Late-Onset Maculopapular Eruptions Associated with Elevated Varicella-Zoster Virus Complement-Fixing Antibody Titers following BNT162b2 mRNA COVID-19 Vaccination in Older Adult Japanese Patients: A Cross-Sectional Study
Abstract
Diverse cutaneous adverse reactions associated with the messenger RNA-based BNT162b2 coronavirus disease 2019 (COVID-19) vaccine have been reported, usually developing within 3 weeks after the first vaccination. However, the long-term cutaneous effects of this vaccine remain poorly understood. We hypothesized that the BNT162b2 vaccine might trigger late-onset (>4 weeks after the first dose) maculopapular eruptions associated with elevated varicella-zoster virus (VZV) complement-fixing antibody (VZV-CF Ab) titers indicative of recent subclinical VZV reactivation. Therefore, we conducted a hospital-based cross-sectional study at the Dermatology Department of Kita-Harima Medical Center, Ono City, Japan, between July 1, 2021, and June 30, 2022, to investigate the correlations among the BNT162b2 vaccine, maculopapular eruptions, and VZV-CF Ab titers. Fifteen eligible patients (EPs) who experienced maculopapular eruptions on the trunk and extremities no earlier than 4 weeks after the first BNT162b2 vaccine dose and 12 control patients (CPs) were enrolled. The mean age of EPs and CPs was 73.7 and 77.6 years, respectively, and the median interval between the first BNT162b2 vaccination and onset of maculopapular eruptions was 90 days. The median VZV-CF Ab titer of EPs was significantly higher than that of CPs (×8 vs. ×4). Although people of all ages, except children aged ≤12 years, received BNT162b2 vaccinations, all EPs were aged ≥57 years. All EPs presented between July 2021 and March 2022. There were no EPs from April 2022 to December 2023. These results suggest that the BNT162b2 vaccine triggers elevated VZV-CF Ab titer-associated maculopapular eruptions as late-onset cutaneous adverse reactions in older adults. Furthermore, the observation that two EPs concurrently experienced delayed large local reactions, also known as COVID arm, along with maculopapular eruptions, supported our hypothesis. Our findings may improve the diagnosis of BNT162b2 vaccine-triggered elevated VZV-CF Ab titer-associated late-onset maculopapular eruptions and facilitate further investigation of diverse BNT162b2 vaccine-induced adverse events.
1. Introduction
Messenger RNA (mRNA)-based BNT162b2 vaccine from Pfizer-BioNTech was developed to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Diverse cutaneous adverse reactions associated with the BNT162b2 vaccine have been reported [2–7], including immediate local injection-site reactions, delayed large local reactions (DLLRs), and maculopapular eruptions, most of which develop within 3 weeks after the first vaccination [2, 5, 7]. However, the long-term cutaneous effects of the BNT162b2 vaccine are poorly understood.
The first and second BNT162b2 vaccinations were administered to individuals aged ≥13 years between May and October 2021 in the regions around Kita-Harima Medical Center, Ono City, Japan. However, since July 2021, patients who received the first BNT162b2 vaccine dose more than 4 weeks earlier presented to the center with maculopapular eruptions on the trunk and extremities. In addition, the patients exhibited high varicella-zoster virus (VZV) complement-fixing antibody (VZV-CF Ab) titers. Maculopapular eruptions, also known as morbilliform eruptions or exanthematous eruptions, are widespread, symmetrically distributed skin lesions of pink to red macules and papules that may coalesce to form plaques involving the trunk and some portions of the extremities [8]. These eruptions are typically observed in drug eruptions and viral skin diseases, such as measles. Many cases of BNT162b2 vaccine-induced acute-onset maculopapular eruptions have been reported [2–7]. However, BNT162b2 vaccine-induced late-onset maculopapular eruptions have not been described. We hypothesized that the BNT162b2 vaccine may trigger late-onset (longer than 4 weeks after the first BNT162b2 vaccine dose) maculopapular eruptions associated with elevated VZV-CF Ab titers. Therefore, we conducted a hospital-based cross-sectional study to investigate the hypothesis.
