A Kinetic and Thermodynamic Study of Cationic Thiazine Dye Removal From Aqueous Solution by Date Stones Variety Ghars
An Agricultural Waste
Abstract
Date stones represent an unused resource that is often incinerated or disposed of directly as waste. Therefore, great attention has been paid to transforming it into useful products. This study was conducted to evaluate the ability of activated carbon prepared from the stones of one of the date varieties, called “Ghars,” as an agricultural waste to remove a basic dye, methylene blue (MB), from aqueous solutions. The surface characteristics of prepared activated carbon Ghars stones (ACGS) were characterized using scanning electron microscope (SEM) and Fourier transform infrared (FTIR) spectroscopy. Physical and chemical factors affecting MB adsorption, such as contact time, the mass of adsorbent, potential of hydrogen (pH) value, and initial concentration of the solution, were studied in a batch system. Isotherm equilibrium data are well described by the Freundlich model (R2 = 0.98), and the experimental adsorption capacity was found to be (qe.exp = 130.3 mg/g) at 298 K. The kinetic study indicates that the adsorption process of MB follows the pseudo-second-order (PSO) model. ΔH° (19.94 J/mol) and ΔG° (−1.101, −1.127, −1.430, and −1.546 kJ/mol) values revealed that the adsorption of MB on ACGS was endothermic nature, feasible, and spontaneous. The positive value of ∆S° (15.67 kJ/mol) confirmed the increased in the randomness at the solid-solution interface during the adsorption process of MB on ACGS. MB adsorption/desorption experiments demonstrated the reusability of ACGS for five consecutive cycles. The results showed that ACGS developed from agricultural waste could be successfully used to remove cationic organic pollutants from wastewater.
1. Introduction
In recent decades, with the rapid development of industry and population growth, the consumption of various types of synthetic dyes has increased significantly. It requires a continuous production of huge quantities of dyes to meet the needs of many industrial sectors, approximately 7 × 105 million tons of 10,000, several types of synthetic dyes are produced yearly. These products are widely used in beverage and food, leather, plastics, cosmetics, paper, pharmaceuticals, and textiles [1, 2].
Dyeing processes, particularly in textile industries, require a large amount of water to color their products, which generates a large amount of various hazardous colored wastewater to be disposed in most cases into the sewers without treatment. It is estimated that 17%–20%of water pollution is attributable to the textile industry [3, 4]. The presence of dyes in water causes damage to the aquatic environment; it can reduce the permeability of sunlight, affecting the photosynthesis of microorganisms, increasing or decreasing water temperature, change the potential of hydrogen (pH), increasing the turbidity of water, and entering the food chain. In addition, synthetic dyes are classified as toxic, mutagenic, and carcinogenic to humans and animals. Due to the harmful and dangerous effects of dyes, several conventional methods have been used to treat dyes from wastewater before reaching aquatic lifethetic dyes are classified as toxic, mutagenic, and carcinogenic to humans and animals. Due to the harmful and dangerous effects of dyes, several conventional methods have been used to treat dyes from wastewater before reaching aquatic life [3, 4], including chemical precipitation, photo-catalysis, chemical oxidation, membrane filtration, coagulation, and electrochemical [5, 6]. These techniques are effectives, but certain limitations limit their use. Such as cost, highly skilled technicians, need for chemicals, and large energy consumption, while adsorption is a promising technique that has many advantages. It is easy to apply using fewer tools, low-cost and high removal efficiency [6–8].
A number of researches have worked to find appropriate biological, physical, and chemical methods to remove and treat these pollutants before discharged into the environment, which has become a real problem. Adsorption technology is an important and reliable strategy for treating different types of effluents as a result of its effectiveness, easy use and low cost economically compared to other methods [9, 10]. Adsorbents are characterized by special advantages, such as low cost, the ability to adsorb several types of pollutants, whether organic or inorganic, high mechanical stability, and porosity that can be modified by chemical or physical methods to improve pore size and distribution in addition to the availability of various functional groups on their surfaces that are required for specific applications. However, adsorbents have some limitations that have restricted their use, such as consuming some environmentally unfriendly chemicals, as many researchers focus on the added value of the amendment without citing the side effects of chemical activators in terms of human safety or even environmental effects, for example, the use of aluminium chloride (AlCl3), iron chloride (FeCl3), zinc sulfate (ZnSO4), and zinc chloride (ZnCl2) as chemical activators may contaminate the final product. Furthermore, the adsorbent becomes saturated and completely inactive after several cycles (adsorption/desorption) and is considered a hazardous solid waste, creating environmental problems [11–14].
