Evaluation of Atrioventricular Valve Regurgitation in Detection of Atrioventricular Septal Defects at First Trimester Ultrasound
Abstract
Objective. To assess the effectiveness of atrioventricular valve regurgitation (AVVR) detection using color Doppler in the four-chamber view (4CV) for identifying atrioventricular septal defects (AVSDs) during 11–13 + 6 weeks’ gestation. This study compares AVVR detection to conventional 2D ultrasound and investigates associations between AVSD and increased nuchal translucency thickness (NT), as well as additional cardiac, extracardiac, and chromosomal abnormalities in the first trimester. Materials and Methods. This prospective study analyzed data from singleton pregnancies diagnosed with AVSD at 11–13 weeks gestation. It included routine ultrasound examinations focusing on fetal anatomy, NT measurement, and blood flow analysis across AVVR using both color and directional power Doppler. Evaluations targeted the 4CV and the three-vessel and trachea views (3VT). Ratios such as LAVV/RAVV and main pulmonary artery-to-aorta (PA/AO) diameter were also calculated. Results. Over three years, 452 fetuses were diagnosed with congenital heart disease in the first trimester, including 25 cases of AVSD (21 complete, 1 partial, and 3 intermediate). Seventeen cases were isolated AVSDs, 13 associated with heterotaxia syndrome, one with tetralogy of Fallot, and two survived. Among these, 92% showed AVVR, 61.54% had NT above the 95th percentile, and 32.0% lacked a nasal bone. AVVR was a more reliable indicator of AVSD than other ultrasonic markers. Conclusion. AVSD exhibits a diverse range of clinical presentations. A comprehensive review of both intracardiac and extracardiac anomalies is essential. AVVR detected during NT scanning in the first trimester can confirm the presence of AVSD.
1. Introduction
An AVSD is distinguished by a singular atrioventricular junction, unlike the distinct right and left junctions found in a normal heart [1]. Key morphological features of AVSD include an ovoid-shaped common junction and displacement of the left ventricular outflow tract, which typically lies between the tricuspid and mitral valves [2, 3]. Additionally, abnormalities are often present in both the muscular and membrane portions of the atrioventricular septum [4].
In newborns diagnosed with congenital heart disease, the prevalence of AVSD ranges from 3% to 7% [5, 6]. This incidence increases in fetuses, particularly those in high-risk groups, where it approximates 17%, compared to 11% in low-risk groups [7, 8]. AVSD often remains undetected until the second trimester of pregnancy or postnatally due to the difficulty of visualizing the full extent of the defect in the four-chamber view during the first trimester. Even skilled clinicians achieve only a 42.9% detection rate in the first trimester, and accurately assessing the left and right ventricular outflow tracts during this period poses significant challenges [9].
AVSD can be identified in the four-chamber view by noting alterations at the heart’s crux, primarily through the appearance of a common atrioventricular valve [10]. To enhance early prenatal detection of AVSD, we advocate a simplified echocardiographic technique focused on examining atrioventricular valve regurgitation. This study delineates the diagnostic features noted in fetuses with AVSD during the first trimester, emphasizing conditions observed during fetal development, rates of prenatal detection, the range of abnormalities, and prognostic outcomes post-diagnosis. Our research also aims to explore the correlations between AVSD and increased nuchal translucency (NT), atrioventricular regurgitation, and abnormal flow in the ductus venosus during the 11–13-week scan.
2. Materials and Methods
2.1. General Information
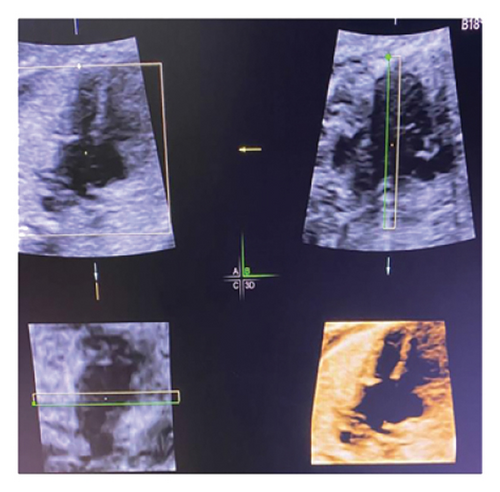
This study was conducted at the Jiangxi Maternal and Child Hospital in China from January 2020 to January 2023. It focused on routine first-trimester chromosomal abnormality screening in singleton pregnancies. During the ultrasound scans, three critical digital images were captured: NT thickness, 4CV of the heart, and three-vessel and trachea view using color Doppler and/or directional power beam (Figure 1(a)). The protocol involved assessing atrioventricular valve anatomy, blood flow, and valve regurgitation and calculating ratios of left to right atrioventricular valve (LAVV to RAVV) and pulmonary artery to aorta (PA to AO), along with ductus venosus measurements. The hospital’s Ethics Committee approved the study, and all participants were fully informed and consented.
