New and Advanced Methods in Detection of Fruit Juice Adulteration: Focusing on Nano-Biosensor
Abstract
One of the most popular products made from fruit is juice, which is consumed as a snack by people of all ages due to its high content of polyphenols and vitamins. Therefore, the prevalence of adulteration in juices is very high, and ensuring the authenticity and quality of juices is a major concern for those responsible for monitoring food safety for the public. The most common frauds in fruit juices include adding water, sugar, organic acids, and other juices clouding agents and exceeding the permitted amount of sulfites. Having knowledge of the types of fraud in fruit juices and their detection methods is essential for developing more efficient detection methods. Chromatographic techniques, such as high-performance liquid chromatography (HPLC) and gas chromatography (GC), are commonly used to determine the purity of fruit juices. Among the new methods for detecting counterfeit fruit juice, the development of bio-nanosensors is particularly notable. These sensors reduce sample volume, analytical time, costs, and solvent consumption and will be discussed further in this study. Therefore, this review will explain the basic analytical approaches and chemometric tools used to assess the authenticity of fruit juice and identify any adulteration in the juice.
1. Introduction
World marketplaces for food in recent decades have made a huge variety of food choices available to customers, and there is a rising demand for high-quality foods [1]. Soft drinks such as juices are one of the most widely used products made from natural products and are consumed by all members of society [2].
According to surveys, the consumption of fruit juice is increasing in Europe and other countries around the world [2]. The popularity of this product is due to its numerous health benefits, which include containing minerals and important vitamins, aiding in cancer prevention, assisting with digestion, providing antiallergic effects, and strengthening bones [3–5]. Food and beverage fraud is an important growing concern in today’s universal market. Fruit juices (orange juice and apples) were among the first 7 foods reported as the most frequent targets of fraud between 1980 and 2010. In addition, nonalcoholic fruit beverage adulteration is one of the issues that have been studied in depth in the field of beverage technology [6–8].
Types of juice fraud that are currently subject to legal sanctions include the addition of sugar and pulp wash solution without indicating this on product packaging (mislabeling), the addition of water, blending of juices, addition of colorants, organic acids, amino acids, and substitution with another juice that has a lower commercial value [9].
Effective control methods are essential in order to protect customers from unclean and falsely fruit juices and hold immense economic value [10]. On the other hand, attention to implementing food-based health standards and conducting research to develop fraud diagnostic tools is also crucial.
Several fruit juice frauds have been identified using a variety of techniques. Physical and observational methods, chromatographic and spectroscopic methods, molecular methods based on Deoxyribonucleic acid (DNA) such as polymerase chain reaction (PCR), and methods based on sensors such as electronic noses (e-noses) and electronic tongues (e-tongues) have all been examined and studied up to this point. Each of these techniques has its own benefits and drawbacks. [11]. In recent years, the use of biosensors in detecting counterfeit products has garnered attention, and they offer advantages such as low cost, simplicity, and sensitivity. Also, advancements in biosensor technology have significantly improved the detection of fraud, ensuring the authenticity and safety of various products [12].
Therefore, the purpose of this study is to investigate the main analytical methods and chemometric tools, with a focus on the use of biosensors for evaluating the authenticity and identifying fraud in fruit juice.
2. Method
The databases searched for articles were “Scopus”, “PubMed”, “Web of Science”, and “Science Direct”. The searched terms were “fruit juice”, “Adulteration,” “Instrumental analysis,” and “Rapid detection.” This study focused on published research articles and papers.
3. Results and Discussion
The present study is focused on published article papers from 2010 to 2023. Table 1 and Figure 1 show the usage of the numerous methods to identify fraud in juices. Complex fraud can only be found using a select few of them.
| Type of juices | Target | Technique | Accuracy | LOD (mg/L) | LOQ (mg/L) | Ref |
|---|---|---|---|---|---|---|
| Shiikuwasha juice | Identification of adulteration from juice to juice | GC | Identification limit: 10% | [13] | ||
| Apple juice | Identification of the addition of pear juice to apple juice | GC | Identification limit: 0.5–3% | [14] | ||
| Pomegranate juice | Identification of the addition of peach and grape juice to pomegranate juice | GC | Identification limit: 10%–50% | [15] | ||
| Orange juices | Detection of juice authentication | GC | 100% | [16] | ||
| Orange juice, plum juice, mango juice, cherry juice, grape juice, and apple juice | Detection of quantification of short-chain volatile organic acids | GC | Identification: 0.025–1 ng | 0.25 | 0.82 | [17] |
| Lemon juice | Adulteration with orange juice | GC | — | — | [18] | |
| Citrus fruit | Distinctiveness of juices | HPLC | 100% | 0.02 | 0.04 | [19] |
