Comparison of Rocuronium and Succinylcholine for First-Attempt Intubation Success in the Emergency Department
Abstract
Background. Succinylcholine and rocuronium are the predominant neuromuscular blocking agents (NMBAs) used for rapid sequence intubation (RSI) in the emergency department (ED). Prior studies have found reduced first-attempt intubation success (FAIS) with rocuronium compared to succinylcholine. Recent large registry data have shown no difference in intubating conditions or FAIS. Objectives. The objective of this study was to compare FAIS rates for rocuronium and succinylcholine when used for RSI in a high-acuity academic ED. Methods. This was a single-center retrospective study. Patients were included if they received either succinylcholine or rocuronium for RSI in the ED from January 2016 to August 2020. The primary endpoint was FAIS. Subgroup analyses were performed evaluating the impact of weight-based dosing on FAIS for each agent, and multivariate analysis was conducted to evaluate the impact of baseline characteristics on the primary outcome. Results. There were 448 patients who received rocuronium and 183 patients who received succinylcholine. No difference was observed in unadjusted FAIS between patients receiving rocuronium (median weight-based dose: 1.22 mg/kg) or succinylcholine (median weight-based dose: 1.43 mg/kg) (361 (80.6%) vs. 150 (82.0%), p = 0.69). There were no differences in FAIS between the weight-based dose categories for rocuronium and for succinylcholine. Conclusions. These findings were consistent with those from recent studies indicating no difference in FAIS between rocuronium and succinylcholine, although the median dose of rocuronium used in this study was higher than traditionally recommended. Larger prospective studies are warranted to further evaluate the effect of weight-based paralytic dosing on FAIS.
1. Background
Emergency airway management facilitated by rapid sequence intubation (RSI) is commonly performed for critical medical or traumatic illness in the emergency department (ED). Neuromuscular blockade renders the patient flaccid, improving intubation conditions and first-attempt intubation success (FAIS) in appropriately selected patients [1–3].
Succinylcholine and rocuronium are the two most commonly used neuromuscular blocking agents (NMBAs) for RSI. Succinylcholine is a depolarizing agent that acts by mimicking acetylcholine and stimulating both autonomic ganglia and muscarinic receptors of the parasympathetic and sympathetic nervous systems, leading to sustained depolarization. The standard succinylcholine dosing for RSI is 1.5 milligram per kilogram (mg/kg) given intravenously. Succinylcholine has historically been the preferred paralytic due to its rapid onset of action (less than one minute), short duration of four to six minutes, and rapid metabolism by plasma pseudocholinesterase. Additionally, succinylcholine has been associated with higher physician satisfaction due to the extent of paralysis and greater FAIS when compared to rocuronium, although many of these studies used lower doses of rocuronium as compared to the current standard [4, 5].
Because of its depolarizing effects on myocytes, succinylcholine can increase potassium by an average of 0.5 milliequivalents per liter. For the majority of patients, this is of no clinical consequence, but for patients with suspected hyperkalemia or those at risk for an exaggerated intracellular potassium efflux with depolarization, succinylcholine is avoided [6–8]. Risk factors for hyperkalemia include extensive muscle trauma, burns, subacute stroke, and history of degenerative neuromuscular disease or long-term immobility, all of which leads to post-synaptic receptor upregulation [6]. Additionally, to avoid prolonged neuromuscular blockade, careful use or dose reduction must be considered in patients with family history of reduced plasma cholinesterase activity [9, 10]. Succinylcholine is also contraindicated in patients who have a personal or family history of malignant hyperthermia [6].
Conversely, rocuronium is a nondepolarizing neuromuscular blocker which competitively inhibits acetylcholine binding to receptors in the neuromuscular junction and has few contraindications outside of hypersensitivity. Rocuronium dosing ranges from 0.6 to 1.2 mg/kg given intravenously for RSI and has an onset of one minute and a dose-related duration of action ranging from 22 minutes for a 0.45 mg/kg dose to 67 minutes for a 1.2 mg/kg dose [11, 12]. Rocuronium is largely eliminated renally, and its effect can be prolonged in renal dysfunction [11, 12].
