Transforming Polycotton Textile Waste into New Bicomponent Fibers: An Investigative Study
Abstract
This study aimed to develop an innovative recycling method for end-of-life polycotton textiles, eliminating the need for component separation. The use of 1-ethyl-3-methylimidazolium acetate ([EMIM][Ac]) as an ionic liquid solvent facilitated the dissolution of cotton, enabling the creation of a spinning dope containing cellulose and polyester fibers. Successful spinning of bicomponent fibers ensued, followed by comprehensive fiber evaluation. The dissolution of cotton was achieved with [EMIM][Ac], and spinning trials were conducted to devise a suitable method for regenerated cellulose. Tensile tests on the produced cellulosic fibers clearly demonstrated an increase in tensile strength with higher cellulose concentration. The introduction of polyester fibers to the spinning dope, comprising [EMIM][Ac] and cotton, posed challenges to the entire spinning process. Tensile tests on the resulting bicomponent fibers revealed a decrease in tensile strength compared to pure regenerated cellulose fibers. This reduction was attributed to increased voids and irregular polyester fiber distribution, corroborated by microscopy images and a wicking test. It was concluded that the quantity and length of polyester fibers significantly influenced the tensile strength of the bicomponent fibers, with lower concentrations and shorter fibers resulting in higher strength.
1. Introduction
Textile products are essential in our daily lives and contribute to our warmth, safety, and individuality [1, 2]. With the increasing global population, textile products will continue to be a crucial part of our lives. However, this population growth will also lead to a surge in the demand for textile products, resulting in millions of tons of waste fabrics if not managed properly [3, 4]. Unfortunately, without viable recycling options, textile products often end up being incinerated or sent to landfills [5]. While several countries have established recycling infrastructure for paper, glass, and metals, there is a noticeable lack of such infrastructure for textile products [6]. This is mainly due to the complexity involved in recycling textile products, which are often made from blend materials with various coatings and finishes [7].
The most widely used blended fabric in the world today is a combination of cotton and polyester (poly(ethylene terephthalate) (PET)), commonly referred to as polycotton. Cotton and polyester are the most prevalent natural and synthetic fibers, respectively [7]. The blending of these two fibers offers advantageous properties by compensating for their individual strengths and weaknesses [8]. Polycotton finds extensive applications in garments, home decoration, and service clothing [9, 10].
Various methods for recycling polycotton have been reported, typically categorized as mechanical or chemical recycling [11, 12]. Mechanical recycling routes involve shredding polycotton fabrics and subsequently spinning them into new threads. However, a drawback of mechanical recycling is the shortening of the fibers [13]. On the other hand, chemical recycling often entails separating the cotton and polyester components by either dissolving or depolymerizing one of the constituents to recover the other [6].
One chemical recycling method for cotton fabrics that has received considerable attention recently is the utilization of ionic liquids (ILs) to dissolve the cotton (cellulose) [14]. ILs are salts with melting points below 100°C [15], and they have been the subject of extensive research in recent times.
In a study conducted by Xia et al. [5], postconsumer cotton and polyester blends were separated using IL 1-allyl-3-methylimidazolium chloride (AmimCl). Prior to separation, the postconsumer textiles underwent a pretreatment with household bleach to eliminate impurities and colors from the fabrics. Following separation, both the cellulose and polyester components were successfully transformed into transparent films with favorable mechanical properties, thereby demonstrating the valorization potential of waste textiles. The authors concluded that high-purity polyester could be fully extracted from the dissolved cellulose without degradation, indicating that ILs are a suitable choice for recycling cotton and polyester blends.
Ingildeev et al. [16] conducted a study comparing the behavior of ILs and the lyocell process in terms of dissolution, spinning process, and physical properties. The ILs used in the study were 1-ethyl-3-methylimidazolium acetate [EMIM][OAc] and 1-ethyl-3-methylimidazolium diethylphosphate [EMIM][DEP]. The study’s findings indicated that fibers produced from ILs exhibited similar physical properties to fibers from the lyocell process but with less fibrillation when using the dry–wet spinning method. Additionally, the researchers successfully spun fibers dissolved in ILs using the wet spinning technique, resulting in fibers with properties comparable to those produced through the viscose process [16].
