The Correlation Between Serum Tumor Markers and Liver Metastasis of Lung Cancer
Abstract
Purpose: To investigate the relationship between the changes of lactate dehydrogenase (LDH), neuron-specific enolase (NSE), keratin-19 fragment antigen 21-1 (cyfra21-1), and liver metastases of lung cancer.
Methods: Eighty patients who had lung cancer that had spread to their liver diagnosed in our hospital from October 2021 to October 2023 (Group A), 80 individuals with advanced lung cancer who have metastasized to other sites (Group B), and 80 individuals with lung cancer who have not spread (control group) were selected as the study objects. LDH, NSE, and serum cyfra21-1 levels of patients in the three groups were detected, and pathological results were used as the diagnostic gold standard. ROC curves were drawn to examine the clinical value of NSE, cyfra21-1, and LDH levels in the distinct types of lung cancer identification of liver metastases.
Results: There was no remarkable variation in pathological types among the three groups (p > 0.05), but there were remarkable variations in TNM stage and lymph node metastasis among the three groups (p < 0.05). The levels of cyfra21-1, NSE, and LDH in Group A and Group B were greater compared to those in the control group (p < 0.05), and the levels of cyfra21-1, NSE, and LDH in Group A were greater compared to those in Group B (p < 0.05). The critical value, sensitivity, specificity, and area under the curve (AUC) of serum cyfra21-1 in the distinct identification of liver metastasis of lung cancer were 8.22 ng/mL, 37.42%, 65.18%, and 0.508, respectively. The critical value, sensitivity, specificity, and AUC of NSE for distinct types of lung cancer identification of liver metastases were 23.96 ng/mL, 64.56%, 81.23%, and 0.723, respectively. The critical value, sensitivity, specificity, and AUC of LDH for differential diagnosis of liver metastasis of lung cancer were 304.78 U/L, 75.65%, 85.73%, and 0.821.
Conclusion: The serum levels of NSE, cyfra21-1, and LDH in patients with liver metastasis of lung cancer were remarkably greater compared to patients without liver metastasis, which can be useful as a clinical auxiliary in determining lung cancer metastasis.
1. Introduction
The occurrence of lung cancer has increased in recent years, becoming a serious problem threatening society, and most of the clinical diagnoses are late, seriously threatening the patient’s life safety [1, 2]. Currently, lung cancer diagnosis is based on tumor markers, which include proteins, associated serum proteins, and peptides and gene types [3, 4]. The aberrant expression results of either normal or tumor genes are known as tumor indicators which play a significant role in disease diagnosis, treatment, and prognosis evaluation [5–7]. Research studies have finalized that the content of redox-type enzyme lactate dehydrogenase (LDH), which is nearly associated with glycolysis, is greater in patients with tumors [8]. The degree of elevation is closely associated with the clinical TNM stage and distant lymph node metastasis of gastric cancer, lung cancer, esophageal cancer, and other tumors [9]. Domestic and foreign scholars have used LDH level change as a diagnostic criterion for lymphatic cancer, and some scholars have also used LDH level in the identification of lung cancer [10–12]. In this study, keratin-19 fragment antigen 21-1 (cyfra21-1), LDH, and neuron-specific enolase (NSE) were investigated. The clinical value of NSE and LDH expression levels in individuals with liver metastasis from lung cancer has been extensively studied, as these biomarkers can provide important insights into disease progression, prognosis, and response to therapy.
2. Materials and Methods
2.1. General Data
We develop a scientific sample collection plan, ensure the accuracy and representativeness of sample selection, and improve the scientificity and credibility of clinical research results. First, we ensure the reliability of the data source, as all samples are from the Respiratory Department of the Third Affiliated Hospital of the Naval Medical University. Second, in the stage of data storage and utilization, doctors and nurses establish a data verification and review mechanism to ensure the accuracy and consistency of clinical data. Eighty patients who had lung cancer that had spread to their liver diagnosed in our hospital from October 2021 to October 2023 (Group A), 80 patients with lung cancer combined with metastasis of other sites (Group B), and 80 without metastasis lung cancer patients (control group) were selected as study objects. There were 48 males and 32 females in Group A. The patients’ age in Group A ranged from 46 to 77 years, with a mean age of 57.3 ± 6.8 years. There were 35 smoking cases. There were 41 males and 39 females in Group B. The patients’ age in Group B ranged from 44 to 78 years, with a mean age of 56.8 ± 7.1 years. There were 34 smoking cases. The control group consisted of 34 females and 46 males. The patients’ age in the control group ranged from 43 to 79 years, with a mean age of 58.8 ± 7.8 years. There were 32 smoking cases. There was no remarkable variation in age, smoking rate, and gender among the three groups (p > 0.05).