Prior studies have reported conflicting data regarding FAIS with rocuronium; however, it is thought that underdosing may have contributed to these outcomes [5, 13–15]. One study reported no difference between rocuronium dosed at 0.6 mg/kg and succinylcholine dosed at 1 mg/kg for incidence of failed intubation attempts in critically ill patients undergoing emergent RSI [16]. Conversely, in a Cochrane review published in 2015, succinylcholine was found to be superior to rocuronium when comparing succinylcholine dosed at 1 mg/kg and rocuronium dosed at 0.6 mg/kg [5]. However, rocuronium dosed at 1.2 mg/kg resulted in equivalent intubating conditions compared with succinylcholine [5]. Another study indicated that RSI with rocuronium dosed at 1 mg/kg followed by reversal with sugammadex allowed earlier reestablishment of spontaneous ventilation than with succinylcholine dosed at 1 mg/kg [17]. A recent study indicated higher FAIS with a direct laryngoscope when rocuronium was dosed at 1.4 mg/kg or greater with no difference in adverse events [18]. An additional study found that when rocuronium was dosed at 1.2 mg/kg, the onset of action was similar to succinylcholine [19]. Outside of these studies, there is limited evidence regarding utilizing rocuronium doses greater than 1.2 mg/kg and its impact on FAIS [20]. During the early months of the COVID-19 pandemic, an emphasis was placed on FAIS to limit staff exposure to the virus. This led to an intentional higher dosing regimen for rocuronium in order to ensure rapid and complete paralysis, limited patient cough, and, presumably, better FAIS [18]. The primary objective of this study is to compare FAIS rates for rocuronium and succinylcholine when used for RSI in adult patients in the ED.
2. Methods
This retrospective, single-center study was conducted at Brigham and Women’s Hospital (BWH) and approved by the Mass General Brigham Institutional Review Board. BWH is a large academic medical center and level 1 trauma center located in Boston, Massachusetts. The ED had 49 licensed beds and averaged 60,000 visits per year at the time of the study. Patient electronic medical records were pulled based on receipt of succinylcholine or rocuronium in the BWH ED, and one encounter was assessed per record. Charts were retrospectively reviewed exclusively by the primary author from January 2016 to August 2020 and if there were any questions, the primary author brought them back to the study team for a consensus. Patients were included if they received either succinylcholine or rocuronium for RSI in the ED. Patients were excluded if they were under 18 years of age, pregnant, received multiple paralytics for intubation, the intubation method was awake intubation using a flexible scope, or there was no intubation note in the chart.
Patients were divided based on whether they received rocuronium or succinylcholine for RSI. The primary endpoint was FAIS, which was defined as confirmed orotracheal placement on first attempt by colorimetric or quantitative end-tidal CO2 and determined by documentation in an intubation note in the patient’s chart. A subgroup analysis of weight-based dosing was performed. Rocuronium weight-based doses were divided into ranges of less than 1 mg/kg, 1.0 to 1.1 mg/kg, 1.2 to 1.3 mg/kg, and greater than or equal to 1.4 mg/kg. Succinylcholine weight-based doses were divided into ranges of less than 1.5 mg/kg, 1.5 to 1.9 mg/kg, and greater than or equal to 2.0 mg/kg. Secondary endpoints included the number of intubation attempts, rates of failed airways indicated by the need for surgical rescue, Cormack and Lehane glottic view, paralytic reversal agent administration for neurologic examination, vasopressor initiation or increased vasopressor requirement after intubation, and time of continuous sedation initiation after paralytic administration. Safety endpoints included change in heart rate, blood pressure, hyperkalemia requiring intervention, and development of complications including cardiac arrest, pneumothorax, esophageal intubation, and main stem intubation.
Baseline characteristics collected included demographic information, renal function, admission diagnosis, intubation category and indication, presumed as well as confirmed COVID-19 infection status, dose of paralytic agent used, and dose of induction agent used. Intubation category was divided into medical vs. trauma, and intubation indication was divided into hypoxia, hypercarbia, inability to protect airway, and procedural. Presumed COVID-19 infection status was defined as whether the patient was suspected or known to be infected with COVID-19 during arrival to the ED for admissions after March 1, 2020. Confirmed COVID-19 status was determined by the results of the SARS-CoV-2 laboratory test for admissions after March 1, 2020. Patients were evaluated for presence of difficult airway as determined by the provider and documented in the intubation note, difficult airway predictors, and contraindications to succinylcholine, including hyperkalemia which was defined as a serum potassium greater than 5.0 mEq/L. Data were collected on Mallampati score, if available, intubation device, intubating provider specialty, and provider type.