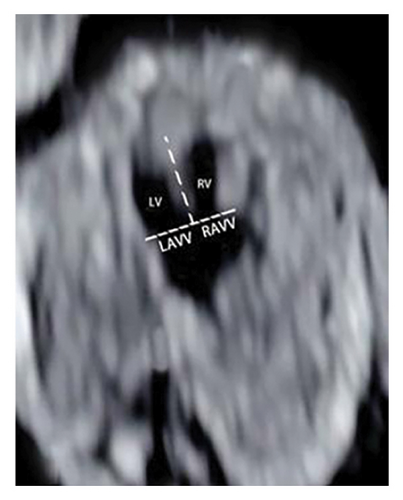
Experienced sonographers with at least five years in early fetal echocardiography conducted the screenings using either a Voluson E8 (GE Healthcare) or a WS80 (Samsung, Korea). The assessment included quantifying the LAVV and RAVV during diastole at their maximum diameter in the 4CV and categorizing common atrioventricular valve regurgitation (AVVR) as none, mild/moderate, or severe based on its extension to the posterior atrial wall.
The study’s protocol for detecting AVVR involved noting the regurgitation jet’s origin relative to the atrioventricular annulus and its position near the ventricular septum. The optimization of sound beam interface reflection, by angling it close to vertical relative to the interventricular septum, was crucial for enhancing detection. Further assessments of suspected AVSD cases were carried out by a fetal cardiologist within 14 weeks, with follow-up between 16 and 19 weeks. Only confirmed cases of congenital heart disease (CHD) by fetal cardiologists, surgery, or postmortem were documented. Mid-trimester evaluations were scheduled for patients with normal first-trimester outcomes, and associated cardiac and noncardiac anomalies, including conotruncal deformities, were recorded.
2.2. Statistical Analysis
The analysis of all collected data was performed using SPSS version 23.0 to identify significant differences. Metrics such as detection rate, false-positive rate, screening accuracy, positive predictive value, and negative predictive value were used to assess the effectiveness of the screening process. Continuous data were analyzed using independent-sample, two-tailed t-tests, while categorical variables were examined with Fisher’s exact tests. The data from the AVSD group underwent initial statistical analysis to provide deeper insights. A subsequent comparison between individuals with heterotaxy and those with situs solitus highlighted notable variations.
3. Results
3.1. Study Population
We enrolled 21,563 consecutive singleton pregnancies with a median maternal age of 28.96 ± 6.24 years (range, 21–42 years) and a crown-rump length (CRL) of 65.04 ± 10.44 mm (range, 46–83 mm). In this cohort, 452 fetuses were screened for congenital heart disease, which included 25 cases of AVSD: 21 complete, 3 transitional, and 1 partial. Two cases were misdiagnosed, and two were missed. Isolated AVSD occurred in 12 cases (42.5%).
Extracardiac malformations were found in 8 cases, and isomeric syndrome in 6 cases. These extracardiac abnormalities included skeletal, neurological, and renal issues. Termination of pregnancy before 14 weeks occurred in 13 out of 25 cases (58.5%). Of the remaining pregnancies, six continued, resulting in three abortions and one case lost to follow-up. There were two live births; one infant died without receiving surgery. Fetal echocardiography was repeated for 12 cases between 14 and 18 weeks, and there were two cases of intrauterine fetal death (IUFD).
Atrioventricular valve regurgitation was observed in 92% of AVSD cases (23/25), with two cases initially misdiagnosed as mild tricuspid regurgitation. Furthermore, 61.54% (16/25) exhibited nuchal translucency above the 95th percentile; 32.0% (8/25) had an absent nasal bone; and 64.0% (16/25) showed ductus venosus A wave inversion.