In another study conducted by Ingildeev et al. [17], cellulose was dissolved in ILs along with synthetic polymers to create spinning dopes of varying concentrations. Polyamides were the synthetic polymers used, and the researchers discovered that aromatic polyamides exhibited the best miscibility with cellulose. The ILs utilized in the study were 1-ethyl-3-methylimidazolium acetate [EMIM][OAc] and 1-ethyl-3-methylimidazolium diethylphosphate [EMIM][DEP], and both the cellulose and polyamides were dissolved in these ILs. The researchers determined that the dry–wet spinning method was the most favorable. The resulting spun fibers exhibited different properties depending on the concentrations of the spinning dopes, and their physical properties fell within the range of commercially available lyocell fibers [17].
In a study conducted by Textor et al. [8], cellulose was dissolved in different ILs, including EMIM AC, EMIM CL, EMIM DEP, and BMIM CL, and then applied as a finishing on polyester fibers. Rheological tests were performed on the various solvents, and according to the authors, EMIM AC exhibited the most favorable viscosity. The coated polyester fibers did not show significant differences with the added cellulose finish, although they were able to achieve coloration using reactive dyes. The finish, however, demonstrated excellent durability [8]. Another similar study by Sharma et al. [18] involved the dissolution of PET fibers and their deposition onto cotton fabrics. In this study as well, good durability was observed. The tensile strength of the fabrics increased, and they retained their hydrophilic properties [18].
There have been reports of successful separation of cotton and polyester using ILs without degrading the polyester component [5]. However, a study by Haslinger et al. [19] utilizing 1,5-diazabicyclo(4.3.0)non-5-enium acetate ([DBNH][OAc]) as an IL to dissolve cellulose and separate it from polyester, reported degradation of the polyester fibers and a loss of tensile properties by approximately 50% [19]. In a study by Hermanutz et al. [20], specific conditions for an effective IL for cellulosic fibers were investigated. According to the article, EMIM acetate (1-ethyl-3-methyl-imidazolium acetate) met all the requirements, including a low melting point, easy processing, uncomplicated recovery, and nontoxicity [20].
The primary objective of this study is to create a bicomponent filament named chemical recycling of polycotton in an ecofriendly manner. Compared to other studies, this project has the potential to offer a key advantage: Eliminating the step of separating polyester and cotton during recycling leads to significant reductions in processing time, chemical usage, and energy consumption, making it more cost-efficient.
2. Material and Methods
2.1. Materials
As a cellulose source, preconsumer cotton material that had undergone mechanical recycling, with fiber lengths below 15 mm, was used. Polyester fibers were purchased from a secondhand store in the form of pillow stuffing and were used without any pretreatment. 1-Ethyl-3-methylimidazolium acetate ([EMIM][Ac] >95%), provided by Sigma–Aldrich, was utilized without requiring further purification.
2.2. Methods
2.2.1. Spinning Trials
In the spinning trials conducted for this study, it is important to note that preconsumer textile waste was utilized instead of postconsumer waste. However, as part of future work, it is suggested that the exploration can be extended to include postconsumer textile waste. This is because factors such as wearing, washing, and ironing can potentially impact the properties of materials, and studying the effects of these factors on the spinning process and resulting fibers would provide valuable insights.
This study involved two primary sections in the spinning trials. The initial phase focused on conducting experiments using solely cellulose and solvent. This approach was necessary to develop the dissolution process and establish the required setup for the laboratory spinning equipment, ensuring the production of fibers with satisfactory quality. Once the setup was considered effective, the second phase commenced, involving the introduction of polyester fibers.
The spinning trials involved several key steps. Initially, cotton was dissolved in the chosen ionic liquid, [EMIM][Ac], with an investigation into its properties and the development of a process to obtain a spinnable homogeneous dope that produces high-quality fibers. Following this, a dry-jet wet spinning setup was developed to ensure the production of uniform, good-quality fibers. Subsequent steps involved testing the produced fibers and incorporating polyester fibers into the process, which required additional development to refine the process accordingly.
2.2.2. Dissolving
Two different methods for dissolution were tested and evaluated. The first, based on the work by Textor et al. [8] and De Silva et al. [21], employed an oil bath and a beaker for the dissolution of cellulose [8, 21]. Stirring was carried out using either a magnetic stirrer or an overhead stirrer, and various temperatures and durations were tested. The second method, based on Ingildeev et al. [17] and Li et al. [22], involved using a vacuum oven for dissolution. In this method, the samples were stirred before placing the beaker in the oven [17, 22].
