The Efficacy of Methazolamide Combined With Ibuprofen for Treating Acute Mountain Sickness
Abstract
Aims: When entering a mountain plateau, people are at risk of developing acute mountain sickness (AMS), for which there are limited prophylactic medicines available. This study aimed at exploring the effectiveness of ibuprofen, acetazolamide, and methazolamide in preventing AMS and at providing valuable insights for the future development of related drugs.
Methods: A total of 137 mountaineers were recruited for this study and divided into six groups: a control group, an ibuprofen group, an acetazolamide group, a methazolamide group, an ibuprofen/methazolamide combination group, and a high-dose ibuprofen/methazolamide combination group. After the assigned drug was taken for three days at a lower elevation (300 m), the participants ascended to a plateau environment at 5050 m. The Lake Louise AMS Score (LLS) system was used to diagnose and evaluate the AMS rates of the mountaineers in each group, and the results were compared through statistical analysis.
Results: The results show that all the medications tested herein were effective in preventing AMS, but their level of effectiveness varied. The prevalence of AMS was 50.00% in the control group, 14.29% in the ibuprofen group, 5.56% in the acetazolamide group, 27.27% in the methazolamide group, 44.8% in the ibuprofen and methazolamide group, and 22.50% in the high-dose ibuprofen and methazolamide group. Acetazolamide demonstrated a significant prophylactic effect on symptoms related to AMS diagnosis, and ibuprofen showed the best efficacy for preventing headache.
Conclusion: Acetazolamide remains an effective medicine for preventing AMS. Ibuprofen combined with methazolamide is less effective than ibuprofen alone to prevent AMS.
Trial Registration: ClinicalTrials.gov identifier: ChiCTR-TRC-12002219
1. Introduction
Acute mountain sickness (AMS) is a syndrome caused by hypoxia that usually occurs when people move from plains to high-altitude environments (>2500 m) or from plateaus to higher altitudes in a short period. The main symptom of AMS is headache, followed by gastrointestinal discomfort, fatigue/weakness, and dizziness. AMS can further develop into high-altitude pulmonary edema, high-altitude cerebral edema, and other fatal diseases if not treated in a timely manner [1, 2].
Acetazolamide is thought to be the drug of choice for the prevention of AMS, and its efficacy has been confirmed in many clinical trials [3–5]. As a carbonic anhydrase inhibitor (CAI), acetazolamide can increase bicarbonate excretion and produce diuretic effects. It stimulates the respiratory tract to increase arterial oxygen pressure and reduces cerebrospinal fluid production while promoting ion transport across the blood‒brain barrier. However, it can also cause sensory abnormalities, increased urination, and other side effects, placing additional stress on the body when adjusting to higher altitudes. Hence, it is essential to explore alternative medications. Methazolamide can improve ventilation and oxygenation levels, reduce the production of reactive oxygen species (ROS), relieve brain edema, reduce hypoxic pulmonary vasoconstriction, and eliminate hypoxic fatigue. Methazolamide activates the antioxidant nuclear transcription factor 2 (Nrf2) and prevents the release of interleukin-1β (IL-1β). These pharmacological effects are beneficial for preventing and treating high-altitude diseases, but further testing is needed to determine whether methazolamide is a safe alternative [6, 7]. Ibuprofen affects the nonselective cyclooxygenase COX-2 or COX-1, nitrogen oxides, and pain receptors in the nervous system and is commonly used to alleviate pain. It has a potential therapeutic effect on headache in AMS patients [8].
Although previous studies have shown the efficacy of acetazolamide, methazolamide, and ibuprofen for preventing AMS, the comparative efficacy of these three drugs has not been explored.
In this study, the effects of ibuprofen, acetazolamide, and methazolamide were compared. As headache is not the only criterion for AMS, a combination of ibuprofen and methazolamide was also tested. The Lake Louise AMS Score (LLS) system [9] was used to diagnose AMS, providing a reference for preventive drug development.
2. Materials and Methods
2.1. Participants and Screening
A total of 137 male adults volunteered for the study. The screening process for inclusion followed specific criteria: (1) only residents without 2500 m of plateau experience in the past 2 years were considered and (2) only healthy males over 18 years of age with no cardiovascular, respiratory, or neuropsychiatric diseases were selected. Participants who had recently used antipyretic analgesics and hormones were excluded. A questionnaire survey was conducted to collect basic information, such as age, height, weight, nationality, smoking and drinking history, and vital signs. Informed consent forms were signed by all participants, and the Army Medical University Ethics Committee approved the project.