In recent years, there is a growing attention in exploring different types of agricultural byproducts to produce adsorbents for treating hazardous elements and dyes from wastewater such as sisal [10], mango peels and seeds wastes [13], coffee waste [15], Degla Beida stones [16], coconut leaf [17], Rumex abyssinicus [2], Ficus carica bast [18], Corncob cyanide [19], palm shell reactive [20], coir-pith [21], queen palm fruit endocarp [22],
Cortaderia selloana flower spikes [23], orange and lemon peels [24], and peach stones [25]. These lasts have been used for the removal of malachite green, Black 5 dye (RB5), lead, 2,4-dichlorophenoxyacetic acid herbicide, pesticides, and methyl orange, respectively.
These materials are characterized by their low or no price and easy availability in large quantities. It represents an important resource for the production of adsorbents, especially for the production of activated carbon. In addition, the surface properties of these materials contain different functional groups, play a vital role in the adsorption of various non-biodegradable pollutants, and are particularly suitable for the treatment of solutions loaded with methylene blue (MB) through different mechanisms. MB is the most consuming dye in the industry; it is widely used in many applications for dyeing paper, cotton, silk, leather, wool, etc. These activities generate a large amount of effluent laden with MB, which is usually disposed of into natural water sources, causing many permanent harmful effects to living organisms, humans, and animals. Therefore, there is great interest in treating MB. This interest has been demonstrated by a large number of research papers, with more than 15,156 articles published on MB treatment since 2010 [23, 26].
The climate of southern Algeria is very suitable for a wide variety of palm trees known as Phoenix dactylifera L, according to the Food and Agriculture Organization (FAO) report 2023, Algeria is ranked the third largest producer in the world. Date fruit-processing industries generate large quantities of solid waste known as date stones, which are not economically exploited and are disposed of by burning or disposing of them with waste. Although there are a large number of date stone varieties with different physicochemical and morphological characteristics, no article mentioned the varieties used in preparing adsorbents; as a result, identification of the variety will be beneficial for the preparation of adsorbents [27–29]. Therefore, the aim of this study was to prepare activated carbon from another variety of date stones called Ghars. Also, for applied it in the field of removing cationic dye (MB) as typical dye pollutants from aqueous solutions. The experimental parameters influencing the MB dye adsorption process were studied in order to know the best performance of the adsorbent. Mathematical models were applied to kinetics and equilibrium isotherm experimental data. Moreover, the physical and chemical properties of the adsorbent were studied using Fourier transform infrared (FTIR) and scanning electron microscopy (SEM). Thermodynamic parameters such as enthalpy ΔH°, Gibbs free energy ΔG°, and entropy ΔS° have been calculated and interpreted.
2. Chemicals and Experimental Preparation of Adsorbent
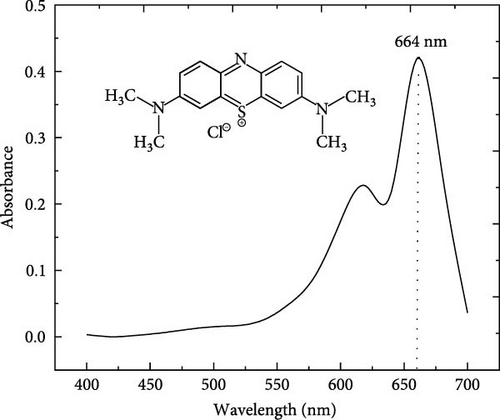
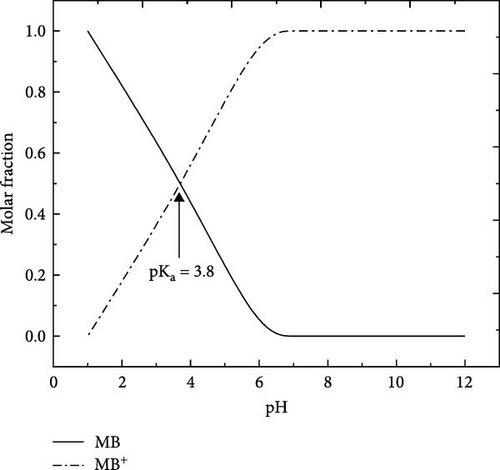
MB (98.5% purity) C16H18N3SCl (molecular weight [MW] = 319.85 g/mol), a basic cationic thiazine dye, was used in this study as an adsorbate model, very soluble in water, neutral for a pH lower than 3.8 which represents its pKa and becomes a positive charge for a pH higher than pka. Figure 1 shows its chemical structure and its visible absorption spectra. At first, distilled water was used as a solvent to prepare MB stock solution (500 mg/L). Experimental solutions were prepared at appropriate concentrations by diluting the stock solution, and the initial pH of experiment solutions was adjusted using both solutions, hydrochloric acid 0.1 M HCl (hydrochloric acid) or sodium hydroxide (NaOH) base 0.1 M NaOH [23, 30].
