Evaluation of CD4+ T Lymphocyte Counts to Predict Survival of ICU Patients with Sepsis Using Sepsis-3 Criteria: A Prospective Cohort Study
Abstract
Background. Sepsis remains a major health condition with a high mortality rate that may be related to immunosuppression. T lymphocyte subsets may reflect the immune function of sepsis patients. The purpose of this study was to investigate the predictive value of CD4+ T lymphocyte counts of ICU patients for their short-term prognosis. Methods. We conducted a prospective, observational cohort study in a general ICU and enrolled patients with sepsis using the Sepsis-3 criteria. Peripheral blood samples were collected within 24 hours of enrollment or measurement of blood cell analysis and biomarkers of CD4+ T lymphocytes and CD8+ T lymphocytes. Severity was classified by the Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment (SOFA) scores. The primary outcome was 28-day mortality. Results. A total of 100 patients with sepsis were enrolled and analyzed. CD4+ T lymphocyte counts gradually decreased based on 28-day mortality (p < 0.001). Similarly, multivariate logistic regression analysis showed that only CD4+ T lymphocyte counts were an independent predictor of 28-day mortality in sepsis patients. The area under the receiver operating characteristic curve of the combination of CD4+ T lymphocyte counts and the SOFA score was 0.78. Conclusion. Our study demonstrated that CD4+ T lymphocyte counts are associated with 28-day mortality. A combination of CD4+ T lymphocyte counts with the SOFA score increased the predictive accuracy for 28-day mortality.
1. Background
Sepsis is presently defined as a life-threatening organ dysfunction syndrome instigated by a dysregulated host response to infection, a condition that is garnering escalating interest in the field of emergency medicine [1]. Across the globe, sepsis is now estimated to result in more than 11 million deaths a year, and septic shock, the most severe form, leaves nearly 40% of patients dead at hospital discharge [2]. Over the past few decades, the mortality rate of sepsis has seen a gradual decrease due to the timely administration of antibiotics, fluid resuscitation, and multiple organ support therapies [3]. However, mortality rates persistently remain high among specific populations, such as immunosuppressed individuals. The Sequential Organ Failure Assessment (SOFA) score often falls short in identifying these patients, leading to a large segment of sepsis patients being erroneously classified as “immunocompetent” [4]. This misclassification results in prolonged intensive care unit (ICU) stays and increased financial burdens for these immunosuppressed patients.
To better identify these at-risk populations, numerous new technologies have been developed [5]. As an important immune cell, CD4+ T cells may play a significant role during sepsis. CD4+ T cells are a subgroup of lymphocytes that coordinates the cells of the immune system by releasing cytokines that are essential for their proliferation, differentiation, and bactericidal functions against pathogens [6]. It remains contentious whether CD4+ T cells are intimately involved in the early stages of sepsis. Some animal studies have shown that CD4+ T cells may directly mediate the host response to sepsis [6, 7], while others have shown that they had no impact on the inflammatory response [8]. Human patients and experimental mouse models of sepsis exhibit profound CD4+ T-cell apoptosis [9, 10]. However, the clinical relevance of these findings has not been sufficiently corroborated by research studies. Therefore, evaluating the relationship between sepsis and T lymphocyte subsets not only can it help to better understand the pathogenesis of sepsis-related immunity but it can also provide new insights as well as strategies for the treatment of sepsis. The purpose of this study was to investigate the predictive value of CD4+ T lymphocyte counts in peripheral blood using the Sepsis-3 criteria on 28-day mortality.
2. Methods
2.1. Patients
The study was performed in the general intensive care unit (ICU) of a 2,700-bed tertiary-level general hospital. From June 2016 to September 2022, we prospectively enrolled patients with sepsis. Patients admitted to the ICU were screened for inclusion and exclusion criteria and enrolled on ICU day 1 or 2. The inclusion criteria were as follows: patients (a) met Sepsis-3 criteria and (b) aged 18 years or older than 18 years. The exclusion criteria were as follows: (a) terminal stage of chronic diseases; (b) patients with autoimmune disease, immunodeficiency, or long-term use of immune suppressants; (c) patients with tumor; and (d) consent unable to be obtained. This study was approved by Guangdong Provincial People’s Hospital Ethics Committee. Written informed consent was obtained from the patients or their immediate family. Consents for patients who were unable to provide consent were provided by their immediate relatives.