| Purple grape juice | Identification of the addition of apple juice in purple grape juice | HPLC | — | 0.62 | 0.64 | [8] |
| Apple and pear juice | Detection of added juice-to-juice adulteration | HPLC | Identification limit: 0.6–7% | 6.44 | 7.09 | [8] |
| Purple grape juice | Identification of the addition of apple juice to purple grape juice | HPLC | Identification limit: 2–5% | 0.2 | 0.66 | [20] |
| Pomegranate juice | Identification of grape, apple, and sour cherry juice adulteration in juice-to-juice | HPLC | — | 0.03 | 0.1 | [21] |
| Indian citrus fruit juices | Identification of juice-to- Indian citrus fruit juices | HPLC | Detected 1-2% | [10] | ||
| Pomegranate juice | Detection of adding peach and grape juice to pomegranate juice | HPLC | — | 0.5 | 1.05 | [15] |
| Lemon juice | Detection of the addition of organic acids & sugars | HPLC | — | [22] | ||
| Orange-based fruit juice | Detecting the presence of sugar and water | ICP | — | [23] | ||
| Pomegranate juice | Detection of differences between homemade and commercial juices | ICP | — | [24] | ||
| Orange juice | Detecting the presence of sugar and water | ICP | — | [25] | ||
| Grape juice | Detection of differences between organic and conventional juices | ICP | — | [26] | ||
| Orange and apple juices | Identifying the presence of added sugars | ICP | Correct classification rate: 80–94% | [27] | ||
| Orange, mango, peach, pear, and pineapple | Detection of the fruit species in fruit juices | DNA-based | Identification limit: 25% | [28] | ||
| Pomegranate | Identification of adulteration using bulking agents | DNA-based | Identification limit: 1% | |||
| Grapefruit and orange juice | Detection of grapefruit juice in orange juice | DNA-based | Identification limit: 2.5–10% | [29] | ||
| Orange | Identification of adulteration using Mandarin juice | DNA-based | Identification limit: 1% | [30] | ||
| Orange mandarin | Detection of orange adulteration with mandarin juice | DNA-based | Identification limit: 5% | [31] | ||
| Pineapple, apple, and orange juices | Identification of the addition of grape juice to pineapple, apple, and orange juices | NIRS | Identification limit: 5% (accuracy of 98%) | [32] | ||
| Lime juice | Differentiating between synthetic and natural lime juice | NIRS | Classification correction: 97% | [33] | ||
| Lime juice | Distinguishing contaminated lime juices using citric acid | NIRS | Classification correction: 88% | [34] | ||
| Grape juice | Detection of juice | FT-IR | Identification limit: 50–100% | [35] | ||
| Orange juice | Identification of the addition of water | FT-IR | Identification limit: 0.5–20.0% | [36] | ||
| Commercial juices | Detection of saccharin | FT-IR | Identification limit: 0.1–2% | [37] | ||
| Pineapple, orange, and apple juices | Identification of the addition of grapefruit to pineapple, apple, and orange juices | FT-IR | Identification limit: 5% | [38] | ||
| Lime juice | Detection of juice | FT-IR | Identification limit: 0.87–0.96% | [39] | ||
| Orange juice | Detection of juice | NMRS | Identification limit: 10% | [40] | ||
| Apple, orange, pineapple, and pomegranate juices | Detection of juice-to-juice adulteration | NMRS | Identification limit: 6.25% | [41] | ||
| Orange juices | Detection of different orange cultivars and identifying fresh orange juice | NMRS | — | [42] | ||
| Orange juice | Identification of freshly squeezed orange juices adulterated with concentrated orange juices | E-nose | Identification limit: 0–30% | [43] | ||
| Lime juice | Detection of adulteration | E-nose | Identification limit: 5% | |||
| White grape juice | Detection of between grape varieties | E-nose | — | [44] | ||
| Orange juice | Detection of adulteration | E-nose | Identification limit: 100% | — | — | [45] |
| Pineapple–grape and orange–grape juices | Detection of juices of orange and pineapple combined with grape | E-nose | Identification limit: 0.1–2.5 μg/mL | — | — | [46] |
| Grape juice | Detection of between grape varieties | E-nose | — | — | — | [47] |
| Orange juice | Detect formaldehyde adulteration | Biosensor | Detection range (0.01–0.3 mg/L) | — | — | [48] |
| Orange juice | Detect formaldehyde adulteration | Biosensor | 0.01–0.3 mg/L | 0.05 | 0.15 | [49] |
| Grape juice | Sugar solution and water | Biosensor | [50] |
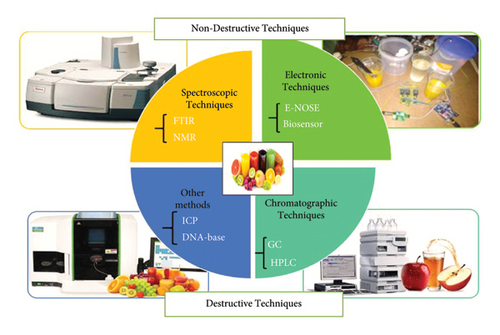
3.1. Destructive Techniques
3.1.1. Chromatographic Techniques
(1) Gas chromatography (GC). Chromatography is an analytical methodology that is deemed reliable and appropriate for identifying adulterants in food. By employing GC techniques, an intended sample made up of several different compounds is identified and separated. When it comes to beverage quality control, mass spectrometry (MS) and flame ionization detectors (FID) are the most often used detection techniques [15]. The validation of product authentication can be accomplished by determining certain marker substances or conducting fingerprinting analysis. The sensitivity and high separation efficiency of chromatography in authentication have been substantiated by numerous studies; however, the technique does encounter some limitations, such as the complex procedure for sample pretreatment and operation, as well as the potential loss of unstable compounds [51]. Numerous researches have reported on the use of GC for the identification of fruit juice adulteration in Table 1. Willems and Low created a technique that used arbutin and oligosaccharide as markers in capillary GC with FID to determine the addition of pear juice to apple juice. As a result, the suggested approach could detect low levels of adulteration between 0.5% and 3% [14].