2. Materials and Methods
2.1. Study Design
This cross-sectional study was conducted at the Dermatology Department of Kita-Harima Medical Center, Ono City, Japan, between July 1, 2021, and June 30, 2022, in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [9]. The completed STROBE checklist for cross-sectional studies is provided in Table S1.
2.2. Study Participants
Fifteen eligible patients (EPs) who presented with maculopapular eruptions on the trunk and extremities that developed no earlier than 4 weeks after the first BNT162b2 vaccine dose to the Outpatient Dermatology Department of Kita-Harima Medical Center between July 1, 2021, and June 30, 2022, were enrolled in this study. Among these EPs, the data of 12 were retrospectively examined between July 1, 2021, and February 3, 2022, whereas 3 were prospectively enrolled between February 4, 2022, and June 30, 2022. To rule out the possibility of drug-induced maculopapular eruptions, patients who were prescribed new medications within 6 months before the onset of maculopapular eruptions were excluded. Twelve outpatients who were followed up for unrelated skin diseases that did not contain maculopapular eruptions and were unaffected by the BNT162b2 vaccine were prospectively enrolled between February 4, 2022, and June 30, 2022, as control patients (CPs). All 15 EPs and 12 CPs had received the first and second doses of the BNT162b2 vaccine when they participated in this study. Almost all EPs (13/15) and CPs (10/12) were individuals residing in the regions around Kita-Harima Medical Center. The regions comprised Miki, Ono, and Kato cities, and the population distribution in these regions is shown in Table 1. The vaccination rate in these regions exceeded 74% at the end of 2021.
| Age | Male | Female | Total |
|---|---|---|---|
| 0−14 | 10,200 | 9,643 | 19,843 |
| 15−64 | 47,845 | 44,296 | 92,141 |
| ≥65 | 22,479 | 28,196 | 50,675 |
| Total | 80,524 | 82,135 | 162,659 |
- The regions around Kita-Harima Medical Center, where this study was conducted, comprise Miki, Ono, and Kato cities. Numbers are the sum of the number for the three cities at the end of January 2021.
Age, sex, comorbidities, the interval between the first BNT162b2 vaccination and VZV-CF Ab titer test, VZV-CF Ab titer, and history of herpes zoster (HZ) were recorded for all participants. In addition, the interval between the first BNT162b2 vaccination and the onset of maculopapular eruptions in the 15 EPs, treatment and outcomes of maculopapular eruptions in the 15 EPs, and dermatological disease and treatment during the recruitment of the 12 CPs were recorded.
2.3. Determination of VZV-CF Ab Titers
VZV-CF Ab titers of the EPs and CPs were determined on the days the patients presented with maculopapular eruptions and were enrolled in the study, respectively. VZV-CF Ab titers were determined by a commercial company (SRL, Kobe, Japan) and considered positive and negative at ×8 or higher and ×4 or lower, respectively [10]. The sensitivity, specificity, and quality control management of the methodology for determining VZV-CF Ab titers of the company are as follows: sensitivity: when the CF test was conducted using VZV antigen (Ag) reference solution and VZV-CF Ab-positive reference serum panel, the value of VZV-CF Ab ranged from ×32 to ×64; specificity: when the CF test was conducted using VZV Ag reference solution and VZV-CF Ab-negative reference serum panel, the value of VZV-CF Ab was <×4; and quality control management: daily difference of the VZV-CF Ab titers of control sample was less than ±1 dilution series.
2.4. Statistical Analysis
Sample size estimation for detecting positive VZV-CF Ab titers was performed assuming that the titers were obtained as ×2x using the Poisson distribution P(X = k) = (λke−λ/k!), where k = {0, 1, 2.…} is the number of occurrences and e is Euler’s number, with rate parameter λ of 2.92 from 38 cases and 1.19 from 16 controls in the preliminary data. The Monte Carlo method was used to obtain the same sample size that exceeded the power of 0.8. At least 12 samples were required for each group. The significance level was set at 0.05. We anticipated no dropouts during the study period. Case-control differences in VZV-CF Ab titers were evaluated using the Mann–Whitney test. All p values were two-sided, and statistical significance was set at p < 0.05. All statistical analyses were performed using R. 3.6.3 (https://www.r-project.org/).