2.2. Data Collection
Clinical characteristics of patients, including age, gender, preexisting clinical conditions, vital signs, and results of laboratory examinations, were recorded on study days 1–7. SOFA and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were calculated. Patients were followed for at least 28 days after recruitment or until death. According to the mortality within 28 days, the patients were divided into nonsurvival and survival groups.
Blood samples were obtained in the morning within 24 hours after being enrolled. Study day 1 was defined as the day when the patients were enrolled. All patients underwent blood sampling on study day 1, and some of them who agreed also had their blood samples taken on study days 2, 4, and 7.
2.3. Flow Cytometry
Peripheral blood was extracted from all the patients and was collected in heparin tubes. The samples were transported to the laboratory at 4°C within 4 h. Erythrocytes were lysed, and cells were processed for evaluation by a researcher who was blinded to our clinical data. Antibodies were purchased from BD Pharmingen (Franklin Lakes, NJ). The manufacturer’s instructions with respect to the use of monoclonal antibodies and their fluorescence minus one controls were followed: aliquots of 100 μL of whole blood were incubated with PC-5.5-labeled anti-CD3 (2 μL clone UCHT1), FITC-labeled anti-CD4 (2 μL clone RPA-T4), and PE-labeled anti-CD8 (2 μL clone HIT8a). Samples were processed on CytoFLEX (Beckman Coulter, Inc.) and analyzed using CytExpert Software version 2.0 (Beckman Coulter, Inc.). Lymphocytes were gated by forward scatter (FSC) and side scatter (SSC), and T-cell subsets were further identified by CD3+, CD4+, and CD8+ staining. Results are expressed as percentages.
2.4. Statistical Analysis
We used statistical software SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL, USA) for all statistical analyses. The baseline characteristics were described as frequencies, percentages, median, and interquartile ranges. Comparisons between groups were made using the Pearson χ2 test for categorical data and the nonparametric Mann–Whitney U test for continuous variables. Repeated measurement data analysis of variance was performed to show the dynamic changes in CD4+ T lymphocyte counts. Binary logistic regression was used to identify the variables associated with 28-day mortality in patients with sepsis. Those variables with a collinear relationship were not included in the multivariable analysis. CD4+ T lymphocyte counts were stratified using the optimal threshold indicated by the receiver operating characteristic (ROC) curve. Patient survival was analyzed using the Cox proportional hazards model. All statistical tests were two-tailed, and p < 0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics
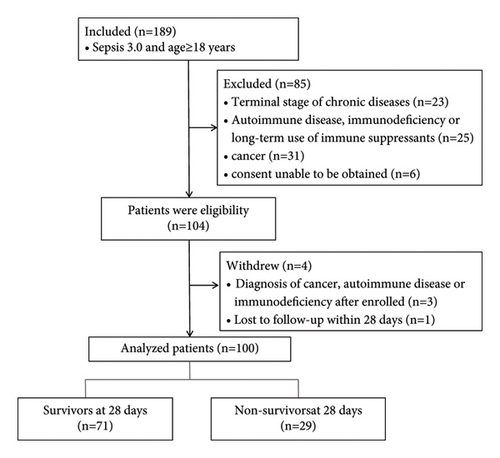
The study enrolled 104 patients, and 4 patients were withdrawn because 3 of them were eventually diagnosed with cancer or autoimmune diseases and 1 lost to follow-up (Figure 1). Thus, 100 patients were analyzed. There were 72 men (72%) and 28 women (28%), all of whom were Chinese, and the median age was 72 (60–78) years. A total of 29 patients died at or before 28 days. The demographic and clinical characteristics are shown in Table 1.