3.2. Karyotype Abnormalities
Cytogenetic analysis and microarray detection were conducted in 12 cases, revealing abnormalities in six fetuses. These included three instances of trisomy 21 (two of which were associated with isolated atrioventricular septal defects, AVSDs) and two instances of trisomy 18 (both with isolated AVSD). Additionally, exon sequencing identified a heterozygous mutation, NM 007126.3: c.1487C > T (p.Pro496Leu), in one case following labor induction. Detailed findings are presented in Table 1.
| Case | CRL (mm) | GA (wk + d) | NT (mm) | Type | AVVR | Other cardiac malformations | Extracardiac deformity | Genetic evaluation | Pregnancy outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | 13.20 | 2.5 | CAVSD | ++ | TOF; PA | CVS, trisomy 21 | TOP | |
| 2 | 59 | 12.30 | 2.1 | TAVSD | − | RAIS | 46, XY | ND | |
| 3 | 71 | 13.20 | 2.3 | CAVSD | +++ | CAT | LAIS | Unperformed | IUFD |
| 4 | 76 | 13.40 | 3.1 | CAVSD | +++ | CAT | RAIS | Trisomy 21 | TOP |
| 5 | 59 | 12.30 | 1.5 | CAVSD | ++ | TOF | Unperformed | TOP | |
| 6 | 65 | 12.60 | 7.2 | CAVSD | +++ | CVS, unperformed | TOP | ||
| 7 | 77 | 13.50 | 2.7 | CAVSD | ++ | Absence of nasal bone | Trisomy 18 | TOP | |
| 8 | 70 | 13.30 | 2.8 | CAVSD | ++ | DORV | CPC | Trisomy 21 | TOP |
| 9 | 80 | 13.60 | 2.6 | CAVSD | ++ | CoA | RAIS | Unperformed | IUFD |
| 10 | 56 | 12.10 | 2.7 | TAVSD | − | Unperformed | TOP | ||
| 11 | 63 | 12.50 | 3.1 | TAVSD | ++ | CAT | RAIS | 46, XY | Postnatal death |
| 12 | 64 | 13.10 | 3.1 | CAVSD | − | Unperformed | TOP | ||
| 13 | 57 | 12.20 | 2.0 | CAVSD | ++ | TOF; PA | LAIS | Trisomy 21 | TOP |
| 14 | 79 | 12.50 | 4.2 | CAVSD | ++ | 46, XX | IUFD | ||
| 15 | 75 | 13.30 | 2.7 | CAVSD | +++ | AMNIO 46, XX | TOP | ||
| 16 | 72 | 13.20 | 1.8 | CAVSD | + | CAT | LAIS | Unperformed | TOP |
| 17 | 65 | 12.20 | 2.5 | CAVSD | ++ | RAIS | CVS 46, XY | TOP | |
| 18 | 75 | 13.30 | 3.8 | CAVSD | +++ | AMNIO 46, XX | TOP | ||
| 19 | 53 | 12.10 | 1.7 | CAVSD | ++ | CAT | CPC; RAIS | CVS 46, XY | TOP |
| 20 | 63 | 12.20 | 2.5 | CAVSD | ++ | CAT | LAIS; SUA | CVS 46, XY | TOP |
| 21 | 53 | 12.00 | 3.4 | CAVSD | ++ | Unperformed | LTF | ||
| 22 | 79 | 13.50 | 1.1 | CAVSD | ++ | CAT | LAIS | AMNIO 46, XX; | TOP |
| 23 | 50 | 11.50 | 1.4 | CAVSD | +++ | 22q11 microdeletion | TOP | ||
| 24 | 46 | 11.30 | 6.5 | PAVSD | − | CTEV; PE | VCP | TOP | |
| 25 | 48 | 11.40 | 2.2 | CAVSD | ++ | Unperformed | TOP |
- CAVSD, complete atrioventricular septal defect; PAVSD, partial atrioventricular septal defect; TAVSD, transitional atrioventricular septal defect; 4CVc, 4-chamber view in color; GA, gestational age; CRL, crown-rump length; CAT, common arterial trunk; CoA, coarctation of the aorta; AMNIO, amniocentesis; CVS, chorionic villus sampling; TOF, tetralogy of Fallot; NT, nuchal translucency; PA, pulmonary atresia; ECHO, echocardiography; PS, pulmonary stenosis; HLHS, hypoplastic left heart syndrome; LAIS, left atrial isomerism syndrome; RAIS, right atrial isomerism syndrome; ND, neonatal death; CPC, choroid plexus cyst; SUA, single umbilical artery; PE, pleural effusion; TOP, termination of pregnancy service; IUFD, intrauterine fetal death; LTF, lost to follow-up.