The primary endpoint and other categorical data were evaluated using two-tailed chi-square test. Ordinal data were evaluated using Mann–Whitney U and Kruskal–Wallis tests, and parametric data were evaluated using unpaired t-test and ANOVA. A logistic regression was performed to compare patients who were intubated on first attempt vs. patients who required multiple intubation attempts to control for confounders that may have impacted FAIS. Statistical significance was defined as a p value of less than 0.05.
3. Results
A total of 774 charts were identified for eligibility based on retrospective review exclusively by the primary author between the period of January 2016 and August 2020. Of these, 113 charts were excluded, resulting in a total of 631 charts for analysis (448 patients who received rocuronium and 183 patients who received succinylcholine) (Figure 1).
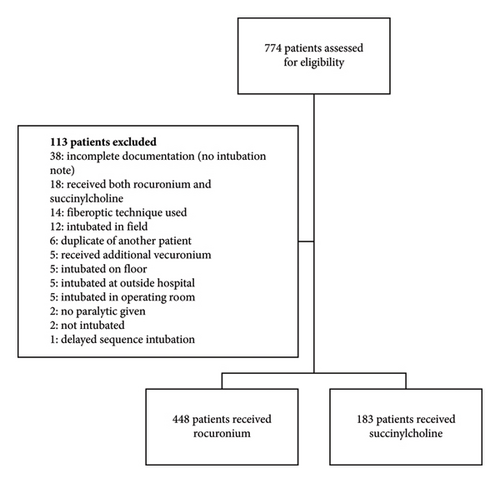
Patient baseline characteristics and comorbidities were similar between the patients who received rocuronium and those who received succinylcholine (Table 1). There were significantly more patients who received succinylcholine that were admitted for angioedema (0 (0%) vs. 3 (1.6%), p = 0.02). Conversely, there were significantly more patients who received rocuronium that were admitted for seizures or status epilepticus (59 (13.2%) vs. 11 (6.0%), p = 0.008). Significantly more patients who received rocuronium were intubated for the indication of hypercarbia (35 (7.8%) vs. 6 (3.3%), p = 0.04). Additionally, there were significantly more patients who received succinylcholine that had a large tongue on presentation as a difficult airway predictor (2 (0.4%) vs. 4 (2.2%), p = 0.04). Patients who received rocuronium received a significantly lower median dose of propofol for induction as compared to those who received succinylcholine (75 mg (60–100) vs. 110 mg (80–150), p = 0.03). No other differences were observed in choice or dosing of induction agents. Significantly more patients who received rocuronium were intubated by an emergency medicine provider, whereas more patients who received succinylcholine were intubated by an anesthesia provider as shown in Table 1. Significantly more patients who received rocuronium were intubated by a resident physician, while more patients who received succinylcholine were intubated by a physician assistant as shown in Table 1. There was no significant difference in the incidence of hyperkalemia prior to intubation (72 (16.1%) vs. 26 (14.2%), p = 0.56). Overall, the median weight-based dose of rocuronium used was 1.22 mg/kg (1.04–1.45), and the median weight-based dose of succinylcholine used was 1.43 mg/kg (1.25–1.67).