2.2.3. Dry-Jet Wet Spinning
The spinning process was conducted using a simple laboratory setup, as depicted in Figure 1. The setup comprised a syringe pump, a syringe equipped with a needle (1.2 mm diameter), a container for the coagulation bath (water), and a take-up roller to induce drawing. The spinning dope was transferred directly from the oven to the syringe, and temperature control was not feasible. The spinning dope was then extruded into the water bath at a fixed flow rate of 30 mL/hr and guided with tweezers up to the take-up roller. Following spinning, the produced fibers were placed in another coagulation bath for a minimum of 6 hr to ensure the complete removal of any residual solvent.

2.2.4. Characteristics
(1) Microscopy. Microscopic analysis was conducted using a Nikon SMZ800 stereomicroscope in conjunction with the NIS Element software. This test aimed to analyze the physical structure of the produced fibers.
(2) FTIR. Fourier transform infrared spectroscopy (FTIR) was conducted using the Nicolet iS10 spectrometer to analyze changes in chemical composition. The spectra were recorded in the frequency range of 4,000–500 cm−1 at a scan resolution of 4 cm−1, with 32 scans for both background and sample scans. This test aimed to detect any residues of ionic liquids in the regenerated cellulose and bicomponent fibers
(3) Tensile Testing. Tensile testing was carried out using a Tinius Olsen Ltd. universal 10KT testing machine located in Salfords, UK. The gauge length was set to 20 mm, and the loading rate was 20 mm/min until the samples fractured. The load range for testing was set to 10 N.
(4) TGA. Thermogravimetric analysis is a thermal analysis method used to measure changes in mass over time in response to temperature changes. The test was conducted using a Thermogravimetric Analyzer Q-500 with TA Instrument software to assess the thermal stability of the produced fibers. The samples were heated at a rate of 10°C/min from ambient temperature to 600°C in a nitrogen atmosphere.
(5) Wicking. The wicking test was conducted to examine the morphology of the fibers and assess the viability of the product area. The test was performed using a modified version of the standard AATCC 197-2013, specifically the vertical method (AATCC 197:2011). The specimen was affixed to a ruler with magnets, which was then submerged in the dye (Rose Bengal). Once the specimen was adequately wetted, a 30-min timer was initiated, and the liquid began to rise in the fiber. After 30 min, the specimen was lifted from the dye, and measurements were taken. Three samples were obtained from each specimen.
3. Results and Discussion
3.1. Spinning Dope Containing Cellulose and IL
The initial attempts to dissolve the sample were conducted using an oil bath and a magnetic stirrer, based on the procedure outlined by Textor et al.’s [8], with some modifications. Cotton was mixed with an 7 wt% ionic liquid (IL) solution, heated to 85°C, and stirred at 500 rpm. However, the viscosity increased rapidly, causing the magnetic stirrer to be ineffective, leading to uneven heating. The resulting solution had air bubbles and impurities. The next attempt, based on De Silva et al.’s [22] method, used incremental additions of cotton up to 4 wt%. Despite efforts, the solution did not completely dissolve after 4 hr. Subsequent trials using an overhead stirrer encountered similar issues with viscous material and inadequate stirring. To address dissolution problems and air bubbles, a modified approach inspired by Ingildeev et al. [17] involved careful mixing of cellulose/IL solutions at room temperature, followed by heating and degassing. Additionally, Li et al.’s [22] method showed promise with more homogeneous solutions [17, 22].
To improve the dissolving process, an alternative method was attempted—dissolving directly in the syringe. Cotton and IL were mixed in the syringe, which was then inserted into a vacuum oven for 24 hr. However, this method resulted in an uneven solution and difficulties in needle attachment. The final method included dissolving in the vacuum oven and transferring the solution carefully to the syringe using a spatula.
