with the pretreatment values, the red area absolute value of the 1 U BTX-treated side was significantly lower than that of the 0.5 U BTX-treated side at weeks 4 (P < 0.05; Table 4, and Figure 2).
| Group | W2-0 | W4-0 | W8-0 | W12−0 |
|---|---|---|---|---|
| 1 U BTX-treated side (n = 26) | −2.65 ± 56.04 | −19.12 ± 51.91 | −17.62 ± 50.58 | −1.92 ± 49.02 |
| 0.5 U BTX-treated side (n = 26) | 4.77 ± 55.87 | 2.5 ± 42.08 | −0.92 ± 57.67 | −7.19 ± 45.92 |
| w/t | 41 | 167 | 70 | 26 |
| p | 0.6126 | 0.0328 | 0.3836 | 0.4949 |
3.4. DLQI
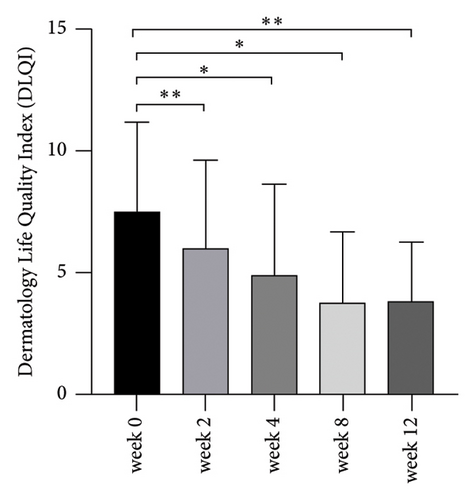
As shown in Figure 3, the DLQI was significantly decreased from weeks 2 to 12 after BTX treatment (P < 0.05; Figure 3).
3.5. Relapses of ETR at 12-Week Follow-Up
As shown in Table 5, the mean CEA was 2.81 ± 0.57 and 0.62 ± 0.75 on the 1 U BTX-treated cheek at baseline and 12 weeks after treatment. The mean CEA scores between patients with the 12 week and baseline on 1 U BTX-treated cheek were statistically different (P < 0.05). Additionally, the mean CEA was 2.62 ± 0.7 and 1.96 ± 0.87 on the 0.5 U BTX-treated cheek at baseline and 12 weeks after treatment. The mean CEA scores between patients with the 12 week and baseline on 0.5 U BTX-treated cheek were also statistically differences (P < 0.05).
| Group | W0 | W12 | T | P |
|---|---|---|---|---|
| 1 U BTX-treated side (n = 26) | 2.81 ± 0.57 | 0.62 ± 0.75 | 5.43 | <0.0001 |
| 0.5 UBTX-treated side (n = 26) | 2.62 ± 0.70 | 1.96 ± 0.87 | 2.988 | 0.0043 |
3.6. Safety
Except for one patient who experienced facial tightness 5 days after the injection, which disappeared spontaneously after 3 weeks without any treatment, and three patients had temporary aggravation of erythema at the injection area after the injections, which disappeared within 3 days, 22 patients participating in the final analysis did not report side effects except for injection pain at the time of the procedure.
4. Discussion
Common treatments for ETR include photoelectric therapies (intense pulsed light, fuel laser or neodymium:yttrium-aluminum-garnet (Nd: YAG), LED red and yellow light, etc.), oral medications (doxycycline hydrochloride, minocycline hydrochloride, carvedilol, hydroxychloroquine, etc.), and topical drugs (0.5% brimonidine, which is not readily available domestically). Actually, we routinely use intense pulsed light or dye laser therapy to target dilated blood vessels and reduce flushing. However, some individuals still experience persistent erythema and burning sensations. Due to the sensitivity of the patient’s skin and impaired barrier function, laser treatments are often intolerable. On the other hand, long-term use of tetracycline antibiotics can lead to some adverse effects such as dysbiosis, bacterial resistance, gastrointestinal issues, dizziness, and drowsiness. Hydroxychloroquine use over time may cause fundus retinopathy [18]. Therefore, there is a need for safe and effective treatments for managing rosacea, especially during the transition from papulopustular rosacea (PPR) to erythema telangiectasia (ETR).
Recent studies have demonstrated that local injections of BTX are safe and effective for ETR [9–13]. The mechanism of BTX in treating ETR involves several neurovascular components. Skin flushing and tingling in ETR patients are associated with some neurotransmitters such as substance P, calcitonin gene-related peptide (CGRP), pituitary adenylate cyclase-activating peptide (PACAP), and transient receptor potential vanilloid (TRPV) receptors 1–4. These factors significantly influence the sensation of discomfort [19, 20]. In addition, vascular dysfunction is crucial in the development and progression of rosacea. Notably, BTX-A addresses these pathogenic mechanisms in several ways: firstly, it inhibits the release of inflammatory mediators, such as substance P and CGRP [21, 22], reducing local skin inflammation and promoting the regression of erythema. Secondly, extracellular BTX-A binds to cholinergic nerve terminal glycoproteins and block intracellular acetylcholine release. Lastly, BTX-A reduces human skin axon reflex and neurogenic vasodilation inhibiting their release and stabilizing overactive blood vessels [10, 23].