The activated carbon used in this study was prepared from date stones variety Ghars were obtained locally (southern Algeria) from agro-food industries as wastes. Initially, the sample was washed several times with tap water and dried for 24 h; the dried date stones were grinded to powder. The resulting powder was subjected to chemical activation by impregnating 40 g of it in 200 mL of 0.05 M NaOH solution for 4 h; after that, the solid obtained by filtration was washed with distilled water several times to remove any excess NaOH residue.
The sample obtained was carbonized in a muffle furnace under a heating rate of 5°C/min to a final temperature of 600°C, where it was maintained for an hour. Then, the sample obtained was washed with distilled water; it was then filtered and dried at 80°C for 24 h. Finally, the sample was sieved; the portion with a size between 315 and 500 μm was chosen to be used in the adsorption experiment; the activated carbon obtained from date stones variety Ghars was denoted activated carbon Ghars stones (ACGS).
3. Technical Characterization of Adsorbent
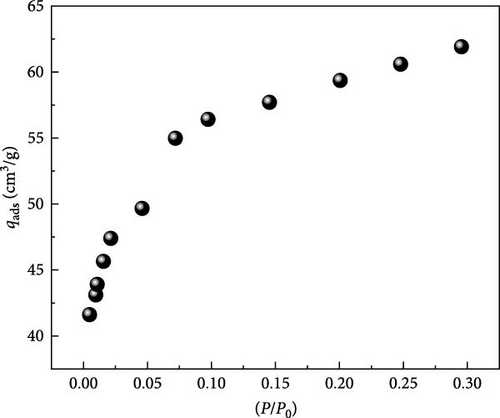
Surface morphology ACGS was performed by SEM (MEB FEG JEOL 6700F), using 350x magnification, an acceleration voltage of 4 kV, and a working distance of 15.3 mm. A 0.2741 g of ACGS was used to measure the nitrogen (N2) adsorption at 77 K using QuadraSorb SI MP Station (Figure 2a).
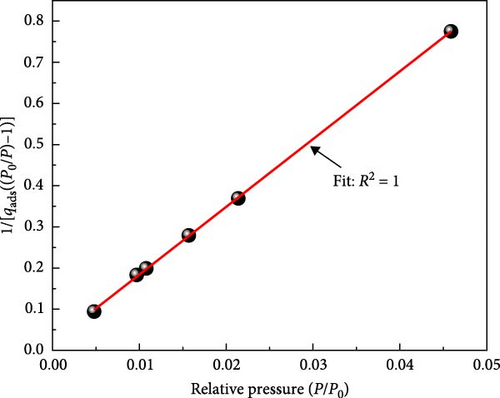
The sample was degassed at 548 K for 12 h; the surface area was calculated using Brunauer–Emmett–Teller (BET) theory (Figure 2b). JASCO FT/IR-4200 spectrophotometer was used in the range of 500–4000 cm−1 to identify the functional groups present on the surface of ACGS before and after the adsorption process. The thermogravimetric analysis (TGA) of treated date stones (5.3919 mg) was conducted using a thermal analyzer (TGA/simultaneously differential thermal analysis [SDTA] 851e Mettler-Toledo). The sample was heated until 950°C under an airflow rate of 40 mL/min using a heating rate of 10°C/min.