3.2. Dry-Jet Wet Spinning of Cellulose Fibers
The initial laboratory configuration for spinning cellulose fibers includes a syringe pump, a syringe equipped with a needle, and a coagulation bath with water serving as the antisolvent. The initial spinning experiment, conducted manually using a plastic syringe, revealed that the solution maintained cohesion even at this early stage, suggesting that the cellulose concentration is sufficient and not excessively low. Drawing, except from the air gap, is not induced at this point
In this phase of the experiment, temperature control within the syringe was impractical, as solutions were directly taken from the oven, resulting in cooling and increased viscosity by the time they were pumped out. To counter this, lower cellulose concentrations (2, 3, and 4 wt%) were chosen, compared to the previous 6–8 wt%, facilitating easier fiber spinning. The enhanced laboratory spinning setup involved drawing, a critical aspect for producing uniform fibers with aligned polymer chains. The vertical arrangement of the pump aimed at better air gap distance control and more efficient needle usage.
During this phase, the syringe solution retained air bubbles after transfer from the beaker to the syringe, impacting fiber production and complicating the spinning process. Another challenge involved the brief coagulation time for fibers before reaching the take-up roller. Although placing fibers in an additional water bath after spinning resolved this issue, it introduced a new problem of fiber entanglement, resulting in breakage during the drying process.
The coagulation bath temperature, influenced by Hauru et al.’s [23] findings (2014), was set at 18°C to enhance mechanical strength by allowing more relaxation time and preventing the loss of fiber orientation caused by reheating. The coagulation time after spinning significantly affects fiber properties; a short duration may leave residual solvent, impacting tensile properties [24]. Initial experiments involved a 2-hr coagulation time, but FTIR spectra revealed residual solvent, leading to an increased coagulation time of 6+ hr.
Spinning pump velocities of 30 and 60 mL/hr were explored based on De Silva et al.’s [21] work. After assessing ease of spinning and take-up roller usage, 30 mL/hr was selected as the standard velocity. The take-up roller speed was set at 0.94 m/min, higher than the spinning pump speed. Testing a higher take-up roller speed resulted in constant spinning breakage. The needle size chosen for biocomponent fibers was a 1.2 mm diameter with a 25 mm length, thicker than needles used in previous experiments [21, 23]. The air gap distance was fixed at 1 cm.
After numerous attempts, successful production of high-quality fibers was achieved. However, a significant challenge remained: the drying process of the fibers. Proper drying is vital to counteract shrinkage during the drying phase, as illustrated in Figure 2(b). If fibers are not correctly aligned and overlap during drying, they develop a crimped structure, posing challenges in accurately assessing their properties. The visual difference that proper drying makes is evident in Figure 2.


To address this, a method was developed for drying fibers after coagulation. Cloth pegs and a clamps rack were employed Figure 3, resulting in straight and uniform fibers, although the bending created weak points that could impact tensile testing.
3.3. Characteristics of Spun Cellulose Fibers
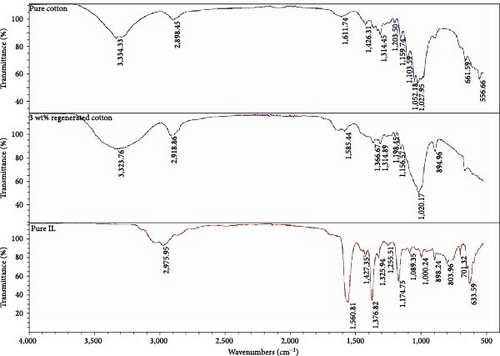
3.3.1. FTIR
FTIR was used to analyze the chemical structure of produced fibers. In the early stage, it was used to see if there was any residual [EMIM][AC] in the fibers, although it should be noted that FTIR may not be sensitive enough to detect small amounts of residue solvents [25]. In Figure 4, FTIR spectra of pure cotton and 3 wt% regenerated cellulose and solvent are shown. The characteristic peaks of [EMIM][Ac] at 1,560 cm−1 corresponding to the C═N bond are not present in the regenerated cellulose spectra, implying that the solvent was successfully removed during coagulation [24]. The broad band of 3,100–3,600 cm−1 corresponds to the OH bonds in both cotton and regenerated cellulose, indicating intermolecular and intramolecular hydrogen bonds. The intensity of the band at 3,100–3,600 is bigger for the regenerated cellulose compared to cotton because of the different morphology of the regenerated cellulose, cellulose type II [26].
