Population Pharmacokinetic Analysis of Selumetinib and Its N-desmethyl Metabolite in Japanese and Non-Japanese Pediatric Patients with Neurofibromatosis Type 1 and Inoperable Plexiform Neurofibromas
Abstract
Background. Selumetinib, a mitogen-activated protein kinase kinase 1/2 inhibitor, has been approved in several countries and regions, including Japan, for the treatment of pediatric patients with neurofibromatosis type 1 who have symptomatic, inoperable plexiform neurofibromas at a body surface area (BSA)-based dose of 25 mg/m2 twice daily. The objective of this population pharmacokinetic analysis was to evaluate ethnic sensitivity in the pharmacokinetics of selumetinib and N-desmethyl selumetinib between Japanese and non-Japanese pediatric patients. Methods. This population pharmacokinetic analysis was based on data from 80 pediatric patients enrolled in two clinical trials, one conducted in Japan and one conducted in the United States, comprising 12 Japanese participants and 68 non-Japanese participants. Both clinical trials used BSA-based dosing schemes. A two-compartment model with first-order elimination and sequential zero-order and first-order delayed absorption for selumetinib, combined with a one-compartment model with first-order elimination for N-desmethyl selumetinib, was used for this analysis. Ethnic sensitivity in pharmacokinetics was evaluated by covariate modeling and comparison of model-predicted exposures. Results. Covariate modeling showed that BSA had a clinically relevant impact on the pharmacokinetics of selumetinib. None of the other investigated covariates, such as race, had a significant impact. The predicted exposure in Japanese and non-Japanese patients showed a considerably overlapping distribution, and no clinically relevant difference in exposure was apparent. Conclusions. These findings support the use of the same BSA-based dosing regimen for Japanese and non-Japanese pediatric patients with neurofibromatosis type 1 and inoperable plexiform neurofibromas. Subsequent to this analysis, selumetinib was approved at the BSA-based dose of 25 mg/m2 in Japan, which is consistent with the recommended dosage and administration in other regions and countries. This analysis used data from trial registered with NCT04495127, and NCT01362803.
1. Introduction
Neurofibromatosis type 1 (NF1) is an intractable and rare autosomal dominant genetic disorder caused by germline mutations in the NF1 tumor suppressor gene [1]. NF1 is characterized by dysfunction of the guanosine triphosphatase-activating protein neurofibromin, leading to overactivation of the rat sarcoma virus (RAS) pathway, which is associated with cell growth and proliferation [2]. Plexiform neurofibromas (PN), a type of benign tumor of the peripheral nervous system, is one of the most common coincident tumor types in patients with NF1 [3, 4].
In Japan, NF1 is estimated to affect 40,000 patients with a prevalence of 1 per 3,000 people [5]. The prevalence of NF1 in Japan is consistent with that in European countries and the United States (US) [6], suggesting no racial differences [5]. In Japan, the diagnostic criteria for NF1 were developed by the Japanese Dermatological Association by translating the National Institutes of Health diagnostic criteria [7] such that they were easy to understand, and adding clear explanations of the concept, reference findings, and important points to consider when making a diagnosis [5].
Selumetinib, formerly known as AZD6244 or ARRY-142886, is a potent and selective mitogen-activated protein kinase kinase 1/2 inhibitor, and it is the only approved treatment for pediatric patients aged ≥2 years (in the US) or ≥3 years (except for the US) with NF1 and symptomatic, inoperable PN. Selumetinib has been approved in several countries and regions, including Japan, the US, and the European Union, for administration at the body surface area (BSA)-based dose of 25 mg/m2 twice daily [8–10]. BSA-based dosing has been used in clinical trials of pediatric populations, including a phase I trial conducted in Japan (D1346C00013; NCT04495127) [11] and a phase I/II trial conducted in the US (D1532C00057; SPRINT; NCT01362803) [12–14].
The pharmacokinetic (PK) profile of selumetinib, along with its active metabolite N-desmethyl selumetinib (3–5 times more potent than selumetinib, circulating at about 7% of selumetinib concentration in plasma, consequently about 20–30% contribution to the selumetinib activity), has been well characterized in several population pharmacokinetic (PopPK) analyses [15–18]. The PK profiles of selumetinib and N-desmethyl selumetinib are comparable among healthy subjects, adult patients with various tumors, and pediatric patients with NF1 and PN. In the pediatric population, at the investigated dose range, there were no significant exposure-response (E-R) relationships between exposure and efficacy (various endpoints including overall response rate and progression-free survival) nor safety (adverse events including those leading to discontinuation, dose interruption, or dose reduction) [18].