2.2. Intervention
The participants were randomly assigned to one of the following 6 groups: the control group (n = 14), ibuprofen group (IBP, n = 14), acetazolamide group (ACZ, n = 18), methazolamide group (MTZ, n = 22), ibuprofen and methazolamide combination group (MTZ + IBP, n = 29), and high-dose ibuprofen and methazolamide combination group (MTZ + IBP, high dose, n = 40). The medication type for each group was determined by drawing lots. The participants were unaware of the type of medication they were taking throughout the experiment. The control group received the placebo. The drugs were taken for 3 days before they reached the plateau. The doses of each drug were as follows: 0.2 g of ibuprofen, 125 mg of acetazolamide, 25 mg of methazolamide, 0.2 g of ibuprofen and 25 mg of methazolamide for the combined group, and 0.3 g of ibuprofen and 50 mg of methazolamide for the high-dose combined group. All drugs were taken twice a day. The drugs were distributed to the subjects on a case-by-case basis, with record-keeping during the process and confirmation that the subjects took the drug. The drug sources used were as follows: ibuprofen tablets from Guangdong South China Pharmaceutical Group Co., Ltd.; acetazolamide tablets from Taro Pharmaceuticals U.S.A., Inc.; and methazolamide tablets from Hangzhou Aoyibaoling Pharmaceutical Co., Ltd. After three days of taking the medication, the groups departed from Chongqing (Alt. 300 m) to reach Yuzhu Peak (Alt. 5050 m). The subjects were monitored for 1 week to assess any side effects of the medication when they reached an altitude of 5050 m.
2.3. Outcomes and Measurements
The subjects completed the acute symptom survey scale, and their vital signs, including temperature, respiratory rate, heart rate, blood pressure, and oxygen saturation, were measured on the second day of their travels to Yuzhu Peak. The LLS was used to evaluate the progress of AMS, whereby symptoms, including headache, gastrointestinal symptoms, fatigue and/or weakness, and dizziness/lightheadedness, were assessed for severity on a four-point scale (0–3). AMS is defined as a LLS of three or more points from the four rated symptoms, including at least one point from headache. The AMS assessment score was as follows: Three to five points indicate mild AMS, six to nine points indicate moderate AMS, and 10–12 points indicate severe AMS.
2.4. Statistical Analysis
The results were analyzed via IBM SPSS Statistics software (version 18.0). Basic vital characteristics among the groups were compared via one-way analysis of variance. The incidence of AMS was statistically compared via the chi-square test. A p value less than 0.05 was considered statistically significant ( ∗p < 0.05, ∗∗p < 0.01).
3. Results
3.1. Basic Information
The subjects had no history of AMS in the past 2 years; no cardiovascular system disease, respiratory system disease, or neurological or mental system disease; and no recent use of antipyretic, analgesic, or hormonal drugs. Additional details for the subjects are listed in Table 1.
Control (n = 14) |
IBP (n = 14) |
ACZ (n = 18) |
MTZ (n = 22) |
MTZ + IBP (n = 29) |
MTZ + IBP (high dose) (n = 40) |
|
|---|---|---|---|---|---|---|
| Age (years) | 23 ± 4 | 26 ± 3 | 25 ± 4 | 24 ± 3 | 25 ± 3 | 23 ± 3 |
| Sex (% male) | 100% | 100% | 100% | 100% | 100% | 100% |
| Height (cm) | 173 ± 5 | 175 ± 5 | 170 ± 3 | 171 ± 4 | 172 ± 4 | 173 ± 5 |
| Weight (kg) | 66 ± 6 | 73 ± 12 | 69 ± 15 | 65 ± 8 | 68 ± 6 | 68 ± 15 |
| History of disease (%) | 0 | 0 | 0 | 2 (9%, rhinitis) | 0 | 0 |
| History of drug allergy (%) | 0 | 0 | 2 (11%, penicillin, sulfonamides) | 0 | 0 | 1 (2%, penicillin) |
| History of AMS (%) | 0 | 0 | 0 | 0 | 0 | 0 |
3.2. Basic Vital Signs of the Subjects in Plain and Plateau Environments
The participants’ vital signs in both the plain and plateau environments are listed in Table 2. Upon arriving at the anoxic plateau, the volunteers’ body temperature remained normal, but their blood oxygen saturation levels significantly decreased (the SpO2 value of the plain environment in the ibuprofen group was suspected to be incorrect). The use of methazolamide may lead to an accelerated respiratory rate, and the heart rate changes among the groups fluctuated greatly, which was presumed to be affected by their state during the test.