3.3. Color Doppler and/or Directional Power Doppler Examination of the Heart in the First Trimester
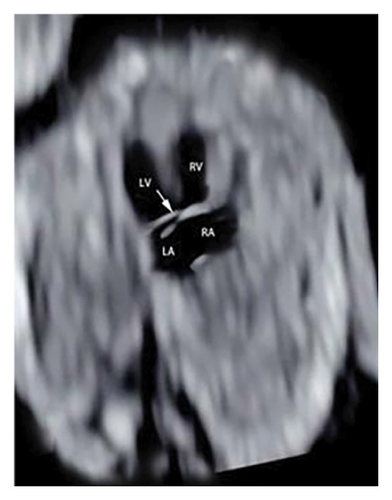
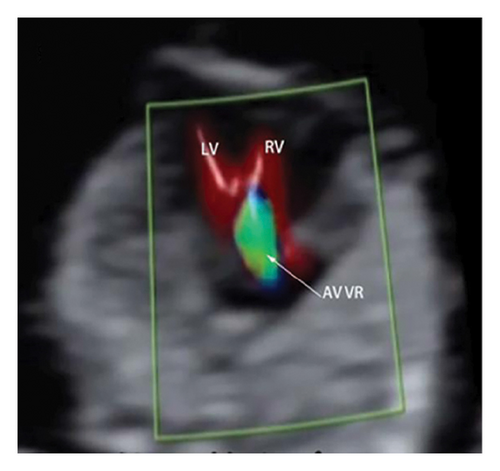
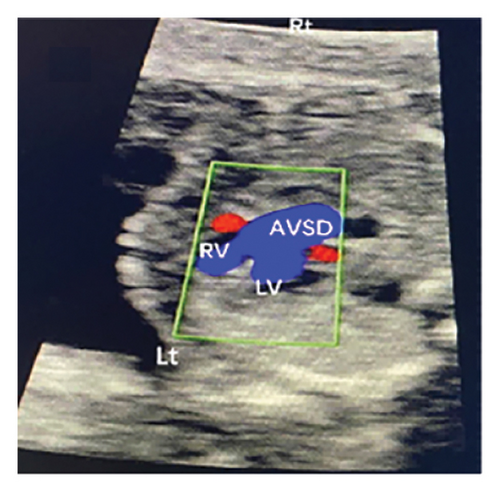
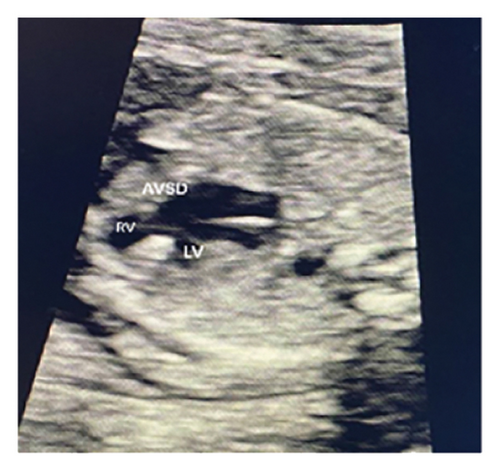
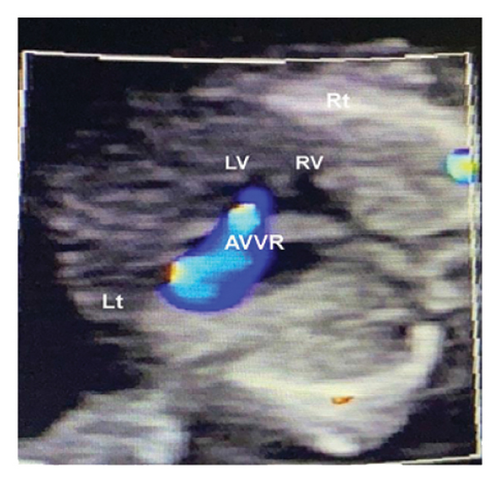
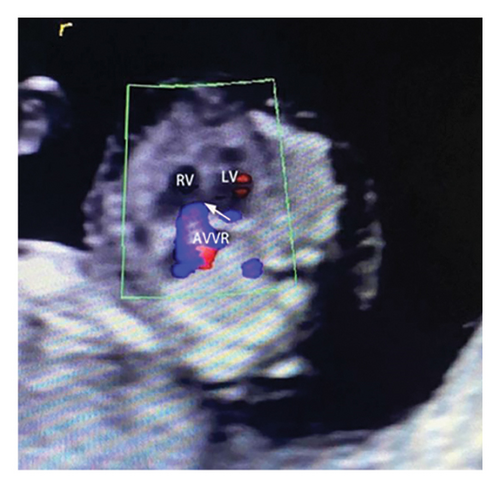
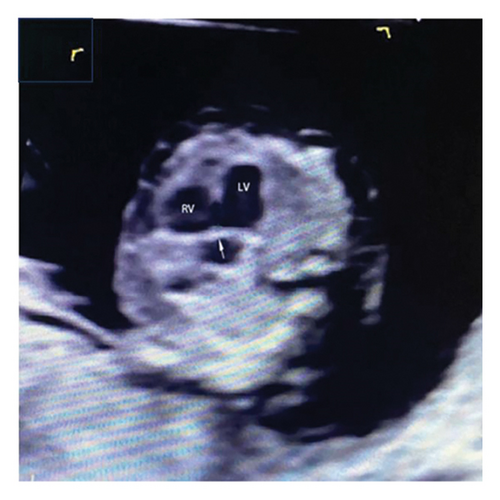
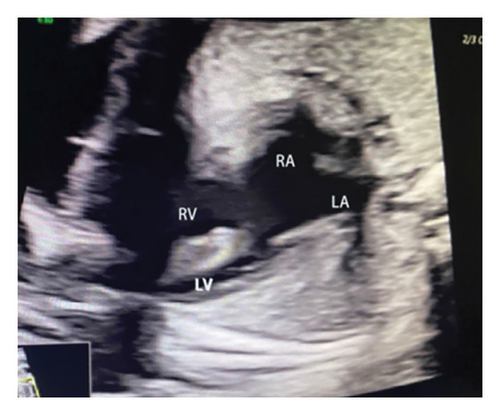
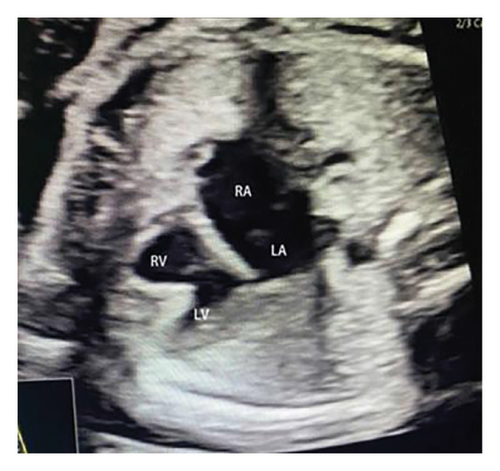
Common atrioventricular valve regurgitation was observed in 23 of 25 cases (92%) during systole (Figures 1(c), 2(c), 2(d), and 3(a)). In another 18 cases (72%), diastolic color Doppler in a four-chamber view revealed a “Y sign,” formed by two ventricles and a common atrioventricular canal (Figures 2(a), 2(b), and 4(a)). Systolic color Doppler detected severe regurgitation in six cases, moderate in 14, and mild in three. Sonographers categorized abnormal three-vessel tract findings into two subgroups based on the number of arterial vessels present: the single-vessel group included seven cases, and the double-vessel group included 18. Fetuses with AVSD exhibited significantly higher rates of increased nuchal translucency (NT), atrioventricular valve regurgitation, and aberrant ductus venosus flow compared to those without these abnormalities. Table 2 details the sensitivity and specificity of four detection methods for atrioventricular septal defects: color Doppler 4CV (4CV-CDF), common atrioventricular valve regurgitation (AVVR), grayscale 4CV, and their combination. Table 3 illustrates key cardiac measurements, including the primary pulmonary artery-to-aorta diameter ratio (PA/AO), the cardiothoracic ratio (CTR), and the calculation of the left atrioventricular valve to right atrioventricular valve ratio (LAVV/RAVV).