| Rocuronium (n = 448) | Succinylcholine (n = 183) | p value | |
|---|---|---|---|
| Age (years) ∗ | 59.6 ± 18.8 | 58.4 ± 17.9 | 0.32 |
| Sex, male† | 260/448 (58.0%) | 108/183 (59.0%) | 0.82 |
| Weight (kg) ∗ | 79.2 ± 21.5 | 80.9 ± 20.2 | 0.36 |
| BMI ∗ | 27.3 ± 6.6 | 28.0 ± 6.3 | 0.16 |
| O2 sat prior to intubation (%)╝ | 98 (95–100) | 98.5 (96–100) | 0.81 |
| Creatinine clearance (mL/min)╝ | 76.2 (44.1–109.3) | 86.4 (58.7–114.2) | 0.02 |
| Admission diagnosis | |||
| AMS† | 55/448 (12.3%) | 14/183 (7.7%) | 0.09 |
| Angioedema† | 0/448 (0%) | 3/183 (1.6%) | 0.02 |
| Burn† | 9/448 (2.0%) | 1/183 (0.5%) | 0.30 |
| Cancer† | 3/448 (0.7%) | 3/183 (1.6%) | 0.36 |
| Cardiac arrest† | 7/448 (1.6%) | 2/183 (1.1%) | 1.0 |
| Endocrine emergency/diabetic ketoacidosis† | 3/448 (0.7%) | 1/183 (0.5%) | 1.0 |
| Fall† | 6/448 (1.3%) | 4/183 (2.2%) | 0.49 |
| Gastrointestinal bleed† | 11/448 (2.5%) | 8/183 (4.4%) | 0.21 |
| Hemorrhagic stroke† | 64/448 (14.3%) | 36/183 (19.7%) | 0.09 |
| Intraabdominal surgical emergency† | 11/448 (2.5%) | 3/183 (1.6%) | 0.77 |
| Ischemic stroke† | 13/448 (2.9%) | 9/183 (4.9%) | 0.23 |
| Motor vehicle accident† | 20/448 (4.5%) | 11/183 (6.0%) | 0.42 |
| Other† | 6/448 (1.3%) | 1/183 (0.5%) | 0.68 |
| Respiratory failure† | 93/448 (20.8%) | 31/183 (16.9%) | 0.32 |
| Seizure/status epilepticus† | 59/448 (13.2%) | 11/183 (6.0%) | 0.008 |
| Shock† | 52/448 (11.6%) | 26/183 (14.2%) | 0.42 |
| Toxic ingestion/overdose† | 15/448 (3.3%) | 7/183 (3.8%) | 0.81 |
| Trauma† | 21/448 (4.7%) | 12/183 (6.6%) | 0.33 |
| Intubation category | |||
| Medical† | 382/448 (85.3%) | 151/183 (82.5%) | 0.39 |
| Trauma† | 66/448 (14.7%) | 32/183 (17.5%) | 0.39 |
| Intubation indication | |||
| Hypoxia† | 71/448 (15.8%) | 26/183 (14.2%) | 0.60 |
| Hypercarbia† | 35/448 (7.8%) | 6/183 (3.3%) | 0.04 |
| Inability to protect airway† | 279/448 (62.3%) | 121/183 (66.1%) | 0.36 |
| Procedural† | 63/448 (14.1%) | 30/183 (16.4%) | 0.45 |
| Dose (mg)╝ | 100 (80–100) | 100 (100–120) | N/A |
| Weight-based dose (mg/kg)╝ | 1.22 (1.04–1.45) | 1.43 (1.25–1.67) | N/A |
| Induction agent | |||
| Etomidate† | 384/448 (85.7%) | 164/183 (89.6%) | 0.19 |
| Dose (mg)╝ | 20 (10 : 20) | 20 (20 : 20) | 0.17 |
| Weight-based dose (mg/kg)╝ | 0.24 (0.18 : 0.30) | 0.24 (0.21 : 0.28) | 0.69 |
| Ketamine† | 33/448 (7.4%) | 6/183 (3.3%) | 0.053 |
| Dose (mg)╝ | 100 (100 : 150) | 100 (100 : 125) | 0.76 |
| Weight-based dose (mg/kg)╝ | 1.6 (1.2 : 1.9) | 1.74 (0.94 : 1.96) | 0.86 |
| Midazolam† | 4/448 (0.9%) | 0/183 (0%) | 0.33 |
| Dose (mg)╝ | 5.5 (4.3 : 7.5) | — | — |
| Weight-based dose (mg/kg)╝ | 0.083 (0.044 : 0.11) | — | — |
| Propofol† | 20/448 (4.5%) | 9/183 (4.9%) | 0.80 |
| Dose (mg)╝ | 75 (60 : 100) | 110 (80 : 150) | 0.03 |
| Weight-based dose (mg/kg)╝ | 1.0 (0.7 : 1.2) | 1.4 (0.8 : 2.0) | 0.06 |