Ethnic differences in selumetinib exposure were observed in healthy adult volunteers, with 51% higher exposure in Japanese versus White participants, which may be partially attributable to differences in body size [19]. In contrast, a previous PopPK analysis [18], which included Asian subjects, suggested that race (Asian) did not have a clinically relevant impact on selumetinib clearance (CL), but BSA had a clinically relevant (>20%, which was based on the bioequivalence criterion) impact on selumetinib exposure. These findings support BSA-based dosing in children.
Here, we report the results of a PopPK analysis that was conducted to evaluate ethnic sensitivity in the PK of selumetinib and N-desmethyl selumetinib between Japanese and non-Japanese pediatric patients with NF1 and inoperable PN.
2. Methods
2.1. Clinical Trials and Datasets
In this PopPK analysis, we pooled the data from clinical trials conducted in pediatric patients with NF1 and PN. Individuals were defined as evaluable for the PopPK analysis if they had at least one postdose plasma sample with a selumetinib concentration above the bioanalytical lower limit of quantification.
The clinical trials are briefly described in Table 1. Trial D1346C00013 enrolled Japanese participants from Japan sites, while SPRINT enrolled non-Japanese participants from US sites. The clinical trials were similarly designed other than their location. For example, key eligibility criteria for both trials were pediatric patients with NF1 and PN, 2 or 3 to 18 years old, and with adequate organ and hematological function. Key endpoints were also similar, and both trials measured safety/tolerability, PK, and clinical efficacy (refer to the publications [11–14] and clinical trial information websites for further details of the study designs).
| Clinical trial description | Dose (mg/m2; twice daily) | PK sampling |
|---|---|---|
| D1532C00057 (SPRINT) phase I | 20, 25, 30 | Cycle 1 Day 1: predose, 0.5, 1, 2, 3, 5, 8, 10–12, 24, and 30–36 h postdose |
| Cycle 1 day 28: predose | ||
| D1532C00057 (SPRINT) phase II stratum 1 | 25 | Cycle 1 Day 1: predose, 0.5, 1.5, 3, 6, and 10–12 h postdose |
| Cycle 1 Day 28: predose, 0.5, 1.5, 3, and 6 h postdose | ||
| D1346C00013 (Japan phase I) | 25 | Cycle 1 Day 1: predose, 0.5, 1.5, 3, 6, and 10–12 h postdose |
| Cycle 2 Day 1: predose, 0.5, 1.5, 3, and 6 h postdose | ||
- One cycle was 28 days. NF1, neurofibromatosis type 1; PK, pharmacokinetic; PN, plexiform neurofibromas.
The clinical trials used a BSA-based dose ranging from 20 to 30 mg/m2 that was determined using a nomogram based on the available capsule strengths of 10 and 25 mg. D1346C00013 and SPRINT phase II stratum 1 used one dose level of 25 mg/m2, which was selected as the recommended phase II dose based on SPRINT phase I. The nomograms for the dose of 25 mg/m2 differed slightly between D1346C00013 and SPRINT. The lowest and second lowest BSA bins and their selumetinib dosages were set as 0.55–0.69 m2 (20 mg in the morning and 10 mg in the evening) and 0.70–0.89 m2 (20 mg twice daily) in D1346C00013, and as 0.55–0.59 m2 (10 mg twice daily) and 0.60–0.89 m2 (20 mg twice daily) in SPRINT, respectively. Agents with the potential to interfere with selumetinib metabolism, such as strong or moderate CYP3A4 inhibitors or inducers (for example, clarithromycin and St John’s wort), were avoided, especially in the PK evaluation period.
Clinical trials in adults were not included in the current PopPK analysis for a number of reasons. The previous analysis, which included adult trials in addition to SPRINT, selected age as a statistically significant covariate. The effect of age was considered because BSA alone could not explain the variability in selumetinib CL across a wide age range (i.e., including adults), despite the collinearity between BSA and age in children [18]. In this younger population up to 18 years of age, where an almost linear relationship was observed between BSA and age (Pearson’s correlation coefficient = 0.858 in our pooled analysis dataset), including age as a covariate in addition to BSA for the same parameter, introduced multicollinearity. In addition, NF1 sometimes has skeletal manifestations, such as scoliosis or long-bone dysplasia, and pediatric patients with NF1 tend to be shorter than healthy children [20], which was also observed in our dataset (Figure S1). By pooling clinical trials of pediatric patients with NF1 and PN only, the disease was not a factor in covariate modeling between BSA and CL.