2.2. Inclusion and Exclusion Criteria
Inclusion criteria were defined as follows: (1) The conditions for lung cancer diagnosis refer to the criteria in the 2013 Edition of the Guidelines for the Detection and Treatment of Primary Cancer in Lung formulated by the Chinese Clinical Oncology Society (CSCO); (2) liver metastases of lung cancer were all confirmed by CT or MRI and pathological examination; (3) patients whose lung cancer has spread to their liver were simple liver metastases, and patients with lung cancer combined with metastases of other sites did not develop liver metastases; (4) clinicopathologic information (i.e., age, sex, race, primary tumor site, tumor size, N stage, metastasis status, marital status, household income, and insurance) of patients was available and known; (5) BMI < 27.9 kg/m2; and (6) considering the sensitivity of medical data, doctors should provide patients with detailed explanations of the diagnostic process, treatment plan, and expected outcomes, while clearly explaining the risks and side effects that treatment may bring. This transparency helps patients better understand their condition and treatment options. This study has been approved by our hospital’s ethics committee, and all enrolled patients have signed written informed consent forms.
Exclusion criteria were defined as follows: (1) in addition to primary malignant tumors in other locations; (2) having a history of chemoradiotherapy; (3) serious diseases combined with other systems (myocardial infarction, cerebrovascular diseases, etc.); and (4) accompanied by other factors affecting the relevant indicators of this study.
2.3. Observational Index
The normal value of serum cyfra21-1 was < 7.0 ng/mL, the NSE normal value was < 10.0 ng/mL, and the normal range of LDH was < 245.0 U/L. cyfra21-1 and NSE were detected by the chemiluminescence method, and the required kit was purchased from Snibe Company; LDH was detected by the rate method, and the required kit was purchased from Ningbo Meikang Biotechnology Co. LTD., and the operation procedures were executed strictly in compliance with the instructions.
2.4. Statistical Analysis
Quantitative data that conform to a normal distribution are represented by (x ± s). Independent-sample t-test was used to compare the groups of lung cancer spreading to the liver, lung cancer with other metastases, and lung cancer without metastasis. Count data are represented as n (%). The data were compared using the χ2 test. The diagnostic efficiency index and the area under the curve (AUC) were computed after the receiver operator characteristic (ROC) curve was plotted. p < 0.05 represented that the variation was statistically significant. All statistical analyses were performed using the SPSS 20.0 software (SPSS, IBM, United States) and Excel 2010 software (Excel, Microsoft, United States).
3. Results
3.1. Pathological Data Comparison Among Three Groups
There were no statistically significant differences in pathological types among the three groups (p > 0.05), but there were statistically remarkable variations in TNM stage and lymph node metastasis among the three groups (p < 0.05, Table 1).
| Group | Case | Pathological type | TNM staging | Lymphatic metastasis | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adenocarcinoma | Squamous carcinoma | I | II | III | IV | Yes | No | ||
| Group A | n = 80 | 48 (60.00%) | 32 (40.00%) | 1 (1.25%) | 7 (8.75%) | 47 (58.75%) | 25 (31.25%) | 77 (96.25%) | 3 (3.75%) |
| Group B | n = 80 | 53 (66.25%) | 27 (33.75%) | 1 (1.25%) | 11 (13.75%) | 41 (51.25%) | 27 (33.75%) | 74 (92.50%) | 6 (7.50) |
| Control group | n = 80 | 50 (62.50%) | 30 (37.50%) | 12 (15.00%) | 55 (68.75%) | 12 (15.00%) | 1 (1.25%) | 27 (33.75%) | 53 (66.25%) |
| χ2 | 0.724 | 115.173 | 101.458 | ||||||
| p value | 0.382 | < 0.001 | < 0.001 | ||||||
3.2. Serum NSE, cyfra21-1, and LDH Levels Comparison Among Three Groups
The cyfra21-1, NSE, and LDH levels in Group A and Group B were greater compared to those in the control group (p < 0.05), and the cyfra21-1, NSE, and LDH levels in Group A were greater compared to those in Group B (p < 0.05, Table 2).
| Group | Case | cyfra21-1 (ng/mL) | NSE (ng/mL) | LDH (U/L) |
|---|---|---|---|---|
| Group A | n = 80 | 8.85 ± 3.42ab | 29.41 ± 8.62ab | 376.45 ± 72.18ab |
| Group B | n = 80 | 6.12 ± 2.43a | 19.13 ± 6.57a | 243.16 ± 54.98a |
| Control group | n = 80 | 4.15 ± 1.86 | 16.01 ± 5.17 | 215.37 ± 42.69 |
- Compared with control group: ap < 0.05. Compared with Group B: bp was < 0.05.