| Altitude (m) | Control (n = 14) |
IBP (n = 14) |
ACZ (n = 18) |
MTZ (n = 22) |
MTZ + IBP (n = 29) |
MTZ + IBP high dose (n = 40) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 300 | 5050 | 300 | 5050 | 300 | 5050 | 300 | 5050 | 300 | 5050 | 300 | 5050 | |
| Temperature (°C) | 36.6 ± 0.3 | 36.7 ± 0.1 | 36.4 ± 0.2 | 36.4 ± 0.4 | 36.7 ± 0.2 | 36.6 ± 0.2 | 36.6 ± 0.2 | 36.7 ± 0.2 | 36.6 ± 0.2 | 36.7 ± 0.4 | 36.6 ± 0.2 | 36.0 ± 1.0 |
| Respiratory rate (times/minute) | 16 ± 1 | 17 ± 1 | 17 ± 1 | 17 ± 1 | 16 ± 2 | 18 ± 1 | 15 ± 1 | 18 ± 1 ∗∗ | 18 ± 2 | 18 ± 2 | 15 ± 2 | 17 ± 3 ∗∗ |
| Heart rate (times/minute) | 74 ± 9 | 91 ± 17 ∗∗ | 90 ± 3 | 84 ± 6 ∗∗ | 85 ± 11 | 84 ± 8 | 75 ± 6 | 94 ± 7 ∗∗ | 86 ± 6 | 87 ± 13 | 88 ± 12 | 85 ± 9 |
| Blood pressure (mmHg) | 120/81 ± 13/9 | 128/82 ± 11/7 | 125/82 ± 9/6 | 130/83 ± 10/6 | 122/79 ± 11/11 | 126/78 ± 10/7 | 112/76 ± 9/5 | 131/85 ± 6/5 ∗∗/ ∗∗ | 125/84 ± 8/14 | 138/93 ± 19/13 ∗∗/ ∗ | 121/74 ± 16/10 | 129/84 ± 16/11 |
| SpO2 (%) | 94 ± 4 | 86 ± 6 ∗∗ | 81 ± 6 | 80 ± 6 | 92 ± 4 | 87 ± 7 ∗ | 95 ± 1 | 81 ± 3 ∗∗ | 85 ± 8 | 79 ± 9 ∗ | 93 ± 2 | 85 ± 6 ∗∗ |
- ∗p < 0.05; ∗∗p < 0.01.
3.3. Incidence of AMS Diagnosed by the LLS
The incidence of AMS according to the LLS scoring system is displayed in Figure 1. The control group presented a 50% incidence of AMS. Acetazolamide had the most pronounced effect (5.56%), followed by ibuprofen (14.29%) and its high-dose combination with methazolamide (22.50%). The prevalence rates in the other groups were 27.27% (MTZ) and 44.83% (MTZ + IBP). The lower prevalence in the treatment groups than in the control group indicated that ibuprofen, acetazolamide, and methazolamide played roles in preventing AMS. Overall, the combination of ibuprofen and methazolamide had a reduced effect than ibuprofen alone.
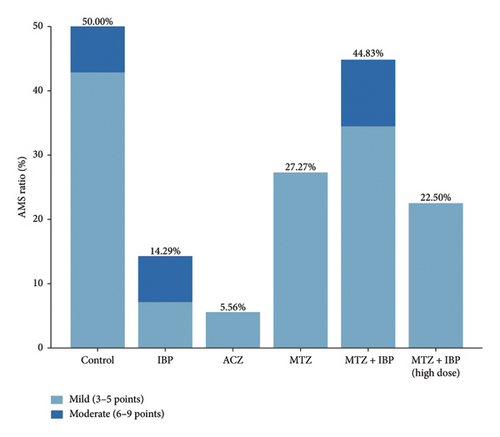
3.4. Symptom Analysis
The incidence of symptoms in each group is displayed in Figure 2. In the control group, headache (64.29%), gastrointestinal symptoms (50%), fatigue and/or weakness (35.71%), and dizziness/light-headedness (64.29%) were observed. Acetazolamide significantly alleviated all symptoms, especially gastrointestinal symptoms (0%) and fatigue (5.56%), and was only slightly inferior to ibuprofen with respect to headache. Ibuprofen had the second-best symptom relief effect and was particularly effective in reducing headache symptoms. However, the proportion of subjects with headaches was higher in the high-dose methazolamide plus ibuprofen group (40.00%) than in the methazolamide (27.27%) or ibuprofen (14.29%) group. Notably, there was a high prevalence of all symptoms in the ibuprofen and methazolamide combination group.