In this regard, we can mention headspace solid-phase microextraction (HS-SPME). HS-SPME is a technique used to prepare samples by extracting volatile organic compounds (VOCs) from a matrix. HS-SPME involves the use of a solid-phase microextraction (SPME) fiber to extract VOCs from the headspace above a sample. The sample is placed in a vial, and the SPME fiber is exposed to the headspace above the sample. The analytes in the headspace are adsorbed onto the fiber, which is then desorbed into a solvent or directly into a chromatographic system for analysis. HS-SPME is a solventless extraction method, making it a clean and efficient technique. It is particularly useful for the analysis of volatile organic compounds (VOCs) in complex matrices, such as biological samples, environmental samples, and food products [52, 53]. In this context, we can refer to the study of Canuti et al. They employed HS-SPME and GC-MS to evaluate Cabernet Sauvignon’s free volatile components. To optimize the procedure, SPME fiber type, temperature, solvent type, and extraction time were studied and 27 taste components were studied to identify the profile of free volatile components in ripe Cabernet Sauvignon grapes [54]. Yamamoto et al. utilized SPME-GC and GC/MS for the detection of volatile components. γ-Terpinene and linalool were employed as chemical markers to identify the addition of Shiikuwasha juice to calamondin juice, with a minimum detection level of 1% [13]. Using HS-SPME and GC-MS, Nuncio-Jáuregui et al.’s study detects adulteration of pomegranate juice with peach and grape juice. A multiplace digestion block and 65% HNO3 were used to digest pure juices and juice blends for two hours. Using deionized water of ultrahigh purity, dilutions of 1 : 10 and 1 : 50 (v/v) were made. For peach juice and grape juice, the study’s lowest detectable levels were 10% and 50%, respectively [15].
Another method in this field is chemometrics. Chemometric methods can be used to detect fraud in fruit juices. In fact, these techniques are widely used in conjunction with fingerprinting analysis to improve quality control and standardization. These methods are often utilized to analyze complex chemical data, identify patterns and relationships within the data, and help identify similarities and differences among samples, enabling classification into categories such as authentic and adulterated. The integration of fingerprinting and chemometric techniques is crucial for ensuring quality and consistency, and they are applied in various fields including food analysis, pharmaceuticals, and environmental monitoring to analyze and interpret complex chemical data. In summary, chemometric techniques play a vital role in enhancing the accuracy and reliability of fingerprinting analysis, particularly in quality control and standardization [55, 56].
Cuevas et al. recently used chemometric techniques in conjunction with fingerprinting analysis to verify the authenticity of premium organic orange juices. This research was conducted by High-Performance Liquid Chromatography-high resolution mass spectrometry (HPLC-HR-MS) and HS-SPME-GC-MS, and the results of principal component analysis, hierarchical cluster analysis, and partial least squares discriminant analysis showed that the results were acceptable. The indicators of this study were flavonoids, fatty acids, aldehydes, and esters, and a combination of mass spectrometry, chemometrics, and data fusion techniques was used. Thus, with 100% accuracy, it was determined that the model was suitable for authentication [16].
(2) High-Performance Liquid Chromatography (HPLC). HPLC is a new chromatography method utilized for quality control in clinical, biochemical, and industrial settings. It is an advanced form of column chromatography that uses high pressures and fine particles for better separation of mixture components. HPLC is a powerful tool in analytical chemistry, aiding the identification, separation, and compound quantification in any sample, including food, drugs, cosmetics, and industrial chemicals [57]. The utilization of HPLC in evaluating the inclusion of adulterants in fruit juice can be achieved by examining the phenolic profile and anthocyanin profile of the juices, as stated in the literature. HPLC is a widely accepted and commonly used analytical technique in the food industry and can be integrated into industrial settings due to its ability to handle large sample volumes and provide high-throughput analysis [58]. This makes it suitable for quality control and authenticity verification in commercial juice production [59]. The initial investment in HPLC instrumentation can be substantial, which may be a barrier for smaller-scale producers or those with limited budgets. While the cost of consumables and maintenance can be significant, HPLC is generally considered a cost-effective method compared to other advanced analytical techniques like mass spectrometry. The cost of implementing HPLC-based adulteration detection must be weighed against the potential economic benefits of ensuring product authenticity and avoiding legal and reputational consequences of adulteration [60].
A powerful analytical technique for separating, identifying, and quantifying various compounds is HPLC coupled with diode-array detection (DAD). DAD is a popular UV detection method used in HPLC. It simultaneously collects data at multiple wavelengths, allowing for the detection of thousands of target analytes. HPLC-DAD provides fast, accurate, and cost-effective analysis for various compounds in beverages, food, and dietary supplements. HPLC-DAD also offers several advantages, including flexibility, speed, accuracy, and cost-effectiveness. It can be used in tandem with various columns and detectors, making it a versatile technique for a wide range of applications [61]. In this case, we can refer to the study of Abad-García et al. They employed reversed-phase HPLC with photodiode array detection to analyze the polyphenols profile of citrus juices to assess their authenticity. The polyphenolic profiles were subsequently utilized to develop classification models using linear discriminant analysis (LDA) and partial least squares-discriminant analysis (PLS-DA). The LDA model reached a perfect accuracy of 100%. Similarly, the PLS-DA model also reported a perfect accuracy of 100% [19]. According to the investigation by Türkyılmaz et al., the phenolic compound phloridzin is not naturally found in grapes but is present in higher amounts in apples compared to other fruits. Adulteration of pomegranate juice can be detected by analyzing its anthocyanin profile. Turkish pomegranate juices were found to contain six distinct anthocyanins: delphinidin 3,5-diglucoside, cyanidin 3,5-diglucoside, delphinidin 3-glucoside, pelargonidin 3,5-diglucoside, cyanidin 3-glucoside, and pelargonidin 3-glucoside. This specific anthocyanin profile of pomegranate juices, determined using HPLC, showed a high level of consistency and seemed to be unrelated to fruit variety or geographic origin [21]. Borges et al. also conducted a similar study on pomegranate anthocyanins, which led to the development of a straightforward methodology for assessing the authenticity of pomegranate juice through HPLC-DAD-MS detection of red grape components [62].