4. Batch Adsorption Experiments
Adsorption experiments were investigated in batch mode using 50 mL of MB dye solution at 25°C during 24 h to reach equilibrium. The effect of ACGS dose was studied in the range (0.02–0.2 g) with an initial MB concentration of 500 mg/L and pH = 6. The kinetic of MB dye adsorption was studied in the range (0–360 min) with an initial MB solution concentration of 500 mg/L and pH = 6. The effect of pH was evaluated in the range (2–10) by using 0.08 g of ACGS and an initial MB concentration of 500 mg/L at pH = 6. Adsorption isotherm was studied at variable initial MB concentrations from 50 to 500 mg/L during 24 h to reach equilibrium. The solid–liquid was filtered after each experiment, and the MB concentration was determined using a spectrophotometer (ultraviolet radiation [UV]–Visible Optizen 1412 V) at the wavelength (λmax = 664 nm).
5. Adsorption Modeling and Thermodynamic Study
6. Results and Discussion
6.1. Characterization of the Adsorbent
SEM was conducted to reveal the nature and the morphology of the adsorbent surface. Figure 3 shows the heterogeneous surface of ACGS material with rough, asymmetrical pores with cavities of different sizes and shapes. The pores were formed during the chemical and thermal (physical) activation of raw material. This porous structure is noticeably important and characterizes adsorbent materials.


According to BET, the surface area was found to be 211.01 m2/g, Figure 2b, shows the valid linear range of BET for ACGS microporous property was at low relative pressures p/p0 ≤ 0.05 [34, 37].
Figure 4 shows the FTIR spectrums of ACGS before and after MB dye adsorption; weak bands were observed in Figure 4a at 1736, 1551, 1402, 1002, and at 884 cm−1 are assigned to C═O in the carboxylic groups, C═C stretching vibration in aromatic, C─O─H stretching vibration in carboxylic group, C─O stretching of carboxylate groups and C─H bending in the aromatic rings, respectively. New peaks appeared after MB adsorption (Figure 4b), at 2921 and 2847 cm−1 are assigned to the aliphatic C─H stretching in methylene group ─C(CH3)3, at 1560 cm−1 is attributed to C─N vibrations of the MB heterocycle and at 832 cm−1 is related to C─H bending in the aromatic rings of the MB molecule. In addition, after MB adsorption, there is a noticeable difference in the FTIR spectrum, lowering of the intensity, disappearance of the peak at 1736 cm−1, and change of positions of some peaks from 1402, 1002, 884 cm−1 to 1425, 995, 876 cm−1, respectively. These results indicate the involvement of the functional groups mentioned above in MB adsorption on ACGS [10, 30, 38].
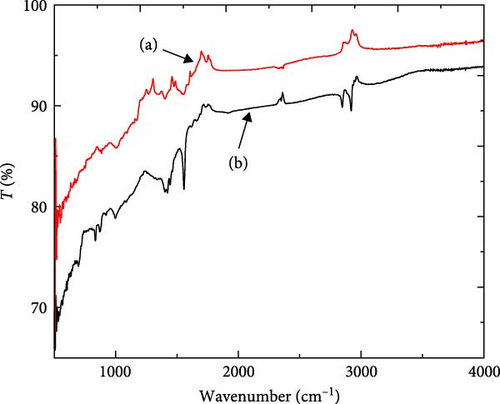
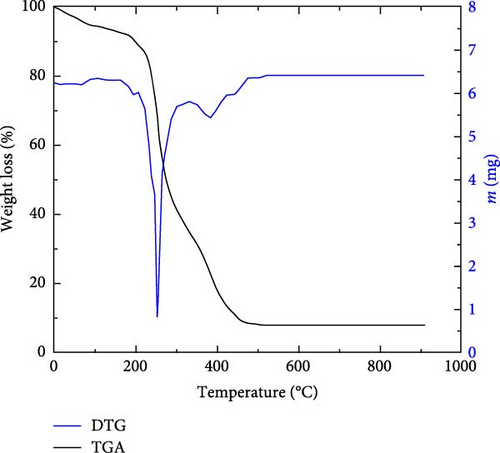
Figure 5 displays the decomposition as a percentage weight-loss of raw date stones variety Ghars treated with NaOH versus temperature. Based on the differential thermogravimetric analysis (DTG)/TGA curves on the literature, losses are attributed to moisture (~87.58°C), hemicellulose (~204.15°C), cellulose (~326.24°C), and lignin (~473 °C) which correspond to 4.8, 6.4, 52.55, and 28.52 (%), respectively [39]. Weight-loss continues until the mass of the sample stabilizes at 7.72 (%) corresponds to (~504.75°C).