| Parameters | All patients, n = 100 | Nonsurvivors, n = 29 | Survivors, n = 71 | p value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Female, n (%) | 28 (28) | 8 (27.6) | 20 (28.2) | 0.953 |
| Age (years) | 72.00 (60.00–78.00) | 72.00 (61.00–77.00) | 72.00 (60.00–79.00) | 0.918 |
| Laboratory findings | ||||
| WBC (×109/L) | 14.97 (9.30–20.10) | 11.86 (8.15–17.92) | 15.94 (8.71–20.47) | 0.118 |
| Lac (mmol/L) | 1.40 (1.10–2.00) | 1.77 (1.23–2.56) | 1.30 (1.10–1.80) | 0.039 |
| Bacteriology positive, n (%) | 53 (53) | 18 (62.1) | 35 (49.3) | 0.246 |
| Vital signs | ||||
| T (°C) | 37.15 (36.70–38.2) | 37.40 (36.65–38.10) | 37.00 (36.70–38.40) | 0.985 |
| Heart rate (cpm) | 100.00 (85.25–110.00) | 105.00 (97.50–110.00) | 96.00 (82.00–110.00) | 0.047 |
| MAP (mmHg) | 89.00 (80.00–99.33) | 85.00 (79.00–98.17) | 89.00 (80.33–100.00) | 0.569 |
| Source of infection, n (%) | ||||
| Bloodstream | 4 (4) | 1 (1.4) | 3 (10.3) | 0.072 |
| Lungs | 64 (64) | 47 (66.2) | 17 (58.6) | 0.474 |
| Abdomen | 14 (14) | 9 (12.7) | 5 (17.2) | 0.540 |
| Urinary system | 10 (10) | 7 (9.9) | 3 (10.3) | 1.000 |
| Central nervous system | 4 (4) | 4 (5.6) | 0 (0) | 0.320 |
| Others | 4 (4) | 3 (4.2) | 1 (3.4) | 1.000 |
| Severity of illness | ||||
| GCS score | 12.00 (7.13–15.00) | 8.00 (3.50–13.50) | 12.00 (9.00–15.00) | <0.01 |
| APACHE II score | 15.00 (10.00–20.00) | 19.00 (15.00–24.50) | 13.00 (9.00–17.00) | <0.01 |
| SOFA score | 5.00 (4.00–8.00) | 9.00 (5.00–12.00) | 4.00 (3.00–6.00) | <0.01 |
| Shock, n (%) | 24 (24) | 12 (41.4) | 12 (16.9) | <0.01 |
- Data are shown as median and interquartile range unless otherwise indicated. Pearson chi-square test was performed for sex, bacteriology positive, and shock while the nonparametric test of two independent samples (Mann–Whitney) were for the others. WBC: white blood cell, Lac: lactate, GCS: Glasgow Coma Scale, APACHE II: Acute Physiology and Chronic Health Evaluation, SOFA: Sequential Organ Failure Assessment, T: temperature, and MAP: mean arterial pressure.
3.2. Comparison of Blood Cell Analysis, N/L, and Lymphocyte Subsets of Survivors and Nonsurvivors in Patients with Sepsis
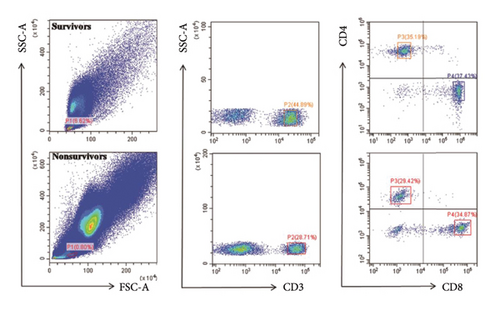
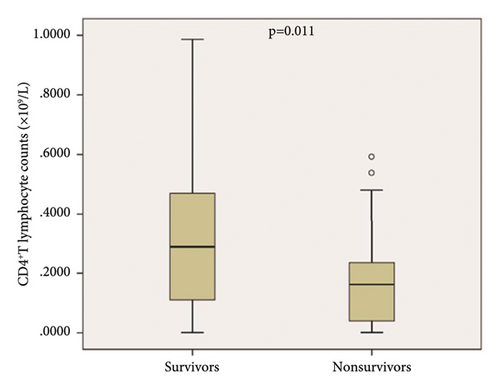
Patients were divided into survivors and nonsurvivors at 28 days. We compared the two groups of the patients, and the results are shown in Table 2. In nonsurvivors, CD4+ T lymphocyte counts were significantly lower than those in survivors (Table 2, p < 0.05; Figures 2(a) and 2(b)). Similar results were also observed when compared with the lymphocyte counts, CD8+ T lymphocyte counts, and lactate (Table 2; p < 0.05). The representative flow dot plots are shown in Figure 2(a).
| Parameters | All patients | Nonsurvivors | Survivors | p value (N/S) |
|---|---|---|---|---|
| Number | 100 | 29 | 71 | |
| N (×109/L) | 13.01 (8.00–17.67) | 10.55 (6.88–16.77) | 13.58 (8.62–18.24) | 0.26 |
| L (×109/L) | 0.85 (0.48–1.30) | 0.50 (0.37–1.07) | 0.91 (0.62–1.40) | <0.01 |
| N/L | 13.04 (8.62–27.99) | 14.01 (9.39–31.02) | 11.82 (8.29–26.25) | 0.248 |
| CD4+ T cells counts (×109/L) | 0.19 (0.09–0.42) | 0.16 (0.03–0.24) | 0.29 (0.11–0.47) | 0.011 |
| CD8+ T-cell counts (×109/L) | 0.15 (0.06–0.30) | 0.11 (0.02–0.25) | 0.17 (0.06–0.30) | 0.031 |
| CD4/CD8 | 1.65 (0.96–2.49) | 1.66 (0.78–2.24) | 1.63 (1.02–2.67) | 0.144 |
- Data are shown as median and interquartile range. Nonparametric test of two independent samples (Mann–Whitney) was performed for the comparison of nonsurvivors and survivors. N, neutrophils; L, lymphocyte; N/L, the ratio of neutrophil count to lymphocyte count.