Plasma samples were analyzed using a previously described validated liquid chromatography-tandem mass spectrometry method with adequate accuracy and precision [21]. The calibration range of the method was 2.00–2000 ng/mL and 2.00–500 ng/mL for selumetinib and N-desmethyl selumetinib, respectively. The same bioanalytical method [21] was used for all clinical trials included in our dataset. When conducting the PopPK analysis, the concentrations of selumetinib and N-desmethyl selumetinib measured in grams were converted into moles using the molecular weights (457.7 and 443.7 for selumetinib and N-desmethyl selumetinib, respectively).
All clinical trial protocols and informed consent forms were approved by the relevant review boards. All patients or their legal guardians provided written informed consent prior to any procedure specific to clinical trials.
2.2. PopPK Modeling
The previously developed PopPK models, especially the structural model in the previous population analysis (Figure 1) [18], were used to guide the model development in the present analysis. We used a two-compartment model with first-order elimination and sequential zero-order and first-order delayed absorption for selumetinib, which was simultaneously combined with a one-compartment model with first-order elimination for N-desmethyl selumetinib. Selumetinib was assumed to be irreversibly converted to N-desmethyl selumetinib. The model included the following parameters: selumetinib clearance of central compartment (CL); selumetinib volume of distribution of central compartment (V2); selumetinib intercompartmental clearance (Q); selumetinib volume of distribution of peripheral compartment (V3); first-order absorption rate constant (Ka); time of zero-order absorption (D1); absolute bioavailability (F1); absorption lag time (ALAG); fraction metabolized (Fm); N-desmethyl selumetinib clearance (CLm); and N-desmethyl selumetinib volume of distribution (V4). Due to an identifiability issue, which is that V4 and Fm cannot be estimated independently, V4 was set to 1 for allowing Fm to be interpreted as a fraction of selumetinib converted to N-desmethyl selumetinib.
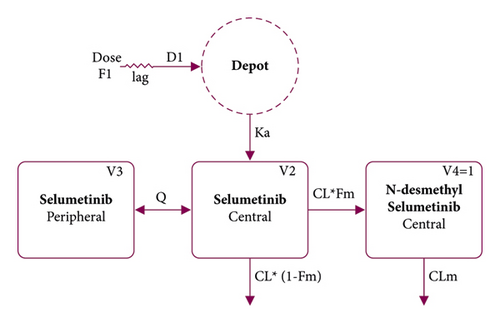
The interindividual variability (IIV) in PK parameters was modeled as exponential errors, and the residual variability in the observations was modeled as proportional errors. Drug concentrations below the lower limit of quantification (BLQ) were treated as missing.
2.3. Covariate Modeling
Covariate candidates for each PK parameter were prespecified in a modeling analysis plan. Based on previous knowledge [18], the effect of BSA on CL was evaluated in the initial covariate modeling as part of the base model. Subsequently, stepwise covariate modeling (SCM) was applied. The significance levels for forward selection and backward elimination were 0.01 and 0.001, respectively. During SCM, the following candidates were explored: on CL and CLm: BSA (CLm only), sex, race, age, aspartate aminotransferase, alanine aminotransferase, albumin, total bilirubin, and creatinine clearance normalized by BSA; on Ka: age; on V2 and V3: BSA, sex, and race. Considering our objectives of the current PopPK analysis, race was primarily assessed as “Japanese” or “non-Japanese,” and patients classified as Black and Asian in SPRINT were pooled into one group (“non-Japanese”) because of the small sample size (Black: n = 5 (7.4%); Asian: n = 3 (4.4%)).
2.4. Model Assessment
Models were selected based on several aspects, including convergence success, goodness-of-fit plots, likelihood ratio test (i.e., the difference in objective function value (ΔOFV)), and the precision and plausibility of the parameter estimates. For the final PopPK model, the uncertainty of parameters and the model robustness were evaluated using the relative standard error of model estimates and bootstrapping by random resampling (1,000 times) with replacement, stratified by clinical trial (D1346C00013, SPRINT phase I, and SPRINT phase II stratum 1). Prediction-corrected visual predictive check (pcVPC) using 1,000 simulations was implemented to evaluate the performance of the model for prediction and simulation.