Several studies have identified fingerprint compounds using HPLC for various juices. These studies demonstrate the application of HPLC fingerprint analysis in identifying and quantifying various compounds in different substances, ensuring quality control and monitoring stability. In a study, Jamie L. Willems identified fingerprint compounds from commercial apple and pear juice samples for structural identification, using a process involving diluting, preconditioning, and fractionation, resulting in retention times of 57.7 for apple and 70.6 for pear. Notably, 4-O-p-coumarylquinic acid has been identified as an unique compound found in apple juice, making it possible to detect the addition of apple juice to pear juice [20]. Spinelli et al., used HPLC to detect the amount of sorbitol 35 and phlorizin 36 to determine the adulteration of purple grape juice with apple juice and concluded that this method is effective in detecting the addition of apple juice to water grapes. In 4 out of 39 cases of industrial grape juice that were analyzed, adulteration was done by mixing apple juice with grape juice [8]. Shui and Leong et al. utilized HPLC to analyze organic acids and phenolic content in a variety of types of juices and beverages. They washed 10 organic acids and 21 phenolic constituents, allowing for chromatographic analysis. This method effectively measures organic acids and phenolic compounds, evaluating authenticity, spoilage, and micronutrient content [63].
HPLC Refractive Index Detectors (HPLC RI Detectors) are used in high-pressure liquid chromatography to detect substances that have limited or no UV absorption. These detectors are particularly useful for detecting chemical components such as alcohols, sugars, fatty acids, polymers, and carbohydrates. They are considered universal detectors because they can detect all compounds that show a different refractive index from the mobile phase in principle [64, 65]. The Zare study examined the substitution of apple juice concentrate with low-cost sweeteners such as date concentrate, fructose syrup, and glucose syrup as adulterants. HPLC and HPLC Refractive Index Detectors (HPLC-RID) were established to monitor the carbohydrate profile. Results showed that glucose/fructose ratio and maltose content were the most effective indicators for the detection of adulteration [66]. Shakeri et al., used HPLC and spectrophotometry to detect fake juices. Their results showed the average of total polyphenol in all samples conformed to the standard. However, the content of flavonoids (hesperidin and eriocitrin) in the samples was lower than the standard level [67].
3.1.2. DNA-Based Techniques
Several methods for food authentication have found success using DNA-based approaches, primarily utilizing microarrays, high-resolution melting (HRM) analysis, PCR, real-time PCR, and next-generation sequencing [68].
High-resolution melting (HRM) analysis is a powerful technique in molecular biology used to detect genetic variations, including mutations, polymorphisms, and epigenetic differences in double-stranded DNA samples. It is a post-PCR analysis method that identifies small differences in PCR melting curves, which are generated by the controlled melting of double-stranded PCR amplicons. HRM analysis is enabled by improved dsDNA-binding dyes used in conjunction with real-time PCR instrumentation that has precise temperature ramp control and advanced data capture capabilities [69]. With a well-designed HRM assay, powerful genotyping can be performed by nongeneticists in any laboratory with access to an HRM-capable real-time PCR machine. Overall, high-resolution melting analysis is a powerful tool for detecting genetic variations and has significant applications in fields such as genotyping, mutation scanning, and epigenetics [70]. It is possible to detect fraud in fruit juice through DNA sequencing of the type of fruit used to make the juice. In this context, we can refer to the research conducted by Faria et al. They examined European market-level juices, including orange, mango, peach, pear, and pineapple, for adulteration, and utilized high-resolution melting analysis. The results demonstrated the effectiveness of this method in detecting fraud, confirming the genetic differentiation of fruits in fruit juices [28, 71].
Due to DNA’s stability in a variety of environmental conditions, farming practices, and production methods, they are thought to be more dependable than chemical analysis [61].
DNA stability is a crucial consideration in DNA data storage systems. While DNA has significant advantages over traditional digital storage media, such as high storage density and capacity, its stability under various conditions is a key factor in ensuring reliable data retrieval and integrity [72]. DNA remains stable under short-term storage at 4°C or room temperature and long-term storage at −20°C and −80°C. Concentration of DNA helps to reduce degradation, especially when stored at −20°C or below. These conditions also highlight the importance of proper storage and handle to ensure the stability and integrity of DNA samples for various applications [73, 74].
Despite these benefits, there are relatively few applications for fruit juice authenticity testing [75]. Since the stability of DNAs in food products and remaining intact in various environmental and cultivation conditions, as well as during the production process, DNA-based techniques have emerged as dependable methods for verifying the authenticity of food [76]. About the identification and detection of adulteration in juice, Table 1 presents the various applications of DNA-based methods. The investigation of juice adulteration has primarily focused on orange juices due to their significant market value, attracting considerable attention from researchers. Numerous studies have been conducted to uncover instances of juice-to-juice adulteration. For example, the use of a PCR restriction fragment length polymorphism (RFLP) assay and a PCR heteroduplex assay facilitated the identification of grapefruit and mandarin juice within orange juice. The PCR heteroduplex assay demonstrated a superior limit of detection of 2.5%, whereas the RFLP assay yielded a limit of detection of 10% [29].