2.5. Ethical Statement
This study was approved by the Ethics Review Committee of the Kita-Harima Medical Center (approval number: 03-57). Overall, 3 EPs and 12 CPs prospectively enrolled between February 4, 2022, and June 30, 2022, provided written informed consent. However, the requirement for written informed consent was waived for the 12 EPs whose data were retrospectively evaluated. Opt-out options for the 12 EPs were provided on the homepage of the Kita-Harima Medical Center’s website, allowing them to refuse the use of their data. All study participants provided consent for the use of their photographs.
3. Results
3.1. Baseline Characteristics
Tables 2 and 3 present the baseline characteristics of the 27 study patients, comprising those of the 15 EPs and 12 CPs, respectively. No significant differences were found in age or sex between the EP and CP groups. The mean age of the EPs and CPs was 73.7 (range, 57–90) and 77.6 (range, 55–89) years, respectively (p = 0.365). Nine (60%) and six (40%) patients in the EP group were males and females, respectively. In the CP group, six (50%) patients each were males and females (p = 0.603). The morbidity rates of representative comorbidities according to disease were as follows: hypertension in 33.3% (5/15) of EPs and 58.3% (7/12) of CPs; diabetes mellitus in 20% (3/15) of EPs and 50% (6/12) of CPs; coronary artery disease in 13.3% (2/15) of EPs and 8.3% (1/12) in CPs; oncological diseases in 13.3% (2/15) of EPs and 8.3% (1/12) of CPs; and autoimmune diseases in 20% (3/15) of EPs and 8.3% (1/12) of CPs. Detailed information on the comorbidities of the 15 EPs and 12 CPs is presented in Tables S2 and S3, respectively.
| Patient number | Age (years), sex | Comorbidities | ||||
|---|---|---|---|---|---|---|
| Hypertension | Diabetes mellitus | Coronary artery disease | Oncological disease | Autoimmune disease | ||
| EP1 | 87, M | + | + | − | − | − |
| EP2 | 58, M | − | − | − | − | − |
| EP3 | 81, F | − | − | − | − | + |
| EP4 | 69, F | − | + | − | − | − |
| EP5 | 73, F | − | − | − | − | − |
| EP6 | 61, M | − | − | − | − | − |
| EP7 | 82, M | − | − | − | − | − |
| EP8 | 90, F | + | − | − | − | − |
| EP9 | 73, M | − | − | − | + | − |
| EP10 | 58, M | − | + | − | − | − |
| EP11 | 84, M | + | − | + | − | − |
| EP12 | 71, M | + | − | + | − | − |
| EP13 | 57, M | − | − | − | + | − |
| EP14 | 78, F | − | − | − | − | + |
| EP15 | 84, F | + | − | − | − | + |
- EP, eligible patient; F, female; M, male.
| Patient number | Age (years), sex | Comorbidities | ||||
|---|---|---|---|---|---|---|
| Hypertension | Diabetes mellitus | Coronary artery disease | Oncological disease | Autoimmune disease | ||
| CP1 | 78, F | − | − | − | − | − |
| CP2 | 55, F | + | + | − | − | − |
| CP3 | 86, F | − | + | − | − | − |
| CP4 | 86, M | + | − | − | + | − |
| CP5 | 89, M | + | − | + | − | − |
| CP6 | 85, F | + | + | − | − | − |
| CP7 | 87, M | − | − | − | − | − |
| CP8 | 66, M | + | − | − | − | + |
| CP9 | 77, M | + | + | − | − | − |
| CP10 | 71, M | − | + | − | − | − |
| CP11 | 72, F | − | − | − | − | − |
| CP12 | 79, F | + | + | − | − | − |
- CP, control patient; F, female; M, male.