2.5. Evaluation of the Ethnic Sensitivity in PK
The ethnic sensitivity in the PK of selumetinib and N-desmethyl selumetinib between Japanese and non-Japanese pediatric patients with NF1 and inoperable PN was evaluated as follows: (1) in the covariate model, if race was not included as a significant covariate or if the impact of including race as a covariate was minimal (<20% change in CL), the ethnic sensitivity in the PK parameter was not considered clinically meaningful and (2) predicted exposure metrics based on the final model were plotted stratified by race (Japanese and non-Japanese), and if the distribution of the exposure metrics overlapped, the ethnic sensitivity in PK exposure was not considered clinically meaningful. The threshold 20% change in CL was based on the bioequivalence criterion.
2.6. Software
The PopPK analysis was performed using a validated installation of NONMEM (version 7.3, ICON, Ellicott City, MD). Data summarization and postprocessing of the NONMEM analysis results were performed using R, version 4.0.2 (The R Foundation for Statistical Computing). NONMEM run execution, bootstrap, and pcVPCs were performed using Perl-speaks-NONMEM, version 4.4.8 (Dept. of Pharmacy, Uppsala University, Uppsala, Sweden). The first-order conditional estimation method with an interaction for estimation was used for PopPK modeling.
3. Results
3.1. Data Analyzed
The final pooled dataset for the PopPK analysis included 80 participants, comprising 12 Japanese participants and 68 non-Japanese participants, with a total of 683 selumetinib and 682 N-desmethyl selumetinib plasma concentration values. Of these, 20 (2.9%) selumetinib and 80 (11.7%) N-desmethyl selumetinib concentrations were BLQ. The demographics and other baseline characteristics for the pooled dataset are summarized in Table 2. In general, these measures were more broadly distributed in SPRINT than in D1346C00013, but both clinical trials showed similar central tendencies.
| Item | D1346C00013 | SPRINT | Total |
|---|---|---|---|
| N = 12 | N = 68 | N = 80 | |
| Age (years) | 13.25 (7.5–18.2) | 10.20 (3.0–18.5) | 10.55 (3.0–18.5) |
| Weight (kg) | 34.00 (20.7–58.9) | 29.55 (15.7–88.7) | 30.09 (15.7–88.7) |
| BSA (m2) | 1.185 (0.82–1.57) | 1.040 (0.67–2.01) | 1.059 (0.67–2.01) |
| CrCL (mL/min/1.73 m2) | 188.55 (126.6–275.7) | 194.43 (128.4–363.4) | 192.01 (126.6–363.4) |
| Bilirubin (mg/dL) | 0.605 (0.31–1.33) | 0.410 (0.21–1.23) | 0.492 (0.21–1.33) |
| AST (µkat/L) | 0.300 (0.19–0.39) | 0.342 (0.12–1.90) | 0.333 (0.12–1.90) |
| ALT (µkat/L) | 0.156 (0.10–0.30) | 0.275 (0.10–0.77) | 0.233 (0.10–0.77) |
| Albumin (g/L) | 44.0 (42–47) | 42.0 (35–49) | 42.0 (35–49) |
| Sex | |||
| Female | 9 (75.0%) | 30 (44.1%) | 39 (48.8%) |
| Male | 3 (25.0%) | 38 (55.9%) | 41 (51.2%) |
| Race | |||
| Japanese | 12 (100.0%) | 0 (0.0%) | 12 (15.0%) |
| White | 0 (0.0%) | 56 (82.4%) | 56 (70.0%) |
| Black | 0 (0.0%) | 5 (7.4%) | 5 (6.2%) |
| Asian-Other | 0 (0.0%) | 3 (4.4%) | 3 (3.8%) |
| Missing | 0 (0.0%) | 4 (5.9%) | 4 (5.0%) |
- Data are presented as the median (range) for continuous items and as n (%) for categorical items. Asian-Other represents Asian Other than Japanese, Chinese, and Indian. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSA, body surface area; CrCL, creatinine clearance.
None of the patients received strong or moderate CYP3A4 inhibitors or inducers in D1346C00013 during the PK assessment period, based on the University of Washington Database dated April 2021. One patient in the 30 mg/m2 cohort of SPRINT phase I took clarithromycin up to cycle 1 day 1, but its impact on PK was unclear as no marked increase of selumetinib or N-desmethyl selumetinib concentration was observed. Therefore, the effects of concomitant medications on the PK of selumetinib or N-desmethyl selumetinib were not further evaluated.