| Lorazepam† | 1/448 (0.2%) | 0/183 (0%) | 1.00 |
| Dose (mg)╝ | 4 | — | — |
| Weight-based dose (mg/kg)╝ | 0.06 | — | — |
| None† | 6/448 (1.3%) | 4 (2.2%) | 0.44 |
| Difficult airway present† | 76/448 (17.0%) | 31/183 (16.9%) | 0.99 |
| Difficult airway predictors present | |||
| Blood in airway† | 28/448 (6.3%) | 16/183 (8.7%) | 0.26 |
| Vomit in airway† | 53/448 (11.8%) | 32/183 (17.5%) | 0.07 |
| Facial trauma† | 15/448 (3.3%) | 7/183 (3.8%) | 0.81 |
| Cervical immobility† | 60/448 (13.4%) | 21/183 (11.5%) | 0.43 |
| Obesity† | 122/448 (27.2%) | 56/183 (30.6%) | 0.39 |
| Airway edema† | 12/448 (2.7%) | 5/183 (1.1%) | 0.97 |
| Small mandible† | 1/448 (0.2%) | 0/183 (0%) | 1.00 |
| Short neck† | 1/448 (0.2%) | 0/183 (0%) | 1.00 |
| Large tongue† | 2/448 (0.4%) | 4/183 (2.2%) | 0.04 |
| Cormack–Lehane view | |||
| Grade 1† | 260/391 (66.5%) | 92/157 (58.6%) | 0.08 |
| Grade 2† | 95/391 (24.3%) | 52/157 (33.1%) | 0.04 |
| Grade 3† | 25/391 (6.4%) | 11/157 (7.0%) | 0.79 |
| Grade 4† | 11/391 (2.8%) | 2/157 (1.3%) | 0.28 |
| Mallampati score | |||
| Grade I† | 16/50 (40%) | 3/8 (37.5%) | 0.89 |
| Grade II† | 15/50 (37.5%) | 5/8 (62.5%) | 0.19 |
| Grade III† | 8/50 (20%) | 0/8 (0%) | 0.32 |
| Grade IV† | 1/50 (2.5%) | 0/8 (0%) | 1.00 |
| Intubation device | |||
| Direct laryngoscopy† | 31/448 (6.9%) | 17/183 (9.3%) | 0.31 |
| Video laryngoscopy† | 381/448 (85.0%) | 155/183 (84.7%) | 0.91 |
| Other or unknown† | 36/448 (8.0%) | 11/183 (6.0%) | 0.38 |
| Intubating provider specialty | |||
| Emergency medicine† | 436/447 (97.5%) | 171/183 (93.4%) | 0.01 |
| Anesthesia† | 8/447 (1.8%) | 12/183 (6.6%) | 0.002 |
| Other† | 3/447 (0.7%) | 0/183 (0%) | 0.56 |
| Intubating provider type | |||
| Attending physician† | 14/445 (3.1%) | 9/182 (4.9%) | 0.28 |
| Resident physician† | 423/445 (95.1%) | 160/182 (87.9%) | 0.001 |
| PGY1† | 81/423 (19.1%) | 22/160 (13.8%) | 0.13 |
| PGY2† | 133/423 (31.4%) | 47/160 (29.4%) | 0.63 |
| PGY3† | 169/423 (40.0%) | 66/160 (41.3%) | 0.78 |
| PGY4† | 37/423 (8.7%) | 25/160 (15.6%) | 0.02 |
| Unknown† | 3/423 (0.7%) | 0/160 (0%) | 0.57 |
| Physician assistant† | 5/445 (1.1%) | 10/182 (5.5%) | 0.001 |
| Fellow† | 3/445 (0.7%) | 3/182 (1.6%) | 0.26 |
| Vasopressor use prior to intubation† | 46 (10.3%) | 13 (7.1%) | 0.22 |
| Contraindications to succinylcholine use | 119/448 (26.6%) | 39/183 (21.3%) | 0.18 |
| Presumed COVID-19 status pre-admit (3/1/20−8/31/2020) | |||
| Positive† | 9/68 (13.2%) | 4/13 (30.8%) | 0.21 |
| Negative† | 38/68 (55.9%) | 6/13 (46.2%) | 0.56 |
| Presumed† | 21/68 (30.9%) | 3/13 (23.1%) | 0.75 |
| Confirmed COVID-19 status post-admit (3/1/20−8/31/2020) | |||
| Positive† | 15/68 (22.1%) | 5/13 (38.5%) | 0.29 |
| Negative† | 48/68 (70.6%) | 7/13 (53.8%) | 0.33 |
| Not tested† | 5/68 (7.4%) | 1/13 (7.7%) | 1.00 |
- ∗Mean ± standard deviation; †n (%); ╝median (IQR).