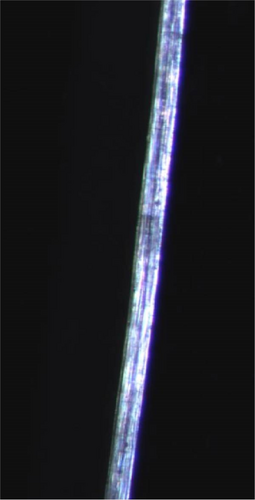
3.3.2. Microscopy
Microscopy was used to more easily visually analyze the samples. Figure 5 shows a 2 wt% fiber, and as can be seen, it is very even. The fibers from 3 and 4 wt% were similar, indicating that the spinning has produced good fibers. The round profile of the fibers can be explained by the rapid coagulation [17].
3.3.3. Tensile Testing
In Table 1, the results of the tensile testing of regenerated cellulose fibers are summarized. The findings clearly indicate that higher concentrations result in stronger fibers, aligning with previous studies [27]. The enhanced molecular weight contributes to increased chain entanglement, which could account for the rise in tensile strength with higher concentrations of cotton [21].
| Samples (wt%) | Linear density (tex) | Tenacity (cN/tex) | Ultimate strength (MPa) | Elongation at break (%) | Youngs modulus (GPa) |
|---|---|---|---|---|---|
| 2 | 7.3 | 17.3 (±1.5) | 94.9 (±8.7) | 9.8 (±1.3) | 2.7 (±0.6) |
| 3 | 10.0 | 19.7 (±1.9) | 150 (±16.5) | 14.5 (±1.9) | 3.1 (±0.8) |
| 4 | 22.6 | 22.2 (±4.2) | 323.0 (±60.8) | 26.0 (±7.5) | 3.5 (±0.7) |
3.4. Spinning Dope Containing Cellulose, Polyester, and IL
The initial attempt to spin bicomponent fibers with regenerated cellulose and polyester involved incorporating hand-cut multifilament polyester, ranging in length from 20 to 40 mm, into the solution before subjecting it to the vacuum oven. Various concentrations were explored: 20 : 80 (polyester : cotton), 30 : 70, 40 : 60, and 50 : 50 while maintaining a constant concentration of 3 wt% for cotton. The oven parameters remained consistent with previous usage at 100°C for 24 hr.
The solution lacks homogeneity, displaying visible clusters of polyester fibers. These clusters posed challenges during spinning, causing clogging as the polyester fibers obstructed the needle, preventing the solution from being dispensed by the syringe pump. Figure 6 illustrates a cluster of polyester fibers causing needle clogging. This situation aligns with the predictions made by Ingildeev et al. [17] for smaller-sized needles, albeit with more holes. Needle clogging stands out as a significant hurdle to overcome for the success of this process.

To achieve a more homogeneous solution, the polyester fibers were cut into smaller lengths, around 10–30 and 5–10 mm, and mixed with the solvent before adding the cotton. This process ensured that the polyester fibers were more uniformly dispersed in the solution, eliminating any clusters of polyester fibers. Table 2 provides a summary of the fibers created and assessed. A 4% concentration constantly clogged the needle, and 3% was stronger than 2%. PC1 and PC2 were produced with a polyester length ranging from 10–30 mm, while PC11 and PC22 had a polyester length of 5–10 mm.
| Sample code | Concertation (polycotton) (ratio, cotton : polyetser) | Lengths of polyester fiber (mm) |
|---|---|---|
| CO3 | 3 wt% (10 : 0) | — |
| PC1 | 3 wt% (8 : 2) | 10–30 |
| PC2 | 3 wt% (9 : 1) | 10–30 |
| PC11 | 3 wt% (8 : 2) | 5–10 |
| PC 22 | 3 wt% (9 : 1) | 5–10 |
| DPC 1 | 3 wt% (8 : 2) | 5–10 |
| DPC 2 | 3 wt% (9 : 1) | 5–10 |
3.4.1. Polycotton Powder
Following the experiments involving polyester fibers, another material source was explored. In this case, polycotton (50/50) obtained from hospital sheets was freeze-dried using liquid nitrogen and subsequently ground into a powder. The dissolution process followed the procedures of previous experiments. The resulting solution, with a concentration of 6 wt% (3 wt% cotton and 3 wt% polyester), exhibited significantly lower viscosity than the solutions prepared earlier. The solution was homogeneous and retained color from the clothing material. The decreased viscosity posed challenges in spinning fibers, and the spinning process did not produce any fibers. Additionally, a higher concentration solution (12 wt%) also failed to yield fibers, with the spinning dope emerging as drops from the syringe. This could be attributed to a lower degree of polymerization of cellulose in the solution. The color had shifted to a darker tone, suggesting a potential impact on the outcome.