The proportion of selumetinib plasma concentrations BLQ was small (<10% of the total sample); therefore, the BLQ data were excluded from the analysis, and no imputation, such as likelihood methods, was implemented. No continuous covariates were missing. Four patients in SPRINT had missing information on race (5.9% of patients enrolled in SPRINT and 5.0% of the total number of patients), and these patients were included in this analysis by classifying their race as “non-Japanese.” No imputation was conducted because only the pooled race group (“Japanese” and “non-Japanese”) was used for PopPK modeling.
3.2. PopPK Modeling
The base model development started from the structural model (Figure 1) [18] with IIV in CL and V2. F1 was fixed as the previously reported value [18] because F1 could not be estimated in this analysis owing to the lack of intravenous dosing data. Fm was also fixed using the previous model’s value [18] to ensure that CLm could be calculated with a reasonable point estimate and precision. The base model comprised the same structure as the previous model, with IIV in CL, V2, V3, Ka, ALAG, and CLm. The residual variability was well described using the proportional error model for both selumetinib and N-desmethyl selumetinib. Therefore, other models, such as the additive model or the combined model, were not explored.
As prespecified, the effect of BSA on CL was evaluated before commencing SCM. This yielded a significant ΔOFV (−35.943, P < 0.0001); therefore, BSA on CL was included as a built-in parameter. Body weight on CL was also checked and yielded a significant ΔOFV (−38.453, P < 0.0001), but the exponent of body weight on CL was 0.509, suggesting a nonlinear relationship with CL. In contrast, the exponent of BSA on CL was 0.836, suggesting a relatively linear correlation with BSA rather than weight. Thus, BSA on CL, not weight on CL, was selected as a built-in parameter. The final covariate model from the SCM included BSA on CL, V2, and CLm. No further covariates were included following SCM.
After SCM, the model was refined. First, because the IIV in V2 in the final covariate model became too small (coefficient of variation: ∼0.3%), the IIV in V2 was removed. Then, the post hoc random effects of CL and CLm were correlated, so covariance between CL and CLm was tested and incorporated.
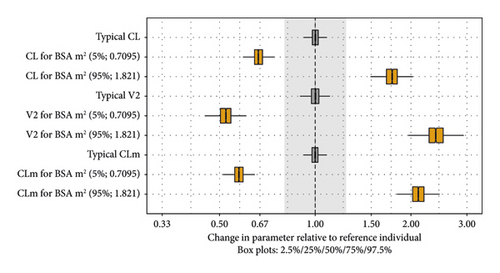
The goodness-of-fit plots for the base and final models are shown in Figures S2 and S3, respectively. We observed some trends for underprediction at lower concentrations at later time points, especially for N-desmethyl selumetinib. This trend was also reported in the previous analysis [18]. Consequently, the misspecifications did not bias the predicted exposure metrics in any clinically meaningful way because these misspecifications occurred at low concentrations and mostly at >20 hours postdose. The pcVPC plot for the final model with all data is shown in Figure 2. The pcVPC plots stratified by clinical trial are presented in Figure S4. The individual fit plots with observed, population-predicted, and individual-predicted values indicated that the model reasonably captured the PK profiles of Japanese and non-Japanese pediatric patients with NF1 and inoperable PN (data not shown). The impact of the covariates is visualized in Figure 3. The parameter estimates of the final model are listed in Table 3.