In this study, we were unable to detect a difference in the primary endpoint of FAIS between patients who received rocuronium and those who received succinylcholine (361 (80.6%) vs. 150 (82.0%), p = 0.69) (Table 2). No difference was observed in percent change in systolic blood pressure or percent change in heart rate as shown in Table 2.
| Rocuronium (n = 448) | Succinylcholine (n = 183) | p value | |
|---|---|---|---|
| FAIS† | 361/448 (80.6%) | 150/183 (82.0%) | 0.69 |
| Change in systolic blood pressure (%)╝ | 2.0 (−10.1–18.5) | 4.2 (−10.1–20.9) | 0.45 |
| Change in heart rate (%)╝ | 3.6 (−1.9–17.5) | 2.4 (−4.9–14.0) | 0.07 |
| Need for vasopressor initiation post-intubation† | 36/402 (9.0%) | 16/170 (9.4%) | 0.87 |
| Need for uptitration of initial vasopressor post-intubation† | 15/46 (32.6%) | 6/13 (46.2%) | 0.51 |
| Need for additional vasopressor post-intubation† | 7/46 (1.6%) | 0/13 (0%) | 0.33 |
| Development of malignant hyperthermia† | 0/448 (0%) | 0/183 (0%) | N/A |
| Development of complications | |||
| Cardiac arrest† | 15/448 (3.3%) | 7/183 (3.8%) | 0.77 |
| Pneumothorax† | 6/448 (1.3%) | 0/183 (0%) | 0.19 |
| Esophageal intubation† | 2/448 (0.4%) | 1/183 (0.5%) | 0.87 |
| Main stem bronchial intubation† | 19/448 (4.2%) | 9/183 (4.9%) | 0.71 |
| Time between paralytic and continuous sedation initiation, hours╝ | 0.17 (0.08–0.37) | 0.15 (0.10–0.32) | 0.30 |
- †n (%); ╝median (IQR).
A logistic regression was performed comparing patients who were intubated on first attempt to those who required multiple attempts (Table 3). Patients who received a higher dose of etomidate for induction were significantly more likely to achieve FAIS (OR: 0.84, 95% CI: 0.71–0.99, p = 0.04). Additionally, patients that lacked the presence of a difficult airway were significantly more likely to achieve FAIS (OR: 6.72, 95% CI: 4.05–11.2, p < 0.01). Finally, significantly more patients who required multiple attempts were ultimately intubated by an attending physician (OR: 0.12, 95% CI: 0.02–0.86, p = 0.04).