3.2. VZV-CF Ab Titers
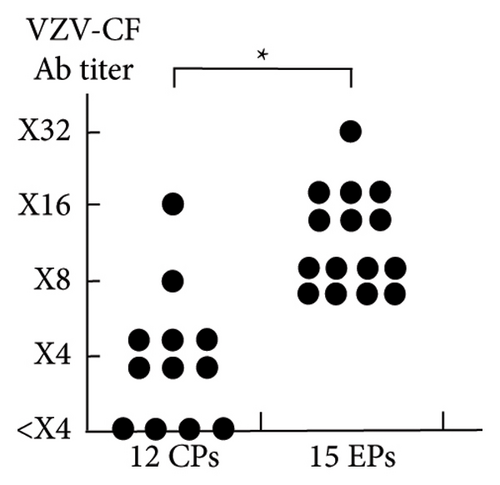
Tables 4 and 5 present the clinical characteristics of the 15 EPs and 12 CPs, respectively. In the 15 EPs, the median interval between the first BNT162b2 vaccination and the onset of maculopapular eruptions was 90 (range, 43–261) days. The median time from the first BNT162b2 vaccination to VZV-CF Ab titer determination was 123 (range, 48–270) and 300 (range, 218–420) days in EPs and CPs, respectively. The median VZV-CF Ab titer in EPs (×8) was significantly higher than that in CPs (×4; p < 0.001; confidence interval of the true location shift: ×4 to ×12: VZV-CF Ab titer below ×4 was considered negative) (Figure 1).
| Patient number | Interval between the first BNT162b2 vaccination and the onset of maculopapular eruptions | Interval between the first BNT162b2 vaccination and VZV-CF Ab titer test | VZV-CF Ab titer | History of herpes zoster | Others |
|---|---|---|---|---|---|
| EP1 | 43 days | 48 days | ×8 | + (10 years before) | |
| EP2 | Approximately 5 weeks | 58 days | ×16 | + (12, 3 years before) | |
| EP3 | Approximately 3 months | 140 days | ×8 | — | |
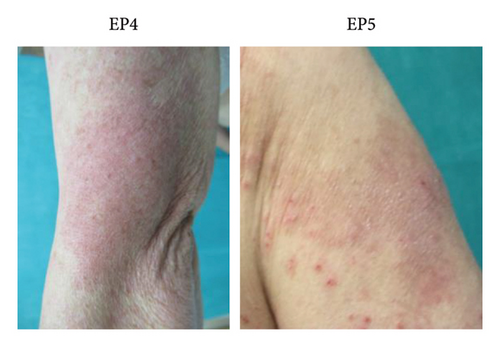
| EP4 | 96 days | 143 days | ×8 | Unknown | The patient had DLLR at the injection site |
| EP5 | 78 days | 82 days | ×16 | — | The patient had DLLR at the injection site |
| EP6 | Approximately 60 days | 68 days | ×8 | — | |
| EP7 | 120 days | 123 days | ×8 | — | |
| EP8 | 118 days | 121 days | ×8 | Unknown | |
| EP9 | Approximately 2.5 months | Approximately 2.5 months | ×16 | Unknown | |
| EP10 | Approximately 5 weeks | 156 days | ×8 | — | |
| EP11 | Approximately 4.5 months | Approximately 5.5 months | ×16 | — | |
| EP12 | Approximately 3 months | 175 days | ×32 | — | |
| EP13 | 69 days | 83 days | ×16 | — | |
| EP14 | 121 days | 140 days | ×16 | — | |
| EP15 | 261 days | 270 days | ×8 | Unknown |
- EP, eligible patient; F, female; M, male; VZV-CF Ab, varicella-zoster virus (VZV) complement-fixing antibody; DLLR, delayed large local reaction.
| Patient number | Interval between the first BNT162b2 vaccination and VZV-CF Ab titer test | VZV-CF Ab titer | History of herpes zoster |
|---|---|---|---|
| CP1 | 251 days | ×4 | + (30 years before) |
| CP2 | Approximately 8 months | <×4 | — |
| CP3 | 218 days | <×4 | — |
| CP4 | 301 days | <×4 | — |
| CP5 | 291 days | ×4 | — |
| CP6 | Approximately 10 months | ×4 | — |
| CP7 | Approximately 10 months | ×4 | + (14 years before) |
| CP8 | Approximately 1 year | ×16 | + (20 years before) |
| CP9 | Approximately 1 year | ×8 | Unknown |
| CP10 | 298 days | ×4 | — |
| CP11 | 373 days | <×4 | — |
| CP12 | Approximately 14 months | ×4 | + (10 years before) |
- CP, control patient; F, female; M, male; VZV-CF Ab, varicella-zoster virus (VZV) complement-fixing antibody.