| Parameter | Final model | Bootstrap | ||
|---|---|---|---|---|
| Estimate | RSE (%) | Shrinkage (%) | Median (2.5th percentile and 97.5th percentile) | |
| Fixed effects | ||||
| CL (L/h) | 7.06 | 3.2 | — | 7.08 (6.57, 7.66) |
| V2 (L) | 15.1 | 4.1 | — | 15.1 (13.5, 16.8) |
| Q (L/h) | 4.28 | 4.8 | — | 4.27 (3.63, 5.25) |
| V3 (L) | 32.7 | 8.6 | — | 32.5 (25.4, 41.8) |
| Ka (h−1) | 4.91 | 8.5 | — | 4.81 (3.34, 8.07) |
| D1 (h) | 0.591 | 10.1 | — | 0.587 (0.443, 0.796) |
| F1 (fraction) | 0.662 | Fixed | — | 0.662 |
| ALAG (h) | 0.316 | 6.4 | — | 0.314 (0.249, 0.374) |
| Fm (fraction) | 0.0748 | Fixed | — | 0.0748 |
| CLm (L/h) | 6.42 | 3.1 | — | 6.41 (5.99, 6.95) |
| BSA on CL | 1.03 | 10.7 | — | 1.03 (0.80, 1.24) |
| BSA on V2 | 1.61 | 9.1 | — | 1.61 (1.32, 2.06) |
| BSA on CLm | 1.37 | 7.9 | — | 1.37 (1.19, 1.58) |
| Interindividual variability | ||||
| IIV in CL (CV%) | 22.6 | 11.8 | 14.0 | 22.0 (16.7, 27.9) |
| Correlation CL ∼ CLm (%) | 74.1 | 13.7 | — | 74.4 (51.8, 90.8) |
| IIV in CLm (CV%) | 23.8 | 11.7 | 14.3 | 23.1 (17.2, 28.5) |
| IIV in V3 (CV%) | 45.8 | 17.5 | 35.1 | 44.9 (26.9, 58.7) |
| IIV in Ka (CV%) | 170.4 | 12.4 | 25.3 | 166.9 (129.8, 202.4) |
| IIV in ALAG (CV%) | 58.3 | 11.9 | 14.5 | 58.6 (39.2, 79.5) |
| Residual variability | ||||
| Selumetinib (CV%) | 48.3 | 3.2 | 9.3 | 48.0 (43.0, 53.4) |
| N-desmethyl selumetinib (CV%) | 39.5 | 3.5 | 9.9 | 39.4 (34.8, 43.6) |
- ALAG, absorption lag time; BSA, body surface area; CL, selumetinib clearance of central compartment; CLm, N-desmethyl selumetinib clearance; CV, coefficient of variation; D1, time of zero-order absorption; F1, absolute bioavailability; Fm, fraction metabolized; IIV, interindividual variability; Ka, first-order absorption rate constant; PopPK, population pharmacokinetic; Q, selumetinib intercompartmental clearance; RSE, relative standard error; V2, selumetinib volume of distribution of central compartment; V3, selumetinib volume of distribution of peripheral compartment.
3.3. Predicted Exposure
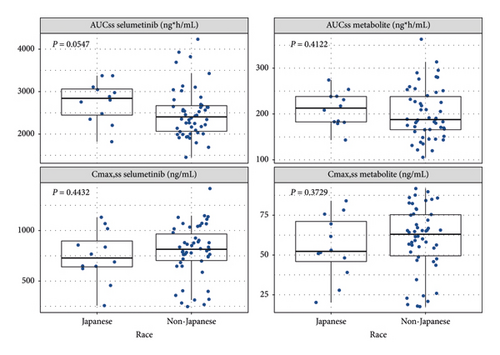
The predicted selumetinib and N-desmethyl selumetinib exposure metrics at steady state (area under the concentration-time curve over a dosage interval at steady state (AUCss) and peak plasma concentration at steady state (Cmax,ss)) based on the assigned dose level are shown in Figure 4. The geometric mean of the model-predicted AUCss of selumetinib was 12% higher in D1346C00013 than in SPRINT phase II stratum 1(P = 0.0547, exact Wilcoxon rank-sum test).
4. Discussion
This PopPK analysis was based on two clinical trials in pediatric patients with NF1 and inoperable PN, one conducted in Japan (D1346C00013) and one conducted in the US (SPRINT). BSA-based selumetinib doses were used in the clinical trials (20, 25, and 30 mg/m2 in SPRINT phase I, and 25 mg/m2 in D1346C00013 and SPRINT phase II stratum 1). As noted above, the nomograms used to attain a dose of 25 mg/m2 differed slightly between D1346C00013 and SPRINT. However, the difference did not affect the PopPK modeling, including the evaluation of the effect of race as a covariate, because the actual dosage in grams (converted to moles) was used.
The findings of the present PopPK analysis were generally similar to those of the previous PopPK analysis [18], which utilized data from a broader population of healthy adult subjects (including Asian people), patients with advanced solid malignancies, and pediatric patients with NF1 and inoperable PN (SPRINT), but not Japanese pediatric patients with NF1 and inoperable PN. In the current analysis, the PK profile of selumetinib was well described by the two-compartment model with first-order elimination and sequential zero-order and first-order delayed absorption, and the PK profile of the metabolite N-desmethyl selumetinib was well described by the one-compartment model simultaneously with parent selumetinib. In addition, the diagnostic plots provided evidence that the final model is fit for purpose (i.e., to predict exposure) and adequately describes the observed plasma concentrations of both selumetinib and N-desmethyl selumetinib.