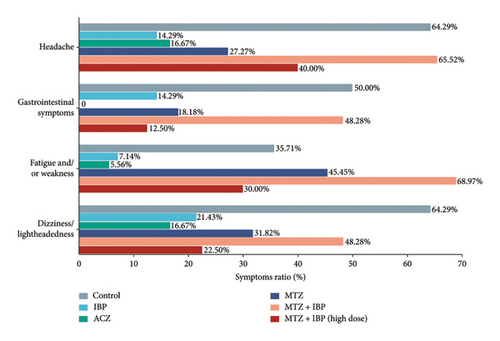
4. Discussion
Previous studies have demonstrated the effectiveness of ibuprofen, acetazolamide, and methazolamide in treating AMS. However, there has been no direct comparison between the efficacy of ibuprofen and that of acetazolamide or methazolamide, nor has the combined effect of multiple drugs been evaluated [7, 10, 11]. In this study, volunteers were divided into 6 groups to compare the therapeutic effects of ibuprofen, acetazolamide, methazolamide, and different doses of combinations of ibuprofen and methazolamide on AMS under the same experimental conditions.
The incidence rate of AMS is related to factors such as altitude [12, 13]. As altitude increases, the prevalence of AMS increases. Beyond an altitude of 2500 m, the prevalence of AMS increases by 13% for every additional 1000 m [13]. Our previous study of 2486 young men who moved from an altitude of 500 m to 3658 m reported an AMS prevalence rate of 33.8% [14]. Another study reported an AMS prevalence rate of 50% at an altitude of 5000 m [15]. Considering the variation in the incidence rate with altitude as well as the incidence in Guo’s study, we believe that the 50% prevalence rate of the control group in this study is credible.
Acetazolamide had the best efficacy in treating AMS, with an incidence rate of 5.56%, which was much lower than that of the control group (50.00%). The incidence rate of AMS revealed that the effect of ibuprofen (14.29%) was better than that of methazolamide (27.27%). These results suggest that acetazolamide may still be the first choice for AMS prevention, which is consistent with the findings of previous studies [3, 5, 10]. However, notably, the high-dose combination of ibuprofen and methazolamide was less effective than ibuprofen alone. As a cyclooxygenase inhibitor [16], ibuprofen can also activate carbonic anhydrase directly. Methazolamide is a CAI [17]. Thus, their antagonistic effect might lead to weakened effectiveness in preventing AMS. In this study, we found that the weakened effectiveness of AMS prevention was more significant at lower doses. These findings imply that the effectiveness of drugs may be compromised when they are used in a nonspecific combination.
The different effects of each drug on the prevention of AMS might be related to their pharmacodynamic preference for different symptoms. Among these drugs, ibuprofen was more effective than acetazolamide for headache relief but less effective for other symptoms. The preventive effect of ibuprofen on AMS might be due to the necessity of headache symptoms in the AMS diagnosis of LLS. Therefore, effective drugs for treating headaches may be options for the prevention and treatment of AMS.
After the administration of acetazolamide, the incidence of gastrointestinal symptoms significantly decreased; in contrast, the occurrence of fatigue and weakness symptoms increased following methazolamide intake. In the treatment group receiving methazolamide and ibuprofen, there were an increased proportion of patients who experienced headaches compared with both the methazolamide-only group and the ibuprofen-only group. These findings also suggest a potential influence of methazolamide on the analgesic effect of ibuprofen, contributing to a reduced preventive effect on AMS.
Our study offers practical guidance for preventing AMS with various drugs, and low doses of acetazolamide are still the first recommendation for AMS prevention. Ibuprofen has also been shown to be effective in preventing AMS, possibly because of its headache-inhibiting effect. Pharmacological prophylaxis can reduce the incidence of AMS, yet its prevalence is influenced by numerous factors. A comparative analysis with past research indicates that the prevalence of AMS escalates with increasing altitude [18, 19]; arriving at high altitude with poor health or ascending rapidly, such as hiking at night [20] or flying [21], also heightens the risk of contracting AMS; residing at a higher altitude [22] or ascending gradually [23] can lower the risk of AMS. Additionally, women are at a higher risk of developing AMS compared to men [24], a difference potentially linked to the effects of sex hormones.
Owing to certain limitations, the sample sizes of each group differed and were not sufficient. Future studies should include a larger sample size and a wider range of ages and subjects of both sexes to accurately reflect the incidence of AMS in plateau areas. Additionally, dosages and other combinations of drugs need to be explored.
Ethics Statement
This study was approved by the Ethics Committee of Xinqiao Hospital, the Second Clinic Medical College of the Third Military Medical University. All clinical investigation obeyed the Declaration of Helsinki.
Consent
We attached the informed consent to the questionnaire, and all participants provided written informed consent before filling in the questionnaire. Ethical issues have been fully considered in the research process to protect the rights of participants.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
W.Z. and Z.G. have contributed equally to this work.
Funding
This work was supported by the National Science and Technology Major Project, China (grant number 2018ZX09J18109) and the Open Cooperation Program from the Key Laboratory of Extreme Environmental Medicine, Ministry of Education (grant number KL2019GY008).
Open Research
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.