In the study conducted by Aldeguer et al., samples were reconstituted with water up to 12% w/w to prepare juice from concentrates (65% w/w). The juice was produced in a laboratory by pressing concentrates or raw samples that came from JUVER SLU in Murcia, Spain, and used a single-nucleotide polymorphism to detect adulterated orange juice with mandarin juice, achieving a 5% detection limit for both fresh and reconstituted juices [31]. In another study conducted by Chandra Roy et al., fraud in mango juice was investigated using a simplex PCR-based method. The study was prompted by concerns about the addition of pumpkin pulp to mango juice in Bangladesh. The results revealed that 4 out of the 6 industrial mango juices tested were adulterated. As a result, it was concluded that this method was effective in detecting fruit juice fraud [77].
3.1.3. Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES)
Finding trace metals can be done via atomic emission spectroscopy with inductively coupled plasma (ICP-AES). Inductively coupled plasma that is employed in this technique is a sort of emission spectroscopy, to excite ions and atoms that can emit electromagnetic radiation at particular wavelengths of a specific element. This report’s brightness indicates how much of the element is present in the target sample. Actually, ICP-AES involves exciting atoms with a plasma. The high temperature of the plasma (6000−10,000 K) causes instantaneous desolvation, vaporization, atomization, and excitation of the sample atoms. Excitation mechanisms include collisional excitation, Penning ionization, and charge transfer reactions, which promote electrons to higher energy levels. As the excited electrons return to lower energy levels, they emit electromagnetic radiation at characteristic wavelengths specific to each element and then measuring the light they emit at different wavelengths to determine the presence of specific elements could help [78]. The development of inductively coupled plasma mass spectrometry (ICP-MS) and ICP-AES made it possible to analyze a variety of components. Researchers can determine the region of origin and the addition of pulp-cleaning solutions to juices using a large number of trace elements in juices and commercially accessible chemometric software tools. Different types of acidic fruits are thought to have unique characteristics. This means that acidic items can be tested to ensure that the labels are accurate using minor and trace constituents [79].
ICP-AES is used to help prevent food fraud and ensures that products meet regional quality standards. The use of ICP-AES in food analysis helps manufacturers comply with regulatory requirements, such as those set by the U.S. FDA, which ensures that food products meet specific safety and quality standards. The findings from ICP-AES studies play a critical role in maintaining food safety and quality by detecting toxic elements, ensuring accurate nutrient labeling, and facilitating regulatory compliance [80, 81].
Table 1 summarizes the application of elemental techniques to identify juice adulteration, highlighting high potassium content as a result of sweeteners or preservatives, low potassium content as water dilution, and elevated calcium content as a result of pulp addition [82]. Additionally, Cristea et al. employed a combination of elemental and isotope analysis using ICP-MS to distinguish between commercially produced and freshly squeezed orange and apple juices. Analyzing water materials with ICP-MS usually entails pretreatment or analysis directly. Nitric acid, hydrochloric acid, and pure water were used to dry, grind, and determine the macromineral content of fruit juices. The apparatus produced a colorless solution after being baked for 12 hours at 200 C. The technique of supervised classification of linear discriminant analysis (LDA) was utilized to assess the dissimilarities between 20 juice samples. The classification accuracy, as determined through validation, exceeded 90% for both apple and orange juices. The content of K and Na was deemed the most significant variables for distinguishing apple juice, with Na concentration having the greatest contribution to the results. Among all cases, the isotopic ratio of oxygen proved to be the most important variable [82]. In a study conducted by Schmutzer et al., the authenticity of 23 commercial orange juices was evaluated through the analysis of their elemental profiles using ICP-MS. After adding 2.5 mL of nitric acid to 2.5 mL of juice, the mixture was digested at 180 C for 12 hours in a Teflon container. Despite some of the juice samples being labeled as “100% fruit juice,” the outcomes indicated that all the juices had a K to Mg ratio of less than 50, indicating adulteration with exogenous sugar [25]. For the detection of fraud in lime juice, the tests of sodium and potassium content were carried out using spectrophotometry [67]. Generally, the Na+ and K+ as diagnostic biomarkers of natural juice in all samples were lower than the standard level. All the analyzed samples in the experiment were nonstandard [67]. Gaiad et al. conducted an extensive study in which they correlated the geographical origin of Argentinean lemon juices with their elemental profile. In this study, 25 trace elements were determined by ICP-MS, and five different multivariate analysis techniques were tested to classify the samples based on their geographical origin [83].
Natural juice is distinguished by a specific range of mineral levels. Consequently, the identification of juice adulteration can also be achieved through elemental analysis based on element markers [84].
3.2. Nondestructive Techniques
3.2.1. Infrared (IR) and Fourier-Transform Infrared (FTIR) Spectroscopic Technique
As an overview, it can be explained that spectroscopic techniques are nondestructive and cost-effective and do not require sample pretreatment. However, they produce repetitive data, including extraneous details such as noise and baseline drift. To eliminate this, data preprocessing methods such as smoothing, differentiation, and baseline correction are used. Chemometric methods are often used to differentiate pure samples from adulterated ones due to minute discrepancies in spectral data [85, 86].