3.3. Dynamic Changes in CD4+ T Lymphocyte Counts
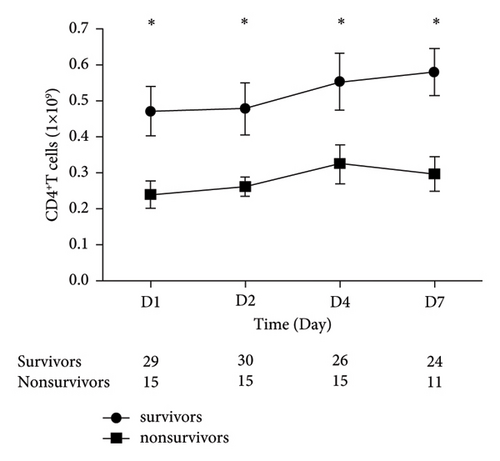
CD4+ T lymphocyte counts of 45 patients were measured on days 1, 2, 4, and 7 after being enrolled. CD4+ T lymphocyte counts in nonsurvivors were significantly lower than those in the survivors on every time point (Figure 3). CD4+ T lymphocyte counts continued to increase in the survivors, notably in the early disease course (1-4d). However, CD4+ T lymphocyte counts began to decline at 4 days in the nonsurvivors.
3.4. CD4+ T Lymphocyte Counts as an Independent Predictor of 28-Day Mortality in Sepsis Patients
Multivariate logistic regression analysis was used to identify the independent predictor of 28-day mortality. These parameters showed significant differences by the univariate analysis, but excluding the possible collinearity indicators between survivors and nonsurvivors, shock, heart rate, Lac, CD4+ T lymphocyte counts, and CD8+ T lymphocyte counts were included in this analysis. The results showed that CD4+ T lymphocyte counts, shock, and heart rate were independently associated with 28-day mortality. The detailed data are presented in Table 3.
| Variable | B | SE | Wald | p value | Odds ratio | 95% confidence interval for B | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| CD4+ T cells | −2.726 | 1.333 | 4.181 | 0.041 | 0.066 | 0.005 | 0.893 |
| Heart rate | 0.039 | 0.015 | 6.692 | 0.010 | 1.040 | 1.010 | 1.072 |
| Shock | 1.157 | 0.591 | 4.104 | 0.043 | 3.181 | 1.038 | 9.747 |
| Constant | −4.565 | 1.605 | 8.093 | 0.004 | 0.010 | ||
3.5. ROC of CD4+ T Lymphocyte Counts for Predicting 28-Day Morality and Survival Analysis in Sepsis Patients
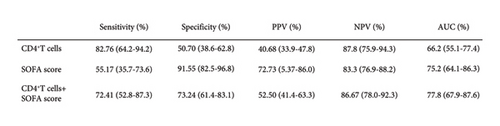
The ROC curve obtained with CD4+ T lymphocyte counts has an AUC of 0.662 (CI 0.551–0.774), and the ROC curve obtained with the SOFA score has an AUC of 0.752 (CI 0.641–0.864). The combination of these two parameters has an AUC of 0.778 (CI 0.679–0.876) for predicting 28-day mortality (Figure 4(a)). ROC curve analysis showed that 0.2810 (×109/L) of CD4+ T lymphocyte counts was the optimal threshold for predicting 28-day mortality in patients with sepsis. The sensitivity, specificity, positive and negative predictive values, and its corresponding confidence interval of 95% for the three ROC curves were used to estimate the prognostic accuracy. The detailed results are shown in Figure 4(b).