Unfortunately, there is no definite recommendation on the injection do seat each spot, and different studies reports different single-point doses for facial flushing and erythema. For instance, Kranendonk et al. [24] used a 40 U/mL concentration of BTX-A solution (BOTOX) injected into a patient’s right cheek at 1 cm intervals, with a single-site injection of 2 U. One week postinjection, the patient developed severe ptosis, likely due to the high concentration and deep injection. Kim et al. [9] conducted a randomized controlled trial where they injected 10 U/mL of BTX-A (NABOTA) or saline into the right and left cheeks of rosacea patients, with 0.5 U per site, totaling 15 U per cheek. The study found significant improvement in both subjective (CEA and GAIS) and objective (erythema index) measures in the BTX-A-treated cheeks, with only mild adverse effects. Calvisi et al. [25] used BTX-A microdrip therapy (∼0.01 mL, 0.2 U) to evaluate mild-to-moderate acne and erythematous rosacea, reporting patient satisfaction with the improvements. Zhang et al. [26] in a systematic review of nine studies on BTX treatment for rosacea found consistent symptom improvement and few side effects. However, the single-site injection doses varied or were not clearly specified across these studies. Given the lack of consensus on the appropriate dosage, further exploration is necessary to determine the optimal dose for BTX treatment in rosacea. Based on the existing studies and considering both efficacy and adverse reactions, we decided to use a 10 U/mL concentration of BTX-A. Intradermal injections of 0.025 ml (1 U) or 0.025 mL (0.5 U) were administered at l cm intervals on either the left or right side of the affected area with the total amount ranging from 15−30 U per cheek.
In this split-face study, we investigated the clinical efficacy and safety of different concentrations (1 U vs. 0.5 U) at each point, of BTX type A for the treatment of patients with ETR for the first time. Compared to pretreatment, the CEA score on the 1 U BTX-treated side exhibited significantly higher improvement compared to the 0.5 U BTX-treated side at 2, 4, and 8 weeks. Additionally, the absolute value of the red zone of VISIA on the 1 U BTX-treated side were significantly different from the 0.5 U BTX-treated side at Weeks 4. Our findings indicate that BTX microdrop therapy is effective for treating ETR, with better results at a 1 U injection volume per point. This aligns with previous research, although earlier studies did not specifically evaluate the optimal single-point dose for erythema and flushing in rosacea. Furthermore, our study introduces a novel therapeutic approach for clinical management of rosacea. The GAIS scores on the 1 U BTX-treated side were significantly higher at 4, 8, and 12 weeks compared to pretreatment, indicating an aesthetic benefit from BTX. Moreover, the DLQI scores showed significant improvement at 2, 4, 8, and 12 weeks posttreatment, consistent with the findings of Gholamreza Eshghi et al. [27], indicating that 1 U BTX treatment significantly enhances patients’ quality of life.
At the 12-week follow-up, CEA scores for both cheeks were significantly lower than at baseline. Although there was a slight gradual recurrence of the redness at 3 months, the levels did not return to baseline, consistent with the findings of Friedman et al. [28].
In this study, some adverse reactions were recorded after BTX treatment, although they were mild and resolved spontaneously. Briefly, one patient developed facial tightness within a week of the injection, which resolved spontaneously after 3 weeks without any treatment. Three patients experienced temporary aggravation of erythema at the injection site, which disappeared within 3 days. We suppose these adverse effects may be related to the area of BTX-A application, the depth of the injection, or the severity of the patient’s condition. In fact, similar adverse reactions were reported in the studies of Kim et al. [9], Park et al. [29], and Dayan et al. [30]. Kim et al. [9] demonstrated that none of the enrolled subjects experienced significant adverse effects such as allergic reaction, facial palsy, or severe muscles paralysis, during or after the study. Mild erythema was common postinjection. Park et al. [29] found that three patients complained of unnatural facial expressions and discontinued participation. Their mild facial paralysis improved within three months without further treatment. Of the total subjects including those who completed the clinical trial early, there was no serious complication during the study period. There were no side effects reported including paralysis or asymmetry. Dayan et al. [30] also noted no adverse effects in two rosacea patients treated with BTX, further confirming its safety.
Our study still has some limitations. For instance, the sample size was small, and the follow-up period was relatively short. Future studies with the larger sample sizes and longer follow-up periods are necessary to determine the optimal injection dose, frequency, and duration of BTX type A for treating ETR. In addition, further research is needed to identify the minimum safe dose per injection site according to the severity of facial erythema.
5. Conclusion
Our study evaluated the appropriate dose, efficacy, and safety of BTX type A at different concentrations (1 U vs. 0.5 U) for each injection point in patients with ETR. Our results suggest that a single injection with a higher dose (1 U) per spot is more effective than 0.5 U, and that intradermally injected BTX can help reduce facial erythema and improve patient quality of life. Despite the small sample size, the split-face study provides valuable insights using CEA and GAIS scores and DLQI assessments. Collectively, our findings indicate that intradermal BTX injection is a useful and safe new option for treating ETR-type rosacea, with the 1 U single-point injection doses being more effective.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Authors’ Contributions
Yuan Jiang and Fengyuan Wang are co-first authors.
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.