| Characteristics | Odds ratio | P value | 95% CI |
|---|---|---|---|
| Age | 1.00 | 0.94 | 0.99–1.01 |
| Sex | 0.77 | 0.27 | 0.48–1.22 |
| Paralytic | 0.87 | 0.60 | 0.52–1.45 |
| Induction agent | |||
| Etomidate | 0.32 | 0.19 | 0.06–1.77 |
| Etomidate dose | 0.84 | 0.04 | 0.71–0.99 |
| Ketamine | 0.30 | 0.49 | 0.01–8.8 |
| Ketamine dose | 0.97 | 0.62 | 0.87–1.09 |
| None | 0.15 | 0.11 | 0.02–1.52 |
| Indication for intubation | |||
| Hypercarbia | 6.73 | 0.02 | 1.29-35.2 |
| Hypoxia | 1.29 | 0.57 | 0.54–3.10 |
| Inability to protect airway | 0.83 | 0.58 | 0.43–1.60 |
| Difficult airway present | |||
| Not difficult | 6.72 | <0.01 | 4.05–11.2 |
| Unknown | 4.02 | 0.01 | 1.44–11.2 |
| Intubation device | |||
| Direct laryngoscopy | 1.75 | 0.29 | 0.63–4.91 |
| Hypercurved video laryngoscopy | 0.48 | 0.06 | 0.22–1.04 |
| Unknown | 0.59 | 0.20 | 0.26–1.32 |
| Intubating provider specialty | |||
| Anesthesia | 1.74 | 0.48 | 0.37–8.20 |
| Intubating provider type | |||
| Attending physician | 0.12 | 0.04 | 0.02–0.86 |
| Resident physician | 0.38 | 0.30 | 0.06–2.35 |
| PGY1 | 2.04 | 0.13 | 0.82–5.07 |
| PGY2 | 1.69 | 0.19 | 0.77–3.73 |
| PGY3 | 2.03 | 0.07 | 0.94–4.36 |
| Physician assistant | 1.82 | 0.67 | 0.11-28.9 |
No difference was observed in FAIS between the weight-based dosing categories for rocuronium (52 (85.2%) vs. 100 (81.3%) vs. 86 (81.9%) vs. 123 (77.4%), p = 0.56) or for succinylcholine (80 (82.5%) vs. 54 (80.6%) vs. 16 (84.2%), p = 0.92) (Table 4). No differences were observed in change in vasopressor requirements or incidence of complications between the weight-based dosing categories for rocuronium or for succinylcholine.
| Rocuronium | Succinylcholine | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| <1 mg/kg (61) | 1.0–1.1 mg/kg (123) | 1.2–1.3 mg/kg (105) | >1.4 mg/kg (159) | p value | <1.5 mg/kg (98) | 1.5–1.9 mg/kg (67) | >2.0 mg/kg (18) | p value | |
| FAIS† | 52/61 (85.2%) | 100/123 (81.3%) | 86/105 (81.9%) | 123/159 (77.4%) | 0.56 | 80 (82.5%) | 54 (80.6%) | 16 (84.2%) | 0.92 |
| Change in systolic blood pressure (%)╝ | 1.3 (−13.9–25) | 1.9 (−14.2–17.8) | 1.7 (−8.3–15.4) | 3 (−7.4–18.5) | 0.91 | 0.8 (−17.2–17.9) | 5.4 (−8.8–19.6) | 16.94 (−7.2–36.7) | 0.15 |
| Change in heart rate (%)╝ | 4.3 (−2.1–15.2) | 2.1 (−2.1–16.5) | 6.3 (−1.5–17.3) | 3.0 (−2.0–18.7) | 0.64 | 1.2 (−5.0–10.7) | 4.4 (−5.8–17.1) | 7.6 (−1.5–20.5) | 0.44 |
| Need for vasopressor initiation post-intubation† | 4/56 (7.1%) | 8/113 (7.1%) | 10/95 (10.5%) | 14/145 (10.1%) | 0.78 | 7 (7.6%) | 8 (13.1%) | 1 (5.9%) | 0.45 |
| Need for uptitration of initial vasopressor post-intubation† | 3/5 (60%) | 2/10 (20%) | 4/10 (40%) | 6/21 (28.6%) | 0.42 | 2 (40%) | 3 (50%) | 1 (50%) | 0.94 |
| Need for additional vasopressor post-intubation† | 2/5 (40%) | 0/10 (0%) | 1/10 (10%) | 4/21 (19.0%) | 0.38 | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Development of malignant hyperthermia† | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Development of complications | |||||||||
| Cardiac arrest† | 2/61 (3.3%) | 3/123 (2.4%) | 2/105 (1.9%) | 8/159 (5.0%) | 0.50 | 2 (2.1%) | 3 (4.5%) | 2 (10.5%) | 0.20 |
| Pneumothorax† | 0/61 (0%) | 3/123 (2.4%) | 1/105 (1.0%) | 2/159 (1.3%) | 0.62 | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Esophageal intubation† | 1/61 (1.6%) | 0/123 (0%) | 1/105 (1.0%) | 0/159 (0%) | 1.0 | 0 (0%) | 1 (1.5%) | 0 (0%) | N/A |
| Main stem bronchial intubation† | 3/61 (4.9%) | 2/123 (1.6%) | 5/105 (4.8%) | 9/159 (5.7%) | 0.39 | 2 (2.1%) | 5 (7.5%) | 2 (10.5%) | 0.14 |
| Time between paralytic and continuous sedation initiation, hours╝ | 0.21 (0.12–0.85) | 0.15 (0.08–0.35) | 0.18 (0.10–0.43) | 0.15 (0.08–0.37) | 0.15 | 0.15 (0.1–0.3) | 0.13 (0.07–0.37) | 0.20 (0.13–0.30) | 0.52 |
- †n (%); ╝median (IQR).