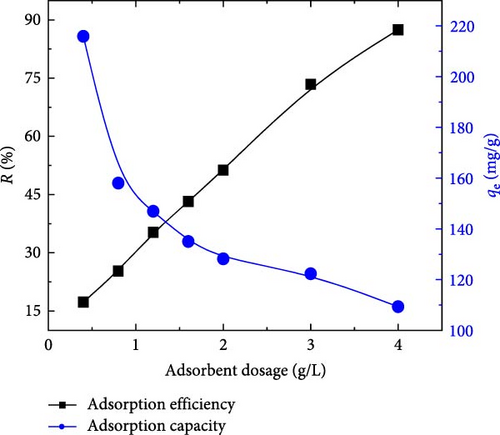
6.2. Effect of Adsorbent Dosage
Figure 6 illustrates the MB removal efficiency R (%) versus the dose of ACGS. It is clear that an increase in the dose of ACGS from 0.4 to 4 g/L led to an increase in MB removal efficiency from 17.12% to 87.45%. This may be due to the increase in the specific surface area of ACGS adsorbent, as a result of the increase in vacant active sites on the surface of the ACGS due to the increase in the mass of the ACGS [40, 41].
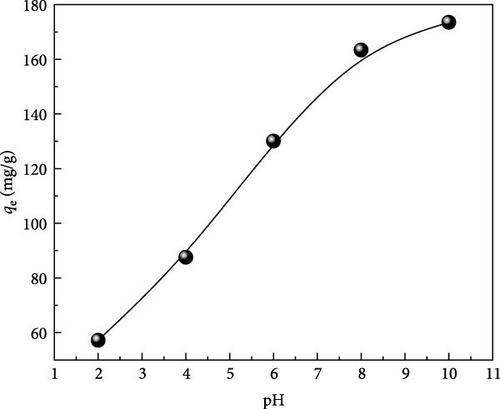
6.3. Effect of pH
From the literature, several authors have stated that the pH is one of the most important parameters affecting the amounts and types of electrostatic charges on the surface of the adsorbent, photo-degradation of dyes, ionization, and chemical speciation of the adsorbate [23, 42].
As shown in Figure 7, at low pH values, the uptake of MB molecules was relatively low (57.18 mg/g) due to the competition of the cationic MB dye with protons (H+) greatly existing in an acidic environment. On the other hand, when the pH changes to the alkaline range, the adsorption capacity of MB changes significantly from 57.18 to 173.54 mg/g; this can be attributed to the reduction of the acidic effect enhancing the electrostatic attraction between the functional groups on the surface of ACGS and cationic dye for higher pH values [31, 38].
6.4. Effect of Contact Time
The effect of contact time on MB dye uptake using ACGS was investigated in the range of 0–360 min, as shown in Figure 8.
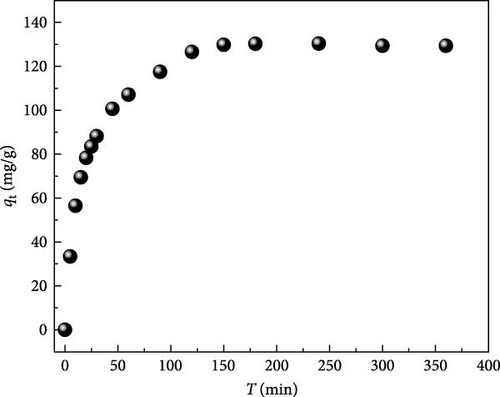
The uptake capacity of MB was rapid during the first 30 min, and the adsorption rate of MB starts to slow down and reaches equilibrium at 150 min; this observation can be explained by the abundantly and unoccupied binding sites on the surface of ACGS at the first stage of MB adsorption process; therefore, the adsorption was fast, in the second stage, where the adsorption was slowing down, mainly due to the gradual packing of sites by MB on ACGS surface until it was completely saturated, in the last stage, from 150 min, all sites are fully occupied and the uptake capacity values of MB remained constant [16, 35].