| Parameter | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| 4CV-CDF | 72% (50.40%∼87.13%) | 99.98% (99.95%∼99.99%) | 85.71% (62.64%∼96.23%) | 99.96% (99.92%∼99.98%) |
| AVVR | 88% (67.66%∼96.85%) | 99.99% (99.96%∼99.99%) | 95.65% (76.03%∼99.77%) | 99.98% (99.95∼99.99) |
| 4CV | 84% (63.08%∼94.74%) | 99.99% (99.96%∼99.99%) | 91.30% (70.49%∼98.47) | 99.98% (99.94%∼99.99%) |
| 4CV-CDF + CR + 4CV | 92% (72.49%∼98.60%) | 99.99% (99.98%∼99.99%) | 95.83% (76.88%∼99.79%) | 99.99% (99.96%∼99.99%) |
- Values in brackets are 95% confidence intervals. NPV, negative predictive value; PPV, positive predictive value; AVSD, atrioventricular septal defect; CDF, color Doppler; ARRV, common atrioventricular regurgitation; 4CV-CDF, color Doppler four-chamber; 4CV, 2D four-chamber; AVVR, atrioventricular valve regurgitation.
| Arteries in the 3VT | CTR | RAVV/LAVV | PA/AO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Decrease | Normal | Increase | Decrease | Normal | Increase | Decrease | Normal | Increase | |
| Two arteries | 2 | 14 | 2 | 4 | 10 | 4 | 4 | 6 | 8 |
| 0.22 ± 0.01 | 0.32 ± 0.04 | 0.53 ± 0.03 | 0.72 ± 0.06 | 1.08 ± 0.02 | 1.34 ± 0.05 | 0.6 ± 0.09 | 1.24 ± 0.1 | 1.75 ± 0.22 | |
| One artery | 1 | 4 | 2 | 4 | 2 | 1 | — | — | — |
| 0.31 ± 0.04 | 0.46 ± 0.06 | 0.82 ± 0.07 | 1.06 ± 0.01 | 1.3 ± 0 | |||||
- CTR, cardiothoracic ratio; LAVV, left atrioventricular valve; RAVV, right atrioventricular valve; PA/AO, main pulmonary artery-to-aorta; 3VT, the three vessels and trachea.
4. Discussion
Fetuses diagnosed with AVSD are a heterogeneous group, displaying variations in their associations with other congenital cardiac anomalies, visceral heterotaxy, and chromosomal abnormalities [4]. AVSD can be identified by distortions in the normal architecture at the crux of the heart, visible in the four-chamber view [10]. This defect typically presents with a common atrioventricular junction instead of separate mitral and tricuspid valves.
4.1. Cardiac Abnormalities: Ultrasound Screening of Fetal AVSD in the First Trimester
Ultrasound screening in the first trimester often employs the 4CV to detect AVSD [11]. The tricuspid valve is positioned lower in the right ventricle compared to the mitral valve on the left, creating an asymmetrical cross. A critical diagnostic indicator is the presence of a common atrioventricular valve in the 4CV, characterized by the lack of normal separation between the atrioventricular valves due to the absence of the atrioventricular muscular septum [12]. A posterior-inferior to anterior-superior sweep confirms that the left and right atrioventricular valves align at the same level during the first trimester [13] (Figures 1(b), 3(b), and 4(b)). Additionally, a “Y” configuration in the blood flow, evident during diastole in the 4CV-CDF, suggests AVSD. This “Y sign”—depicting two ventricles and a common atrioventricular canal—is crucial for diagnosis. In this study, anomalies in the 4CV of 21 fetuses led to the detection of the “Y sign” in 18 of 25 cases (72%), with 12 cases presenting as isolated AVSD.