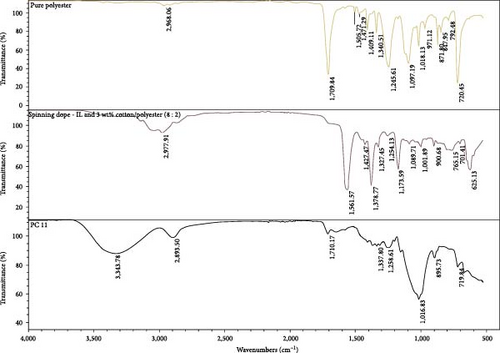
3.4.2. FTIR
In Figure 7, FTIR spectra of pure polyester, spinning dope containing [EMIM][Ac], and 3 wt% polycotton (cotton, polyester 8 : 2) are presented alongside PC11 fibers. The characteristic peaks of [EMIM][Ac] at 1,560 cm−1 are not present in PC 11, indicating that most of the solvent was removed successfully during coagulation. Moreover, a small peak at 1,710 in the PC 11 fibers indicates the presence of polyester.
3.4.3. Microscopy
Microscopy pictures of produced bicomponent fibers are shown in Figures 8(a), 8(b), 8(c), and 8(d), the differences in structure can be observed. The fibers are sometimes smooth and even, while in other cases, there are clusters of polyester fibers. In some areas of the fibers, there is a complete lack of polyester fibers, and in other areas, they are evenly distributed. This variation may explain the significant standard deviation in tensile strength. In Figure 8 (e), voids can be observed. These could result from air bubbles during the spinning dope process. The voids represent weak spots in the fibers and could contribute to the lower tensile strength of the bicomponent fibers.

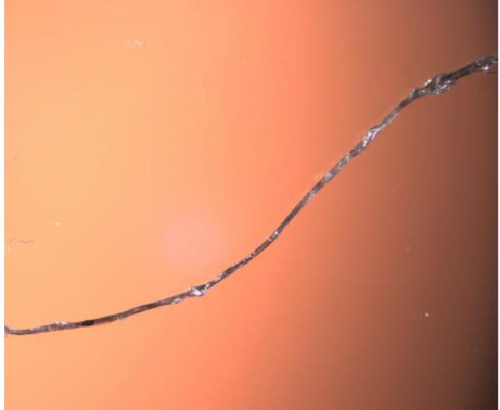

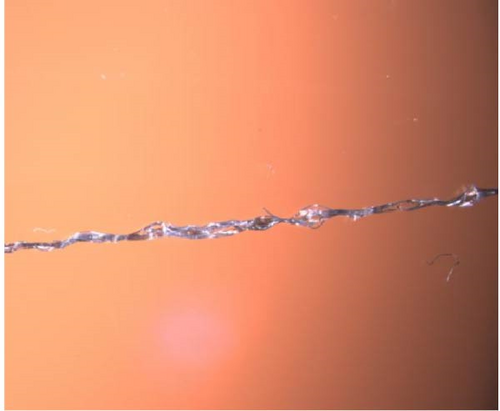
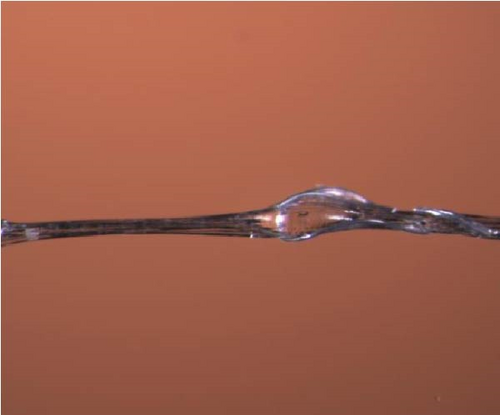
3.4.4. Tensile Testing
The results of the tensile testing for the biocomponent fibers are presented in Table 3. The findings indicate that shorter lengths of polyester fibers result in higher tensile strength. There is also a correlation observed between the concentration of polyester fibers and the tensile strength. This can be explained by the more uniform structure of the fibers when there are fewer polyester fibers present. In comparison to fibers without polyester (CO3), a decrease in tensile strength is observed. This can be attributed to a more irregular morphology and a potential increase in voids during the spinning process.