6.5. Kinetic Study
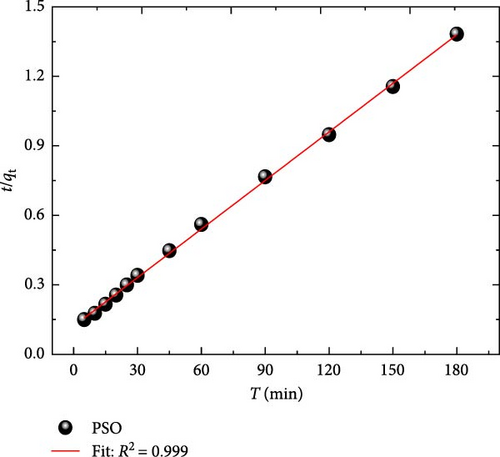
From Figure 9a, the plot of (qt) as a function of (t0.5) is divided into two phases and does not cross through the origin; this means that the intraparticle diffusion was not the only the uniqueness rate-limiting step, which confirms that more than one mechanism exist [43, 44]. The first linear (R2 = 0.987) corresponds to the instantaneous adsorption, and the second linear (R2 = 0.988) corresponds to the intraparticle diffusion that represents the limiting phase of the adsorption process. Table 1 summarizes the intraparticle diffusion constants; the rate diffusion (Kid1 = 11.54) of the first stage was greater than the rate diffusion (Kid2 = 06.09) of the second stage, which confirms that MB molecules were first adsorbed on the external surface of ACGS until saturation, then, the MB molecules begin to diffuse into the interior of ACGS cavities. Finally, the diffusion resistance of MB molecules increases, which leads to a decrease in the diffusion rate; this corresponds to the thicker boundary layer (C2 = 59.74) of the second phase evaluated from the intercept compared to (C1 = 25.52) the first phase [45, 46].


| PFO | PSO | |||||
|---|---|---|---|---|---|---|
qexp (mg/g) |
qe, cal (mg/g) |
k1 (1/min) | R2 | qe, cal (mg/g) |
k2 (mg/g min) |
R2 |
| 130.3 | 95.23 | 0.759 | 0.953 | 140.8 | 0.00042 | 0.999 |
| Intraparticle diffusion | ||||||
|
|
R2 |
|
|
R2 | |
| 11.54 | 25.52 | 0.987 | 6.096 | 59.74 | 0.998 | |
- Abbreviations: ACGS, activated carbon Ghars stones; PFO, pseudo-first-order; PSO, pseudo-second-order.
The parameters of PFO and PSO models were evaluated from the slope and the intercept of the linear curves in Figure 9b,c; the values of both models are summarized in Table 1, and the correlation coefficients confirm the applicability of the PSO model (R2 = 0.999) to the experimental data of MB adsorption by ACGS compared to the PFO (R2 = 0.953). Additionally, the PSO model can be relied upon to describe the adsorption of MB on ACGS based on the near value of the adsorption capacity (qe, cal) calculated using the PSO data to the experimental value (qe, exp).
6.6. Adsorption Isotherm
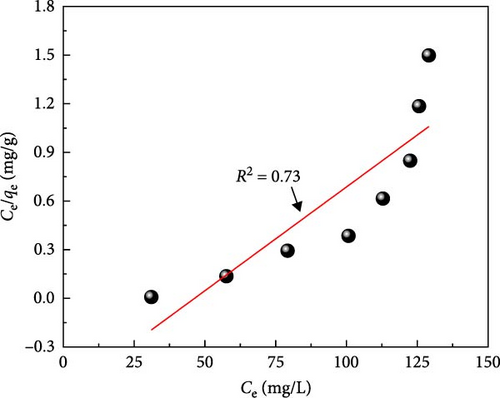
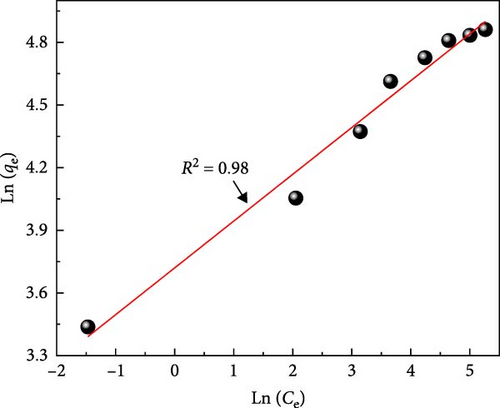
Figure 10a depicts the equilibrium adsorption isotherm of MB on ACGS fitted to Langmuir and Freundlich models, as shown in Figure 10b,c. Table 2 shows a higher value of the correlation coefficient (R2 = 0.98) obtained from the plot of Ln (qe) vs. Ln (Ce) using the linear form of the Freundlich model as compared to the Langmuir model (R2 = 0.76), confirms that the adsorption isotherm was better described by the Freundlich model which suggests a multilayer coverage of MB dye molecules on the heterogeneous surface of ACGS [47].