4.2. Association of Atrioventricular Septal Defect and Atrioventricular Valve Regurgitation
Analyzing the specified cross sections (4 CV and 3 VT) using color and directional power Doppler modes significantly enhances diagnostic accuracy and ease, in contrast to the limitations of solely employing 2D imaging [14, 15]. Tricuspid regurgitation serves as a predictor for complex congenital heart diseases [16]. Research by Davey et al. [17] indicates that approximately 81% of fetuses with AVSD exhibit common atrioventricular valve regurgitation during the second and third trimesters, increasing to 96% postnatally. In preoperative echocardiography, Kozak et al. [18] found that 93% of 104 children with AVSD displayed mild or greater common atrioventricular reflux in preoperative echocardiography. Similarly, Yang et al. [19] reported that 91.89% of 37 fetuses with AVSD demonstrated common atrioventricular valve regurgitation in the first trimester.
In this study, the degree of AVVR was qualitatively assessed and categorized as none/mild or moderate/severe. It was observed in 92% (23 out of 25) of fetuses with AVSD, with classifications of 3 mild, 13 moderate, and 6 severe cases. In the two cases where AVSD was not detected, normal blood flow patterns were identified during diastole in the 4CV, without any signs of common atrioventricular valve regurgitation.
Furthermore, this research highlights that while mild tricuspid regurgitation is typically clinically insignificant, mild common atrioventricular valve regurgitation could be the sole ultrasonographic indicator of AVSD in early pregnancy. This necessitates precise identification of both tricuspid and common atrioventricular valve regurgitation. This study evaluates the sensitivity and specificity of four detection methods for AVSD: AVVR, 4CV in grayscale, and their combinations.
4CV-CDF, 2D 4CV, and AVVR for AVSD screening demonstrated sensitivities and positive predictive values of 72%, 85.71%, 84%, 91.3%, and 88%, 95.65% respectively. The combination of methods proved to be the most accurate, achieving sensitivities and positive predictive rates as high as 92% and 95.83%, respectively. This suggests that the combined approach greatly improves early diagnosis, particularly in detecting complete types of AVSD, with partial and transitional types rarely identified [20, 21].
4.3. Additional Cardiac Abnormalities
This study retrospectively analyzed 25 cases of fetal AVSD in the first trimester. Among these, 21 cases were identified with a complete AVSD, representing 84% of the total. Within this subgroup, 16 fetuses (76.2% of the complete AVSD cases) exhibited significant large vessel malformations, including conditions such as double outlet right ventricle (DORV) and PTA.
Further investigation is required to elucidate the relationship between AVSD and the great arteries, particularly regarding their impact on the outflow tracts [22]. Advanced imaging techniques like HD Flow or color Doppler significantly improve the visualization of the three-vessel trachea view (3VTV), which experienced sonographers can readily observe in early pregnancy. Integrating 3VTV with the 4CV enhances the accuracy of detecting severe cardiac defects affecting the outflow pathways and aortic arch. Such abnormalities are often indicated by an abnormal number of vessels, aberrant sizes, or irregular spatial configurations [23]. The diagnosis of pulmonary stenosis or atresia was confirmed through postmortem or Doppler assessments, or inferred from noticeably smaller pulmonary arteries relative to the aorta, as observed in the 3VTV [24, 25].
Eighteen fetuses with AVSD presented both the aorta and pulmonary artery. In four cases (22.2%), the inner diameter ratio decreased, while in eight cases (44.4%), it increased (Table 3). Additionally, six cases exhibited atrial isomerism, one had an inverse atrial arrangement, and five showed evidence of pulmonary atresia or stenosis.
Within the subgroup displaying an abnormal vessel count, seven cases showed diminished dimensions of the great arteries or the aortic arch. These abnormalities, specifically aortic coarctation or an anomalous course of a vessel (e.g., right aortic arch), were identified during initial trimester screening and confirmed via follow-up echocardiography in the second trimester.
The ratio of the left atrioventricular valve (LAVV) to the right atrioventricular valve (RAVV) was normal in 12 cases, left-dominant in 8, and indicative of a single ventricle in 2 cases (Figure 1(a)) [26]. The study suggests that an unbalanced AVSD occurs when the atrioventricular valve predominantly opens towards the larger ventricle, leading to hypoplasia of the contralateral ventricle (Table 3) [27].