The frequency of the IR radiation matches the vibrational frequency of specific bonds, leading to absorption. It is a powerful tool for analyzing molecular structures and identifying functional groups by measuring the interaction of infrared radiation with matter. Its applications span various fields, including organic chemistry, materials science, and biological research [68, 75].
Smoothing is a process used to reduce the influence of noise in FTIR spectra. It involves reducing the degree of change in spectral intensity at individual data points. Differentiation is used to enhance peak detection and resolution, and the points where the derivative changes from positive to negative are identified as peaks. Baseline correction is crucial in FTIR spectroscopy as it removes distortions in the baseline that can affect the accuracy of results. The baseline represents the signal intensity in the absence of sample absorption and should ideally be a straight line at zero absorbance [87, 88].
Near-infrared spectroscopy (NIRS) is a noninvasive, spectroscopic method that uses the near-infrared region of the electromagnetic spectrum (from 780 nm to 2500 nm) to analyze molecular overtone and combination vibrations. It is commonly used in various fields [89].
NIRS has demonstrated significant potential in detecting food adulteration due to its speed, nondestructive nature, and high accuracy. Its applications are diverse, ranging from detecting adulterants in herbs and spices to identifying fraudulent activities in the dairy and meat industries [90].
NIRS has been compared to laboratory-based methods, such as Fourier transform infrared (FTIR) spectroscopy and has shown comparable or even better performance in some cases. This highlights the potential of NIRS as a more accessible and rapid alternative for food adulteration detection [90].
IR spectroscopy allows for fast, nondestructive, and low sample volume analysis of biochemical patterns, enabling composition-based statistical categorization and fruit juice verification with minimal operational expense [36].
In this regard, we can refer to various studies. Table 1 provides a compilation of various techniques for identifying juice adulteration. The usefulness of NIRS in determining the authenticity of fruit juice has been established in prior studies. Shafiee et al. optimized data mining on lime juice using a Support Vector Machine (SVM), achieving 97% accuracy in classification and detecting adulterants with less than 0.504% prediction error. However, their work lacks validation methods, highlighting the limited application of portable near-infrared spectroscopy (NIRS) devices in fruit juice quality control [33]. In a study conducted by Jahani et al., a handheld NIR spectrophotometer was employed to detect adulterated lime juices, achieving a classification correction rate of 88% through the utilization of the PLS-DA method with standard normal variate (SNV) transforming [34]. Accordingly, the utilization of partial least squares regression (PLSR) on FT-IR data yielded a prediction error ranging from 5.55% to 8.40% [35]. In their study, Ellis et al. managed to achieve a prediction error as low as 1.7% by employing a combination of FT-IR and PLSR to quantify sugar concentration (ranging from 0.5% to 20.0%) in pure orange juice [31]. A recent study by Calle et al. employed FT-IR to uncover instances of juice-to-juice adulteration, a type of fraud that is particularly challenging to detect. As a result, their validation method produced a detection limit of 5%, a discrimination accuracy of over 97%, and a prediction error of less than 1.4% [38].
Vardin et al. utilized chemometrics and Fourier transform infrared spectroscopy to analyze pomegranate juice concentrate adulteration. The spectra diverged most in the 1685–1780 cm1 range, classifying pure and fake samples and distinguishing between pure and fake samples. The partial least square analysis predicted grape juice concentrate quantity as an adulterant and predicted titratable acidity and total solids. These techniques are useful for evaluating pomegranate juice concentrate [91].
Mohammadian showed in 2021 that lime juice samples may be successfully authenticated using FT-IR spectroscopy in conjunction with VIP-PLS-DA and counter propagation artificial neural networks (CPANN) [39].
Thus, IR spectroscopy is a powerful tool for detecting juice fraud, and it faces challenges related to complexity, cost, data analysis, sample variability, and portability. Addressing these challenges through advancements in instrumentation, data analysis, and modeling techniques can improve the effectiveness of IR spectroscopy in combating juice adulteration [92, 93].
3.2.2. Nuclear Magnetic Resonance (NMR)
For metabolomics research and food fingerprinting, nuclear magnetic resonance (NMR) spectroscopy is one of the most popular analytical methods [94]. It entails examining how atomic nuclei with nonzero spins absorb energy when exposed to a magnetic field. The nuclei of nearby molecules have an effective impact on how much energy is absorbed by the atomic nuclei, which somewhat alters the external magnetic field [95]. NMR spectroscopy offers comprehensive structure information for food samples and metabolites [96]. Its key benefits include being noninvasive, having great accuracy and precision, being simple to use, and acquiring data quickly. However, compared with different analytical methods (such as FT-IR or MS), NMR spectroscopy exhibits limited sensitivity, and the instrumentation is relatively expensive with significant operating expenses [94].
The lower sensitivity of nuclear magnetic resonance (NMR) compared to other methods can affect its use in detecting certain types of adulteration or in different food matrices in several ways. NMR’s lower sensitivity means that it may not be able to detect small amounts of adulterants or contaminants, which could lead to false negatives or inaccurate results. This is particularly important in cases where the adulterant is present in trace amounts. NMR’s sensitivity can be further compromised when dealing with complex food matrices that contain a large number of components. In such cases, the signals from the adulterant might be masked or overwhelmed by the signals from the other components, making detection more challenging [97]. NMR often requires specific sample preparation methods, which can be time-consuming and may not be suitable for all types of food samples. This can lead to variability in the results and affect the sensitivity of the method [98].