| Langmuir isotherm | Freundlich isotherm | |||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | RL | R2 | KF (mg/g)/(L/mg)n | 1/n | R2 |
| 78.12 | 0.004 | 0.315 | 0.766 | 41.26 | 0.224 | 0.980 |
- Abbreviations: ACGS, activated carbon Ghars stones; MB, methylene blue.
Also, the value of (1/n) was found to be 0.224 in the range of 0–1 indicates that the adsorption intensity of MB molecules on ACGS was favorable under the studied conditions [48]. Table 3 summarizes the adsorption capacity values of MB removal using various adsorbents reported by other researchers.
| Adsorbents | qexp (mg/g) | References |
|---|---|---|
| ACC | 103.66 | [10] |
| ACDB | 163.6 | [16] |
| Cortaderia selloana flower spikes | 47.9 | [23] |
| Coconut leaf-activated carbon (SAC) | 127–149 | [31] |
| MB-Cucumis sativus peels | 21.45 | [44] |
| Coconut shell (SATCS) | 50.6 | [9] |
| Silk fibroin orange peel foam | 113.8 | [49] |
| Algerian palygorskite powder | 57.5 | [50] |
| Carbon/montmorillonite nanocomposites powder | 138.1 | [51] |
| Barley malt bagasse biochar powder | 161.0 | [52] |
| Nitrogen-Cocos nucifera | 134.7 | [53] |
| Marula seed husk | 33.0 | [54] |
| RDP | 105 | [55] |
| ACGS | 130.3 | Present work |
- Abbreviations: ACC, activated carbon/cellulose composite; ACDB, activated Degla Beida stones; ACGS, activated carbon Ghars stones; MB, methylene blue; RDP, raw date pits; SAC, sulphuric acid-treated activated carbon; SATCS, sulfuric acid-treated coconut shell.
6.7. Thermodynamic Study
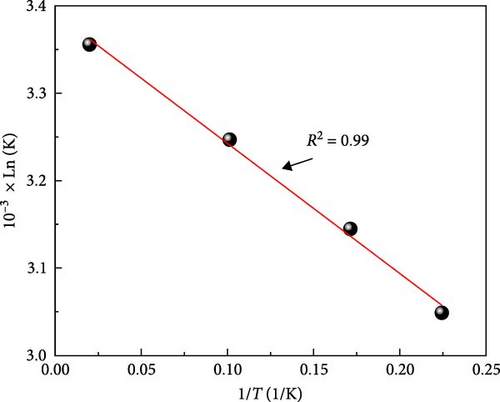
The values of ΔH° and ΔS° can be determined from the intercept and the slope of the linear plot of ln (k) versus (1/T), as seen in Figure 11. The values of thermodynamic parameters are presented in Table 4.
| Thermodynamic parameters | ΔG° (kJ/mol) | ||||
|---|---|---|---|---|---|
| ∆H° (J/mol) | ∆S° (J/mol K) | 298 (K) | 308 (K) | 318 (K) | 328 (K) |
| 19.94 | 15.67 | −1.101 | −1.1274 | −1.430 | −1.546 |
- Abbreviations: ACGS, activated carbon Ghars stones; MB, methylene blue.
The negative values of ΔG° at all temperatures indicate that the adsorption of MB on ACGS is thermodynamically feasible and spontaneous; the increase in temperature produces a decrease of ΔG°, which favors the degree of spontaneity of the adsorption process. Generally, the values of ΔG° give an idea about the nature of the adsorption; the physisorption is in the range of −20 to 0 (kJ/mol), while the chemisorptions in the range of −80 to −400 kJ/mol, the values of ΔG° from −1.101 to −1.546 kJ/mol show that physisorption was the predominant mechanism. The endothermic nature of MB adsorption on ACGS was confirmed by the positive value of ΔH° from 298 to 328 K. The positive value of ∆S° confirmed the increased in the randomness at the solid-solution interface during the adsorption process of MB on ACGS [10, 30, 56, 57].