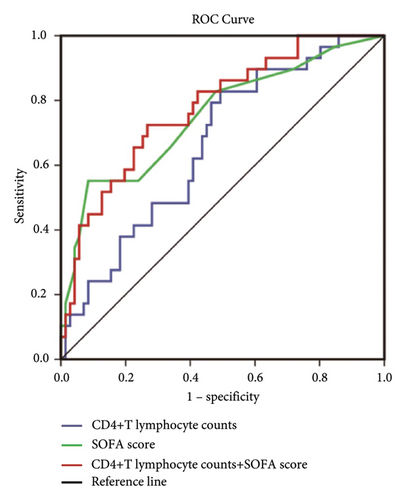
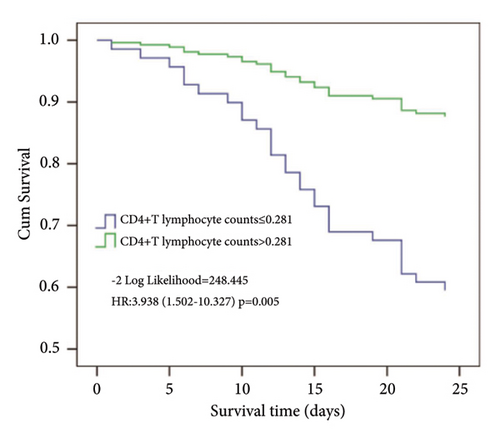
Using cutoff values determined by the ROC curve, patients with the CD4+ T lymphocyte counts lower than 0.281 (×109/L) had a lower probability of survival at day 28 than patients with higher CD4+ T lymphocyte counts (Figure 5).
4. Discussion
Previous studies found that the decrease in peripheral blood lymphocytes in sepsis patients is associated with the severity of the disease and the poor prognosis [11]. Martignoni found that CD4+ T cells may facilitate the early clearance of bacteria by regulating neutrophil function. The decrease of CD4+ T cells indicates the immunosuppression or immune paralysis of the organism [12]. Recently, the criteria for sepsis were updated [2] and the definition of sepsis was changed to a life-threatening organ dysfunction due to a dysregulated host response to infection (Sepsis-3). Changing the criteria may ultimately also affect the performance of prognostic biomarkers. As far as we know, the current study is one of the earliest studies correlating CD4+ T lymphocyte counts to a prognostic value of sepsis patients using the Sepsis-3 criterion.
In the present study, T-cell subgroups were selected as the target to explore their predictive performance on short-term mortality under the new sepsis criteria. We show here that CD4+ T lymphocyte counts and CD8+ T lymphocyte counts were significantly lower in nonsurvivors compared with nonsurvivors. Multivariate logistic regression analysis showed that only CD4+ T lymphocyte counts were independently associated with 28-day mortality in patients. Area under the curve of CD4+ T lymphocyte counts (0.662 and 0.551–0.774) indicated that it is an effective marker for predicting the short-term mortality of sepsis, although its predictive value was not too high.
Many biomarkers showed a relevant correlation with the clinical outcome of patients with sepsis, but their time courses may be more reliable than absolute levels [13]. Clinical studies had found that the immune status of sepsis was constantly changing, which is different from the early stage and the progression of diseases [14]. The present results have shown the dynamics change of CD4+ T lymphocytes on study days 1, 2, 4, and 7 of some patients with sepsis. Interestingly, the results showed that it is significantly associated with an increase in overall and sepsis-attributable mortality rates. As shown in time-serial measurements, CD4+ T lymphocyte counts in survivors were progressively increased. However, in the nonsurvivor group, the counts were increased at the beginning but decreased thereafter. This suggests that with the progression of the disease, the continued decline in CD4+ T lymphocyte counts is indicative of a poor prognosis of sepsis patients.
It is suggested that organ failure should be the core of sepsis. In this connection, SOFA is now widely accepted and is a relatively accurate measure of the severity of the disease [2]. As one of the scoring systems for sepsis patients, the SOFA score was proposed by scholars of ESICM (European Society of Intensive Care Medicine) in Paris in 1994, which has a history of 23 years [15]. Recently, Francesca’s study reported that the SOFA score shows a moderate prognostic stratification ability in sepsis patients [16]. Our study showed a similar result that the area under the curve of the SOFA score for predicting 28-day mortality is 0.752 (0.641–0.863). In comparative analysis of sensitivity and specificity of CD4+ T lymphocyte counts versus SOFA score, we found that the former is more sensitive at 82.76% for the prognosis, while the latter is more specific at 91.55%. More importantly, we have demonstrated that the SOFA score when combined with CD4+ T lymphocyte counts was a better factor for predicting 28-day mortality compared with the SOFA score and CD4+ T lymphocyte counts alone. There are six scoring systems (respiration, coagulation, liver, cardiovascular, central nervous system, and renal) in the assessment of SOFA [14], but the systemic nature and inflammatory response of sepsis involved large number of organs and cell systems [17], which is far more than the 6 items included in the SOFA score. It has been proposed that future iterations of the sepsis definitions should include an updated SOFA score with more optimal variable selection, cutoff values, and weighting, or more superior scoring systems [2]. More and more evidence showed that the function of the immune system played an important role in the occurrence and development of sepsis, and it is closely related to the prognosis of patients [9, 18]. Thus, it can be confidently assumed that the immune system could and should be a part of the improvement of the SOFA score, and CD4+ T lymphocyte counts can be used as an indicator of assessment.