According to the covariate analysis, BSA was identified as an important covariate impacting CL, V2, and CLm (>20% impact). Other covariate-parameter relationships were not identified as statistically significant. CL is the driving parameter for the area under the concentration-time curve of selumetinib, and the linear relationship between CL and BSA was estimated to have an exponent of 1.03. Therefore, the appropriateness of the BSA-based dosing nomogram was reconfirmed. Previous analyses, which included adult data, have identified other statistically significant covariate-parameter relationships, for example, age on V2 and alanine aminotransferase on CL [15], concomitant medications on CL and hepatic function on Fm/V4 [16], and age and race on CL [18]. The current dataset only included pediatric patients with a relatively narrow distribution range of covariate candidates, which could have resulted in the inability to detect small effects as statistically significant due to low power.
In the previous report [18], race was a statistically significant covariate for selumetinib CL, which was 19% lower in Asians than in non-Asians, but it was not considered clinically relevant because the change was <20%. In contrast, with the available data, the current model did not identify race as a statistically significant covariate for any parameter. During SCM, after incorporating the significant effects of BSA on CL, V2, and CLm, the adjusted effect of race on selumetinib CL comprised a reduction of 10%–14% in Japanese, with the largest ΔOFV of −3.301 (P = 0.0692), which was not statistically significant or considered clinically relevant (i.e., change of <20%). Based on the final model, the additional effect of race (Japanese) on CL was 12% by reestimating final model parameters, with a ΔOFV of −2.937 (P = 0.0866), which was not statistically significant and below the threshold for clinical relevance. Overall, race had a small adjusted effect size in the current analysis with a change of <20%, which is consistent with the previous analysis [18], supporting its no clinical relevance in pediatric patients with NF1 and inoperable PN.
In contrast to the previous analysis [18], age on CL was not identified as a statistically significant covariate. In the previous analysis, age was a statistically significant (but not clinically relevant) covariate on CL with a negative exponent (i.e., lower CL in older subjects). Generally, drug clearance can be considered to be well correlated with BSA (or allometric scaling based on body weight) [22]. Considering its pathogenesis, NF1 with PN might not affect drug clearance capacity in pediatric patients, but NF1 could affect physical growth, such as height, in pediatric patients. The height and BSA values were relatively smaller in our pooled dataset (Figure S1). This suggests that BSA in pediatric patients with NF1 and PN would deviate below the growth curve of a population consisting of healthy adults and adult patients with advanced solid malignancies. Consequently, a covariate model comprising BSA alone is expected to underpredict CL in pediatric patients with NF1 and PN. This potential underprediction could be compensated in the previous final model, which incorporated age and race. If tested during covariate modeling in the previous model, a categorical covariate of NF1 with PN versus others or pediatric versus adult patients might have been identified as a statistically significant covariate on CL, possibly correcting the underprediction caused by using BSA only. Covariate testing was conducted for a categorical covariate of healthy versus disease in the previous model, and nonhealthy adults were included in the analysis population; this might be compensated by retaining age as a statistically significant covariate on CL in the final previous model.
To further investigate the potential ethnic differences in PK, the model-predicted exposures were compared between D1346C00013 and SPRINT phase II stratum 1, both of which used the 25 mg/m2 dose. The geometric mean of the model-predicted selumetinib AUCss was 12% higher in D1346C00013 than in SPRINT phase II stratum 1 (P = 0.0547, exact Wilcoxon rank-sum test). However, due to the variability, this was not considered clinically relevant.
The use of different dosing nomograms in D1346C00013 and SPRINT meant that patients in SPRINT with a BSA of 0.60–0.69 m2 were administered relatively higher BSA-normalized doses than other BSA bins, resulting in doses of 29.0–33.3 mg/m2. There were two patients within a BSA range of 0.60–0.69 m2 in SPRINT phase II stratum 1, and their predicted AUCss were 3690 and 3427 ng·h/mL, which were the fourth and fifth highest values in that clinical trial, respectively. These values were not considered to affect the analysis conclusion, which was primarily based on the overall variability in the distributions of AUCss, and which would not dramatically change even if these values had been handled differently (e.g., by excluding these values from the analysis).