In axial images of the fetal thorax, the CTR was calculated by dividing the thoracic area by the diastolic heart area [28]. The results showed three cases with a decreased CTR, four with an increased CTR, and 18 with a normal CTR.
4.4. Extracardiac Abnormality
The occurrence of AVSD during fetal development differs from those identified after birth. About 45% of individuals diagnosed prenatally may exhibit heterotaxy syndromes, mainly left atrial isomerism [9]. Consequently, it is vital to incorporate the assessment of atrial situs, ventriculo-arterial connections, ventricular size, and aortic arch caliber while performing fetal echocardiography in instances of AVSD. We found 13 cases were connected with a heterotaxia syndrome. The mortality rate is elevated among individuals with additional cardiac abnormalities, specifically, the coexistence of left atrial isomerism. Detecting these linked abnormalities is paramount, enabling parents to receive proper guidance regarding outcomes during counseling sessions. The occurrence of elevated nuchal translucency, atrioventricular valve regurgitation, and aberrant flow in the ductus venosus was notably more frequent in fetuses with AVSD compared to those without this condition.
4.5. Karyotype Abnormalities
This study found that over 50% of fetuses diagnosed with either partial or complete AVSD also had Down syndrome. Additionally, approximately half of these fetuses exhibited heterotaxia syndromes, underscoring a strong correlation between AVSD and Down syndrome, and a significant incidence of heterotaxia syndromes within this group [29].
In cases of ectopic syndrome, the fetuses presented various combinations of intracardiac and extracardiac abnormalities, including five instances of chromosomal abnormalities and one case of 22q11 microdeletion (Table 1).
Biomarkers such as fetal tricuspid regurgitation, NT greater than the 95th percentile, or reversed A-wave in the ductus venosus are valuable for early detection of heart defects. Studies have indicated that an NT greater than 3.5 mm predicts complex congenital heart disease with 66% sensitivity; predictive sensitivities for catheter A-wave inversion and tricuspid regurgitation are 50.0% and 35.2%, respectively, with a combined sensitivity reaching 77.0% [30].
These indirect ultrasound signs, however, were not significantly linked to any specific complex congenital heart diseases. The occurrence of increased NT was notable but markedly less frequent than common atrioventricular valve regurgitation. The success rate of surgical repair for AVSD is lower compared to other CHD, which sometimes leads to the consideration of pregnancy termination as a management option [4]. Earlier diagnosis tends to improve psychological recovery following the termination of a pregnancy due to fetal malformations.
The current study provides insightful outcomes that enhance understanding of the diagnostic processes for AVSD using first-trimester ultrasound. Notably, no pathological examinations or invasive procedures were conducted on some of the fetal hearts in this study; the classification of AVSD was based solely on echocardiography results, which can be somewhat subjective.
5. Conclusions
This study examines diagnostic features in first-trimester fetuses with AVSD. Assessing common atrioventricular regurgitation provides a straightforward and swift screening approach for AVSD during the first trimester. The mortality rate is notably higher in cases with concurrent cardiac abnormalities, particularly when AVSD is associated with left atrial isomerism. Accurately identifying these abnormalities is crucial for effectively counseling parents about potential outcomes.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Z.G. and X.Z. were responsible for conceptualization. Y.Y. and Z.Y. were responsible for methodology and investigation. J.X. and X.X. were responsible for resources and data curation. Z.G., J.C., and X.Z. were responsible for review and editing. H.L. was responsible for visualization. X.Z. was responsible for supervision. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This research was funded by the Key Research and Development Program of Jiangxi Province (no. 20192BBGL70006 to Zhen Guo), Application and Cultivation Program of Jiangxi Province (no. 20212BAG70007 to Zhen Guo), Provincial Health Commission Program of Jiangxi (grant no. SKJP20203582 to Zhen Guo), Key Research and Development Program of Jiangxi Province (grant no. 20202BBG73015 to Yang Yan), Provincial Health Commission Program of Jiangxi (grant no. SKJP202211156 to Yang Yan), Provincial Health Commission Program of Jiangxi (grant no. SKJP202130777 to Zhen Guo), and Provincial Health Commission Program of Jiangxi (grant no. SKJP20203582 to Zhen Guo).
Open Research
Data Availability
Data presented in this study are available on request from the corresponding author.