Vigneau and Thomas exhibited that the 1H-NMR spectroscopic technique, when paired with PLSR, was proficient in attaining an estimation error of 3.47%. This was evident when they endeavored to differentiate between unadulterated orange juice and orange juice that had been adulterated with clementine juice [40]. Recently, Marchetti et al. amalgamated 1H NMR with PLSR to ascertain the proportions of apple, orange, pineapple, and pomegranate juices within their amalgamations. The PLSR model demonstrated exceptional performance, with an estimation error of less than 10% and R2 values ranging from 0.821 to 0.987. In some cases, NMR is used to authenticate alcoholic beverages, check beer composition, production date, and ensure quality control. This study emphasizes that NMR can be used as a new method to monitor food items, such as juices. The spectral detection data cover many compounds, and the results of examining different juices are ultimately compared with the database [41].
De Oliveira in Portugal studied how orange juices are stored to determine their principal chemical components using NMR. The formation of formic, succinic, fumaric, lactic acids, and acetic as well as ethanol, which was not present at zero hours, occurs during storage for up to 24 hours. Additionally, it was shown that, as expected, juices held at 24 C generate these compounds more frequently than juices stored at lower temperatures [42].
The food industry is constantly seeking innovative methods to improve food quality, enhance safety, and extend shelf life. Zhang et al. highlighted the current and future application of nonthermal technologies in the food industry, emphasizing the potential of NMR in food processing [99]. Similarly, Marcone et al. provided insights into the diverse food-based applications of NMR technology, emphasizing its versatility in analyzing various food components [100].
Although the existing literature provides valuable insights into the potential applications of advanced technologies, including NMR, for quality assurance in the food industry, there is a need for more focused research on commercial instruments or technologies based on NMR. Addressing the identified knowledge gaps and future research directions will be critical for advancing the use of NMR in ensuring food safety, quality, and regulatory compliance in the food industry [101].
3.2.3. Electronic Techniques
(1) E-Nose. Fruit juices have the potential to be contaminated with overripe and focused on fruit juices. The identification of possible adulteration in these juices has been examined below. The detection of adulteration in cherry tomato juices was investigated by Hong et al. [102] through the examination of overripe tomato juices as an adulterant. This investigation utilized a PEN2 e-nose device equipped with 10 selective metal-oxide semiconductor [26] sensors. Before conducting the e-nose assessment, a pretreatment was performed using desiccant anhydrous sodium carbonate to enhance the signal performance of the MOS sensor array. This step was necessary as MOS sensors are highly sensitive to water vapor. The process of collecting data persisted for 70 seconds, and a purge time of 60 seconds was implemented after each sample. The results obtained from the direct e-nose measurement displayed better differentiation compared to the e-nose measurement that employed desiccant. Desiccant anhydrous sodium carbonate is used before e-nose assessments to improve the signal performance of the sensors. This is because the presence of moisture can affect the accuracy and reliability of the sensors, particularly metal oxide semiconductor (MOS) sensors, which are commonly used in e-nose devices. The desiccant helps to remove excess moisture from the air, ensuring that the sensors respond more accurately to the VOCs being measured. Desiccants can selectively absorb specific compounds, allowing for more targeted detection of adulterants or spoilage indicators. The desiccant method can be slower due to the need for absorption and desorption steps, which may delay the analysis process. In contrast to this method, direct measurement methods are generally faster, as they do not require additional steps such as absorption and desorption, allowing for quicker analysis. Direct measurement methods might not be as sensitive as the desicant method due to missing subtle changes in odors [103, 104].
Although principal component analysis (PCA) achieved a variance of over 90% in the e-nose data for both pure and adulterated juices, the fusion data from the combination of e-nose and e-tongue, analyzed using analysis of variance (ANOVA), were found to be the most effective technique for differentiating the adulteration [102].
PCA is a linear dimensionality reduction technique that transforms a set of correlated variables into a smaller set of uncorrelated variables called principal components. The primary goal of PCA is to reduce the dimensionality of a dataset while preserving the most important patterns or relationships between the variables without any prior knowledge of the target variables [105]. On the other hand, analysis of variance (ANOVA) is a statistical technique used to compare the means of three or more groups to determine whether there are any statistically significant differences between them [106].
Ordukaya and Karlik [107] successfully classified numerous alcohol-adulterated juice samples by e-nose. The study compared LDA and SVM efficiency, finding that SVM outperformed LDA by 98.33%. LDA and SVMare both popular classification methods used in data analysis. LDA is suitable when the data follow a multivariate normal distribution and the assumptions are met, while SVM is more flexible and can handle nonlinear classification and outliers better. LDA is a dimensionality reduction technique primarily used in supervised classification problems. It aims to identify a linear combination of features that optimally separates classes within a dataset [108, 109].
Also, in the study conducted by Shen et al. [43], the authors used e-nose, PCA, and LDA models to differentiate between freshly squeezed orange juices and those with orange juice concentrate, achieving 97.9% calibration and 91.7% validation accuracy.