4. Discussion
Our study was unable to detect a difference in FAIS between patients who received rocuronium and those who received succinylcholine. This finding was consistent with a previous retrospective study where the rate of FAIS was similar between succinylcholine at a median weight-based dose of 1.65 mg/kg and rocuronium at a median weight-based dose of 1.19 mg/kg [19]. Other studies have indicated that the prior findings of increased FAIS with succinylcholine could have been related to underdosing of rocuronium, and higher doses of rocuronium may be equally efficacious to succinylcholine [5, 13–15]. Additionally, a recent study showed increased FAIS with rocuronium at doses of 1.4 mg/kg or greater using direct laryngoscopy [18]. As a result, our findings are consistent with recent literature indicating similar rates of intubation success with higher doses of rocuronium. There was no difference in FAIS across various weight-based dose categories for both rocuronium and succinylcholine, but this could have been related to low sample size in the <1 mg/kg dosing category for rocuronium and the ≥2.0 mg/kg dosing category for succinylcholine. In the logistic regression, higher etomidate dose was significantly associated with FAIS, which could be attributed to better intubating conditions as a result of adequate induction.
In the ED, providers encounter barriers to determining whether patients have conditions that may put them at higher risk of developing hyperkalemia, especially during emergent intubation. Providers often use rocuronium in the absence of information regarding the patient’s prior medical conditions or the events surrounding presentation to the ED. In our study, there were no differences in contraindications to succinylcholine use between patients who received rocuronium and those who received succinylcholine. Due to the retrospective nature of this study, there was no standard practice for the timing of pre- and post-intubation potassium levels, and consequently, the resulting variations in timing limit the interpretation of these results.
Weight-based dosing has been shown to improve the likelihood of FAIS, particularly for rocuronium, and all efforts to accurately estimate patient’s weight should be made in order to optimize intubation conditions for ED patients [14]. Strategies for accurate dosing include documenting a measured or reported weight during triage whenever possible and avoiding the use of fixed dose paralytic, as this can lead to underdosing in overweight patients and overdosing in underweight patients. In our study, most of the patients who received either paralytic agent were appropriately dosed based on weight per dosing evaluated in recent studies [5, 18, 19]. In patients with difficult intubation conditions, ensuring adequate dosing may be crucial to increase the chance of FAIS.
There were several limitations to this study. First, the study size was determined based on the population sizes of prior studies comparing rocuronium and succinylcholine in lieu of a power analysis. The majority of information regarding intubation was gathered from a charted note in the electronic medical record, which could have introduced potential bias into the study. Additionally, because it was a retrospective study, there was the possibility of incomplete documentation. The retrospective nature of the study also made it difficult to capture rationale for selecting one paralytic agent over the other, especially in relation to potential contraindications to succinylcholine, COVID-19 status, or provider preference. Finally, since this study was performed at an academic medical center with a variety of training programs, there were differences in airway management regarding level of experience and medical specialty, as well as a large variation in induction agents and doses of NMBAs. However, a logistic regression was performed to control for these confounders and to strengthen the study.
5. Conclusion
In this single-center academic cohort, we were not able to detect a difference in FAIS between rocuronium and succinylcholine with both typical and escalating weight-based intubating doses. Larger prospective studies are warranted to further evaluate the effect of weight-based paralytic dosing on FAIS.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Open Research
Data Availability
Data were obtained from the electronic medical records at Brigham and Women’s Hospital after approval from the institutional review board. The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research, supporting data are not available.