6.8. Reusability of Adsorbent
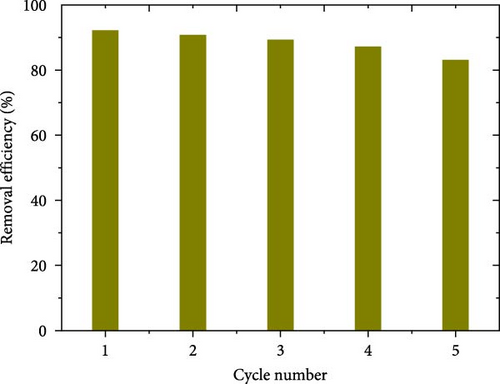
The reusability of the adsorbent used is an important step to determine the stability and the performance of the adsorbent over several times. In order to study the adsorption/desorption processes, 0.08 g of ACGS was added to 50 mL of MB (100 mg/L) at pH = 6. Based on the study of the effect of contact time, as seen in Figure 12, the mixture was stirred for 1 h, which represents the time required to reach equilibrium. The ACGS saturated with MB was separated from the solution using a centrifugation technique and then dried in an oven at 65°C; the obtained sample was contacted with 0.1 M of HCl as an eluent agent at an agitated rate of 150 rpm for 4 h. After that, the ACGS was separated and washed several times with distilled water, then dried in an oven at 65°C to be reused again in MB adsorption process, the process adsorption/desorption was repeated five times. Figure 12 shows that the reusability of ACGS for the removal of MB dye is possible and the removal efficiency is greater than 83.14 (%) after five successive cycles, while the decrease 92.19%–83.14% can be attributed to the gradual decrease in the number of adsorption sites on the ACGS surface due to incomplete removal of MB molecules attached to the surface of the ACGS during the MB desorption process, which will lead to weakening of the electrostatic interactions [58, 59].
6.9. Adsorption Mechanism for MB
The adsorption mechanism of MB on ACGS can occur through various interactions such as electrostatic attraction forces, H-bonds, π–π interactions, and porous diffusion [8]. According to FTIR analysis, the carboxyl and hydroxyl functional groups existing on the surface of ACGS are expected to play an important part in the adsorption of MB through electrostatic attractions between the positive charge of the cationic form of MB (pH < pKa), as shown in Figure 1b, and the negative charges present on ACGS surface as a result of the deprotonation of hydroxyl (─OH) and the carboxylic (─COOH) functional groups. The structure of the MB molecule contains nitrogen atoms and aromatic rings that can, respectively, interact with the H-bonds of functional groups and the C═C double bond presented on the surface of ACGS contributing to the adsorption of MB. In addition, Based on the electron scanning image, the surface morphology of ACGS and porous filling could also contribute to the adsorption of MB [13, 60, 61]. The possible adsorption mechanisms for MB onto ACGS are shown in Figure 13.

7. Conclusion
In this work, activated carbon (ACGS) was produced from date stone varieties Ghars as an agricultural waste for the removal of MB dye from aqueous solutions. Spectroscopic analysis showed that ACGS has a rough surface filled with cavities and the presence of hydroxyl (─OH) and carboxylic (─COOH) functional groups on its surface; these characteristics are certainly helpful in binding different types of contaminants. The MB uptake strongly depends on the pH of the solution, and the maximum adsorption capacity of 130.3 mg/g was obtained at optimum conditions of an adsorbent dosage of 0.08 mg/50 mL, an initial MB concentration of 500 mg/L, initial pH = 6 and contact time of 150 min. H-bonds formation, π–π interactions, and porous diffusion are the possible mechanisms governing the MB adsorption. Kinetic data results showed that the MB adsorption by ACGS was best represented by the PSO model (R2 = 0.999), and the equilibrium data were best described by the Freundlich isotherm model (R2 > 0.99), which validates the multilayer coverage of MB dye molecules on the heterogeneous surface of ACGS. ACGS reusability is possible, and the removal efficiency is greater than 83.14 (%) after five successive cycles. Activated carbon obtained from date stone varieties Ghars is a promising adsorbent as a low-cost and promising alternative to remove cationic dyes from aqueous solution. However, further investigations and improvements are needed before industrial use of wastewater, such as testing its efficiency in removing other pollutants such as cationic and ionic heavy metals, using other chemical activators at different concentrations, and using fixed bed column mode in the adsorption process.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
There is no funding source for this research.
Open Research
Data Availability Statement
No underlying data were collected or produced in this study.