The findings of this PopPK analysis should be interpreted in the context of the following limitations. One major limitation of this analysis is that the E–R relationship could not be assessed. An E–R analysis would not have been robust because of the small sample size (N = 12) and immature efficacy and safety data at the time of the analysis (6-month follow-up). In pediatric patients with NF1 and PN treated with selumetinib, the median (range) time to initial response and best response were 8 (4–40) and 18 (4–94) 28-day cycles, respectively [14]. Therefore, it often takes longer than 6 months to observe a response to selumetinib treatment in pediatric patients with NF1 and PN. E–R relationship pooling Japanese pediatric patients with non-Japanese pediatric patients may be evaluated when long-term follow-up data from D1346C00013 and/or new data from other clinical trials in pediatric patients emerge. However, no apparent E–R relationship could be anticipated in the Japanese patient population considering (1) no clear E–R relationship was established in the non-Japanese pediatric population [18]; (2) the pathogenesis and clinical practice of NF1 and PN are the same regardless of country or race; and (3) the PK profile was similar between Japanese and non-Japanese pediatric patients.
Another limitation of the present analysis is that the covariate effect of race (“Japanese” vs. “non-Japanese”) was exactly the same as the “clinical trial” effect (i.e., “D1346C00013” vs. “SPRINT”), which means that the effect of “race” cannot be distinguished from the effect of “clinical trial”. D1346C00013 was designed to mirror the protocol of the SPRINT phase II stratum 1 in terms of the inclusion and exclusion criteria, intervention, dosage and administration (with a slightly modified nomogram), and schedule of activities (e.g., PK sampling points and the bioanalytical method). This allows the covariate effect of race to be interpreted as a “pure” (i.e., not confounded) race effect to the extent possible. In addition, the post hoc distribution of the IIV parameter of CL in the SPRINT dataset showed that no major trend remained in the race categories, including “Asian-Other” (Figure S5). This further supports that the covariate effect of race could be assessed appropriately without pooling Japanese subjects from D1346C00013 and Asian subjects from SPRINT into one category.
5. Conclusions
Only BSA had an impact of >20% on CL, V2, and CLm, and it was thus considered to be clinically relevant. None of the other covariates, such as race, had any significant impact on selumetinib and/or N-desmethyl metabolite PK. The predicted exposures in D1346C00013 and SPRINT phase II stratum 1 showed a considerably overlapping distribution. The geometric mean AUCss of selumetinib was 12% higher in D1346C00013 than in SPRINT phase II stratum 1, but given the variability, this was not considered clinically relevant. No ethnic difference was suggested from the results of the covariate modeling and predicted exposure, supporting the appropriateness of using the same BSA-based dosing nomogram for both Japanese and non-Japanese pediatric patients with NF1 and inoperable PN. Subsequent to this analysis, selumetinib was approved at the BSA-based dose of 25 mg/m2 in Japan, which is consistent with the recommended dosage and administration in other regions and countries.
Conflicts of Interest
All authors declared support for the present manuscript from AstraZeneca, Alexion, Merck Sharp and Dohme, and the National Cancer Institute. TS and MH are employees of AstraZeneca K.K. ML are an employee of AstraZeneca. IGG is a former employee of AstraZeneca. MH declared stock or stock options in AstraZeneca.
Authors’ Contributions
TS and MH were responsible for conception/design; TS was responsible for analysis and drafting of the manuscript. All the authors were responsible for interpretation and review/approval of the manuscript.
Acknowledgments
We would like to acknowledge the SPRINT study team for providing data from their clinical trial used in this manuscript. The SPRINT study (NCT01362803) led by Drs. Brigitte Widemann and Andrea Gross, was sponsored by the NCI Cancer Therapy Evaluation Program and conducted under a Cooperative Research Development Agreement between NCI and AstraZeneca with additional support from the Neurofibromatosis Therapeutic Acceleration Program and Children’s Tumor Foundation. We would like to thank Drs. Souichi Suenobu, Yoshihiro Nishida, Keita Terashima, and Masaharu Akiyama for conducting the D1346C00013 study (NCT04495127), which was sponsored by AstraZeneca. We would like to thank Dr. Sandrine Hardouin of Alexion Pharmaceuticals, AstraZeneca rare disease unit, for supporting this publication. We would like to thank OPEN Health for editorial support. We would like to thank Dr. Emily Woodhouse of Edanz, Japan, and Dr. Nicholas D. Smith of EMC K.K., Japan, for providing editorial support, which was funded by AstraZeneca. Funding for this work was supported by AstraZeneca as part of an alliance between AstraZeneca and Merck Sharp and Dohme LLC, a subsidiary of Merck and Co., Inc., Rahway, NJ, USA (MSD).
Open Research
Data Availability
The individual data used to support the findings of this study have not been made available because some of the data are owned by a third party or restricted by US-applicable laws and regulations.