(2) Biosensor. Biosensor or biological sensor is used to detect and measure biological compounds such as proteins, enzymes, nucleotides, glucose, and hormones, and various types of it have been introduced in different studies. Electrochemical enzymatic biosensors are a type of biosensor that operate based on the electron transfer between the enzyme active site and the substrate, which is then transduced to an electrical signal [110]. Other nanostructures are flowerlike α-Fe2O3 synthesized using various methods, including template-free microwave-assisted solvothermal methods, which utilize low-cost and environmentally benign chemicals. These nanostructures have high surface areas and abundant hydroxyl groups on their surfaces, making them effective adsorbents. The adsorption mechanism involves ion exchange between the surface hydroxyl groups and the metal ions. Other synthesis methods include one-step biphasic interfacial reactions and solvothermal reactions, which can produce hierarchical architectures and 3D porous structures [111]. Biosensors offer advantages such as high sensitivity, low detection limits, and good reproducibility, making them effective tools for identifying adulteration in fruit juices [112, 113]. One study utilized an electrochemical enzymatic biosensor based on flowerlike α-Fe2O3 nanostructures to detect formaldehyde adulteration in orange and mango juices. The study used fresh juices, extracted through manual pressing and filtration, to analyze formaldehyde levels. The biosensor demonstrated good sensitivity (744.15 μA mg−1 Lcm−2), linear detection range (0.01–0.3 mg/L), RSD 0.73%, and good reproducibility [48]. Another study developed an electrochemical biosensor based on multiwalled carbon nanotubes and synthesized nanocomposite (CNT–Fe3O4) for the selective quantification of formaldehyde in orange juice. The oranges’ edible portion was manually pressed to extract the juice, which was then used for the analysis. For the selective detection of formaldehyde in orange juice, the biosensor shows low RSD values (1.70), acceptable recovery rates (90%), low identification limit, as well as excellent sensitivity (527 μA mg/L−1 cm−2) [49].
Biosensors play a crucial role in detecting adulteration in grape juice. One study developed a constant phase element (CPE) sensor using a thin film coating of poly(methyl methacrylate) (PMMA) to detect adulteration with sugar solution and water. CPE sensor is a type of sensor used in various applications, particularly in electrochemistry. This sensor is used to monitor microbial growth, which can be useful in detecting fraudulent activities in the food industry, such as knowingly selling contaminated or adulterated products as pure. The CPE sensor works by measuring the impedance of a sample, which is affected by the presence of microorganisms. This impedance measurement can be used to detect changes in the sample that might indicate contamination or adulteration [114]. The sensor used principal component analysis (PCA), box plot, and LDA to analyze the constant phase data and distinguish different types of adulteration [50]. Additionally, a microfluidic platform combined with thin-film silicon photosensors was proposed for the detection of pathogen infections in grapes. This platform monitored the concentration of azelaic acid (AzA) to detect infections. The biosensor achieved fast and accurate detection of AzA in grape juice, allowing for the early detection of pathogen infections [50]. Additionally, impedance sensors have been used to detect changes in the ionic properties of adulterated fruit juice, allowing for automated detection and prevention of adulteration [115]. Furthermore, a portable nanofiber-light addressable potentiometric sensor has been developed for the detection of Escherichia coli (E. coli) in orange juice, which is important for preventing bacterial contamination [116].
The integration of flowerlike α-Fe2O3 nanostructures and constant phase element (CPE) sensors has shown promising results in various fields, including glucose determination. The unique morphology and structural properties of α-Fe2O3 nanostructures contribute to improved sensing capabilities, as demonstrated by Li et al., [117]. Additionally, the electrocatalytic performance of Fe2O3-NPs/CPE sensors, as evidenced by Ramírez et al., showcases the potential of this combination for accurate and sensitive detection of analytes such as glucose. These findings underscore the potential of integrating α-Fe2O3 nanostructures with electrochemical enzymatic biosensors for various bioanalytical applications [118].
Despite these advancements, there are still knowledge gaps that warrant further investigation. While the studies have demonstrated the potential of α-Fe2O3 nanostructures in biosensing applications, there is a need for comprehensive studies to elucidate the underlying mechanisms of the enhanced sensing properties. Furthermore, the long-term stability and reproducibility of the Fe2O3-NPs/CPE sensor need to be thoroughly investigated to assess its practical viability in real-world applications.
Generally, advances in juice authenticity testing are important. Moulard, Raggio, and Folse investigate brand authenticity and its antecedents and outcomes. The findings suggest that authenticity positively influences consumer trust in brands and their products [119]. Similarly, the study by Porral and Lévy-Mangin on food private label brands emphasizes the role of consumer trust in loyalty and purchase intention. The findings indicate that consumer trust plays a critical role in driving loyalty and purchase intention [120].
4. Conclusion and Future Prospects
One of the most contentious topics in the food sector today is the identification of the authenticity and quality of juices. For an industry that is continually increasing and rising, it can be claimed that evaluating the purity of fruit juices presents a considerable problem. The most broadly used techniques for the determination of the purity of fruit juices are chromatographic (HPLC and GC) and spectroscopic methods. In recent years, sensor-based techniques have been introduced that can identify adulterations in fruit juice more quickly. However, they may not be as accurate as the spectroscopic method. In addition to economic loss, food fraud also causes social concern. Because of daily need for food in all age groups from children to older people, the concerns for health and safe food are raised. The food industry is directly related to health, and counterfeit foods have attracted a lot of attention in the last few decades. On the other hand, although it is always recommended to prepare goods or food with standard labels, even these labels can be fake. Therefore, the development of fraud detection methods in food such as juices can play an effective role in people’s health, and proving the authenticity of food will be done correctly and quickly.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors acknowledge the financial and technical support of the Tehran University of Medical Sciences, Tehran, Iran. This work is based upon research funded by Iran National Science Foundation (INSF) under project No. 4025197.
Open Research
Data Availability
Data are presented in the paper.