3.3. Value of NSE, cyfra21-1, and LDH Levels in the Detection of Liver Metastasis of Lung Cancer
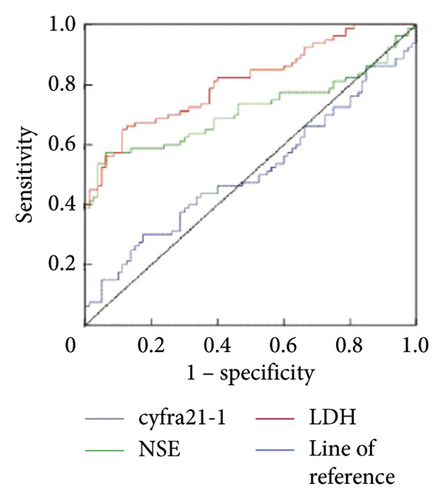
ROC analysis is a powerful tool for assessing the diagnostic performance of index tests, which are tests that are used to diagnose a disease or condition. The AUC value is a summary metric of the ROC curve that reflects the test’s ability to distinguish between diseased and nondiseased individuals. The critical value, specificity, sensitivity, and AUC of serum cyfra21-1 in the distinct identification of liver metastasis of lung cancer were 8.22 ng/mL, 37.42%, 65.18%, and 0.508, respectively. The critical value, specificity, sensitivity, and AUC of NSE for differential diagnosis of liver metastasis of lung cancer were 23.96 ng/mL, 64.56%, 81.23%, and 0.723, respectively. The critical value, sensitivity, specificity, and AUC of LDH for differential diagnosis of liver metastasis of lung cancer were 304.78 U/L, 75.65%, 85.73%, and 0.821, respectively (Table 3, Figure 1).
| Index | Cutoff | Sensitivity (%) | Specificity (%) | Missed diagnosis rate (%) | Misdiagnosis rate (%) | |
|---|---|---|---|---|---|---|
| cyfra21-1 | 8.22 ng/mL | 37.42 | 65.18 | 62.58 | 34.82 | 0.508 |
| NSE | 23.96 ng/mL | 64.56 | 81.23 | 35.44 | 18.77 | 0.723 |
| LDH | 304.78 U/L | 75.65 | 85.73 | 24.35 | 14.27 | 0.821 |
4. Discussion
In clinical practice, lung cancer is a prevalent digestive system malignant tumor that accounts for 17.8% of cancer mortality and 12.6% of new cancer cases [13, 14]. With a high morbidity and mortality rate, it is a malignant tumor disease [15, 16]. A tumor is a malignant condition that is consumptive. Studies have revealed that tumor cells may still perform intense anaerobic glycolysis in the absence of oxygen and use a lot of glucose to produce the energy needed for their own growth [17–19].
LDH is a significant REDOX enzyme in anaerobic colysis, which helps to catalyze the pyruvate and lactic acid bioconversion [20, 21]. LDH can be classified as LDH 1∼5 isoenzymes according to their molecular structure. Under normal circumstances, LDH content in cells is greater compared to that in serum, and the concentration is higher in skeletal muscle, liver, myocardium, red blood cells, and kidney [22, 23]. When a tumor occurs, serum LDH increases abnormally significantly, mainly because the LDH in the tumor cells spills into the blood after the tumor cells themselves are damaged, and following the tumor’s invasion or infiltration of the normal cells, LDH is released into the blood [24]. Therefore, it is speculated that increased serum LDH can be identified in various tumor tissues based on the preponderance of anaerobic fermentation of tumor cells. Following the rule out of acute myocardial infarction, chronic viral hepatitis, pulmonary embolism, cirrhosis, and malignant tumors of the liver and blood system, hepatic metastases of consideration should be given to lung cancer when serum LDH is unusually elevated [25, 26]. According to studies, there is a strong correlation among the lung cancer with liver metastasis risks and the serum tumor markers cyfra21-1 and NSE. Our research shows that the levels of cyfra21-1, NSE, and LDH in patients with lung cancer spreading to the liver and lung cancer with other site metastases are higher than those in patients without metastasis. The levels of cyfra21-1, NSE, and LDH in patients with lung cancer spreading to the liver are higher than those in patients with lung cancer with other site metastases. This is consistent with the report by Kono et al. in 2022 that elevated levels of cyfra21-1 lead to poor prognosis in non–small-cell lung cancer, which is a sensitive biomarker for lung cancer.