| Sample code | Tenacity (cN/tex) | Elongation at break (%) |
|---|---|---|
| PC1 | 3.4 (1.9) | 11.9 (4.7) |
| PC2 | 10.6 (1.6) | 17.3 (4.9) |
| PC11 | 4.8. (1.6) | 12.2 (3.3) |
| PC22 | 11 (1.9) | 13.2 (2.8) |
The elongation at break is notably high and increases with higher cellulose concentration. This can be attributed to the amorphous structure and low crystallinity of the fibers [28]. Higher draw ratios could result in increased orientation of the polymer chains and higher crystallinity, leading to higher tenacity and lower elongation at break [29]. Another possible explanation for the low crystallinity could be the temperature change when the fibers transition from the first coagulation bath to the uptake roller and then reenter the second coagulation bath, which provides the fibers with more relaxation time [23].
3.4.5. TGA
The TGA curves of CO3 (3 wt% regenerated cellulose fiber), polyester, PC11, and cotton are presented in Figure 9. A steep decomposition is observed in all samples containing cellulose within the temperature range of 250–360°C, consistent with previous findings [24]. In the samples with regenerated cellulose, a larger weight loss is noticeable at 70–150°C due to water content in the fibers, which is higher for regenerated cellulose compared to cotton. This suggests that the crystallinity of the native cellulose in cotton is higher than that in regenerated cotton [30]. For the PC11 sample, a two-step decomposition profile is evident, with the initial step corresponding to cellulose and the second to polyester.
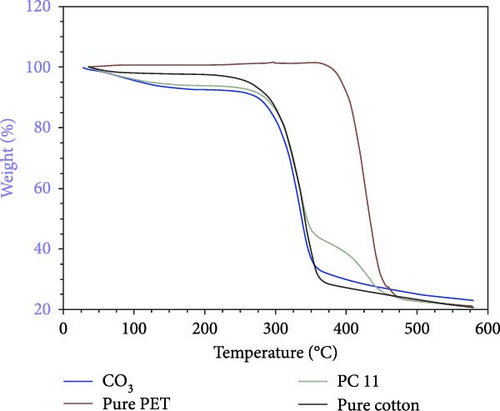
3.4.6. Wicking
The wicking test was conducted to evaluate the morphology and ability to transport liquids along the fibers. The results from the wicking test are summarized in Table 4. The results clearly show that the fibers without polyester have better wicking capacity. This can be explained by the fact that water is probably more likely to transport easier along a smooth and continuous surface and the shortest path rather than going along with wrapped fibers, as shown in Figure 10, and uneven surfaces [31]. Another reason for the poor wicking capability of the fibers with polyester could be the lumps of polyester fibers that act as a hindrance for the liquid.
| Sample code | Wicking height (mm) |
|---|---|
| CO3 | 16.3 (±5.3) |
| PC11 | 3.3 (±0.5) |
| PC22 | 5.3 (±2.1) |
4. Conclusion
This study investigated a novel recycling method for polycotton, where the separation of the two components was not necessary. The study concluded that it was possible to spin fibers from a spinning dope of dissolved cotton and polyester fibers, creating bicomponent fibers.
The addition of polyester fibers to the spinning dope posed challenges. The resulting spinning dope was heterogeneous with polyester fibers, and more air bubbles were present, making the spinning process more challenging due to a lack of continuous spinning. The spinning dope broke because of weak spots created by the heterogeneous solution. The needle got clogged by clusters of polyester fibers, further disrupting the continuous spinning process.
Microscopy and the wicking test revealed that the produced bicomponent fibers were uneven, with irregular distribution of polyester fibers. Some parts of the fibers had almost no polyester fibers, while others had clusters of polyester fibers. Tensile tests on the bicomponent fibers confirmed the uneven structure and irregularity, showing lower tensile strength compared to fibers without polyester, indicating that the addition of polyester fibers decreased tensile strength, possibly due to poor miscibility. Tensile tests demonstrated that lower concentrations and shorter lengths of polyester fibers resulted in higher tensile strength, suggesting that the distribution and alignment of polyester fibers were crucial for creating strong fibers.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Open Access funding enabled and organized by Bibsam 2023.
Open Research
Data Availability
No underlying data were collected or produced in this study.