We have further shown that besides CD4+ T lymphocyte counts, shock and heart rate were also the independent risks associated with 28-day mortality. Consistent with our findings, a retrospective study by Hayase et al. reported that tachycardia was a significant and independent predictor of a reduced survival rate in sepsis patients [6]. Recent studies have shown that controlling heart rate can reduce mortality in patients with septic shock and have a significant effect [18]. Furthermore, Jayaprakash et al. found that elevated modified shock index during early sepsis is associated with the development of the SOFA score and mortality [18]. This indicated that cardiac function plays an important role in sepsis, underscoring therefore the needs to obtain the necessary information.
Sepsis is a heterogeneous biological syndrome which is incompletely understood. Many of the established and emerging biomarkers are helpful for sepsis patients [18]. Notwithstanding, we have identified a biomarker that can reflect the immune function, predict the prognosis of sepsis patients, and increase the predictive accuracy of the SOFA score. Mounting data show that, in most ICU patients, sepsis-associated immunosuppression is associated with increased morbidity and mortality [18]. The immunomodulatory therapy of sepsis is one of the focuses of the current research study. Taken together with previous studies [7, 11, 18], we believed that CD4+ T lymphocyte counts can also play an important role in monitoring the therapeutic effect of immune regulation.
However, there were several limitations in this study. Firstly, it was a single-center study and the sample size was relatively small. Secondly, our study enrolled only ICU patients with the diagnosis of sepsis and who had higher acuity than the general population of infectious patients. Thirdly, we did not carry out further research on the cellular function of the remaining CD4+ T lymphocytes. Finally, this study was lacking a healthy control group.
5. Conclusions
In conclusion, our study strongly supports that CD4+ T lymphocyte counts are associated with mortality in sepsis patients. CD4+ T lymphocyte counts in combination with the SOFA score significantly increase the predictive accuracy for 28-day mortality. Furthermore, measurement of CD4+ T lymphocyte counts is a promising independent prognostic marker for sepsis patients.
Abbreviations
-
- APACHE II:
-
- Acute Physiology and Chronic Health Evaluation II
-
- CI:
-
- Confidence interval
-
- GCS:
-
- Glasgow Coma Scale
-
- ICU:
-
- Intensive care unit
-
- Lac:
-
- Lactate
-
- MAP:
-
- Mean arterial pressure
-
- PBMCs:
-
- Peripheral blood mononuclear cells
-
- SOFA:
-
- Sequential Organ Failure Assessment
-
- T:
-
- Temperature
-
- WBC:
-
- White blood cell.
Ethical Approval
The present prospective observational study was conducted with approval from Guangdong Provincial People’s Hospital Ethics Committee (no. GDREC2015374H(R1)).
Disclosure
The funding agents had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
W.J and G.H designed the research. G.H, X.L, C.Z, H.L, Y.Z, C.H, L.X, and H.Z performed the research and collected the data. L.X carried out the flow cytometric analysis. H.Z, Y.X, and W.J analyzed the data. G.H, P.H, and W.J wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We wish to thank Professor Eng-Ang Ling, National University of Singapore, for his assistance in the preparation of this manuscript and Qifei Deng, Sun Yat-sen University, for providing statistical advice. We would like to express our sincere gratitude to Professor Li Xin for the invaluable guidance and support throughout the research process. Special thanks are due to the funding provided by the grant 2023YFE0114300, with Professor Li Xin serving as the principal investigator in receipt of this generous support. Their contribution has been instrumental in the successful completion of this research work. The authors received financial support for the research, authorship, and/or publication of this article from the National Key Research and Development Program intergovernmental key projects (2023YFE0114300), the Natural Science Foundation of Guangdong (2022A1515012428), and the China International Medical Foundation-Clinical Development Research Fund (Z-2018-31-2102-2).
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.