cyfra21-1 is the soluble fragment of CK19. CK are intermediate filaments that are present in normal and malignant epithelial cells, including bronchial epithelial cells, and are part of the cytoskeleton. CK protect epithelial cells from mechanical stress and also play important roles in cell signaling, the response to stress, and apoptosis. CK are classified by differences in molecular masses and isoelectric points. CK19 is a soluble Type I CK and has an isoelectric pH of 5.2 and a molecular mass of 40 kDa. In lung cancer, the malignant transformation of epithelial cells activates Caspase 3, which is a protease that regulates the apoptosis cascade. During this transformation, CK19 is cleaved as a result of the increased protease activity of Caspase 3, and its soluble fragment cyfra21-1 is released into the blood. In chronic airway inflammatory disease, cyfra21-1 is released from an injured bronchial epithelium. Recently, cyfra21-1 has been highlighted as a potential biomarker of epithelial damage in patients with idiopathic pulmonary fibrosis (IPF). According to some academics, people with lung cancer who have metastasized to their liver have a greater serum level of NSE than patients who have metastasized to other sites [27, 28]. Additionally, studies have shown that compared to other metastatic sites, lung cancer patients with liver metastases had significantly higher levels of NSE and LDH as well as positive expression rates for these markers [29, 30]. However, the above study selected the serum NSE and LDH levels in lung cancer patients after liver metastasis, so it could not be determined whether lung cancer with liver metastasis caused by the increase of NSE or lung cancer with liver metastasis could cause the expression of NSE and LDH, and the pathogenesis of liver metastasis of lung cancer was not clear. It is evident, though, that lung cancer patients who have markedly elevated serum NSE and LDH levels may have complex metastases [31, 32].
The study results showed that the serum levels of NSE, cyfra21-1, and LDH in patients with lung cancer with distant metastasis were remarkably greater compared to those in patients without distant metastasis (p < 0.05). The three tumor indicator levels in patients with liver metastasis were remarkably greater compared to those in lung cancer patients with metastases from other sites (p < 0.05). These results suggest that when the levels of NSE, cyfra21-1, and LDH are elevated, distant metastasis, especially liver metastasis, should be vigilant and prompt, and efficient action must be taken. In this study, it was found that there were no statistically remarkable variations in pathological types of lung cancer patients with liver metastasis or metastasis from other sites (p > 0.05), but there were statistically significant differences in TNM stage and lymph node metastasis (p < 0.05). These results represent that tumor metastasis is closely related to TNM stage, but the specific relationship between metastasis at different sites and the degree of TNM and lymph node metastasis needs further investigation, so as to provide basis for metastasis detection of lung cancer. cyfra21-1 is an important tumor indicator in the detection of lung cancer. Some research studies have reported that cyfra21-1 has a better specificity in the distinct detection of benign and malignant lung diseases [33–35]. In this study, the sensitivity and specificity of three tumor indicators were detected, and it was found that for liver metastases in particular, cyfra21-1 demonstrated a high sensitivity and specificity for lung cancer metastases. Obesity is a potential confounding factor that may affect serum biomarker levels. When recruiting patients, we chose BMI<27.9 kg/m2. Our research provides important insights into lung cancer liver metastasis, and these biomarkers may improve disease stratification and response prediction in the near future. It may also significantly improve the treatment outcomes of patients who were previously difficult to treat.
4.1. Limitation
Our study has limitations. First, our sample size was not very large; second, we did not do longer-term follow-up; and finally, we did not do intervention studies.
5. Conclusion
In summary, the serum NSE, cyfra21-1, and LDH levels in patients with liver metastasis of lung cancer are more remarkably increased compared to those in patients without liver metastasis, which provides some additional utility for diagnosing lung cancer metastases clinically. The detection methods of serum LDH, NSE, and cyfra21-1 are simple and feasible. In the case of no significant changes in imaging features, the serum changes of LDH can be dynamically observed to grasp the detection of distant metastases of lung cancer and remove the obstacle in this diagnosis, which has significant clinical guidance value.
Ethics Statement
This study protocol was approved by the Ethics Committee of Naval Military Medical University (202110NMM).
Consent
Informed consent was obtained from all patients.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jiquan Chen designed the study. Xiaoyuan Bu, Xintong Shi and Yingjun Wu wrote the original draft. Yinxiang Wu and Lu Li collected raw data. Liping Gao and Zhiwei Xiao performed statistical and bioinformatics analyses. Jiquan Chen supervised the study. Xiaoyuan Bu, Xintong Shi, and Yingjun Wu contributed equally to this work.
Funding
This study did not receive any funding in any form.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data could be obtained by contacting the corresponding author.