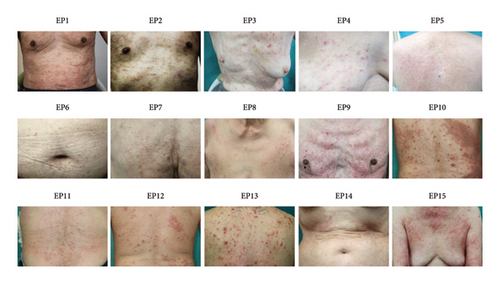
3.3. Eruptions
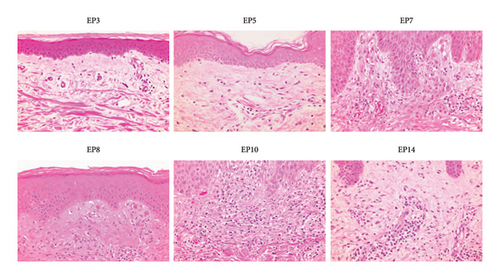
Figure 2 shows the clinical findings of maculopapular eruptions in the 15 EPs. Maculopapular eruptions in all EPs, except in EPs 1, 2, and 9, were pruritic. Skin biopsies were obtained from six maculopapular lesions in six EPs (EPs 3, 5, 7, 8, 10, and 14), and histological analysis revealed mild perivascular lymphocyte infiltration in the upper dermis in all lesions (Figure 3). In addition to maculopapular eruptions, EPs 4 and 5 concurrently experienced late-onset DLLRs at the injection sites (Table 4 and Figure 4) along with late-onset maculopapular eruptions. All EPs, except EPs 3, 10, 11, and 15, were treated with topical corticosteroids and oral antihistamines. These treatments improved their symptoms, except for the symptoms in EPs 6 and 7, albeit with varying healing times (Table S4). The maculopapular eruptions in EP7 were resistant to topical corticosteroid and oral antihistamine therapy but resolved after oral betamethasone/d-chlorpheniramine maleate treatment. The efficacies of topical corticosteroid and oral antihistamine treatment in EP8 and oral prednisolone in EP6 were unknown because these patients were lost to follow-up. EPs 3, 11, and 15 were treated only with topical corticosteroids, and they exhibited a good response to treatment. EP10 was initially unresponsive to topical corticosteroid and oral betamethasone/d-chlorpheniramine maleate treatments but responded to oral prednisolone treatment.
CPs were being treated for various dermatological diseases during study participation (Table S5).
3.4. Transition in the Monthly Number of EPs
The monthly number of EPs during the study period between July 1, 2021, and June 30, 2022, was 1, 1, 4, 2, 1, 3, 0, 1, 2, 0, 0, and 0 in July, August, September, October, November, December, January, February, March, April, May, and June, respectively. There were no EPs from July 2022 to December 2023.
4. Discussion
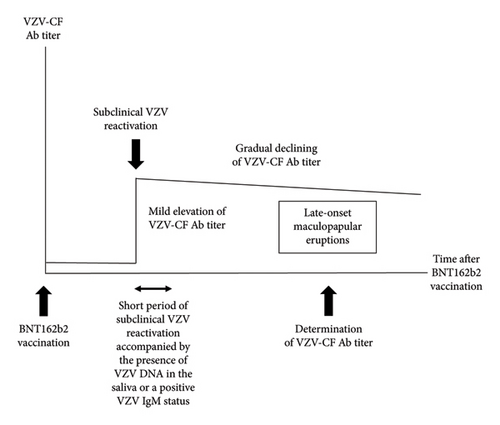
All 15 EPs and 2 of the 12 CPs had positive VZV-CF Ab titers. VZV-CF Ab titers are elevated after the onset of HZ, reaching the levels of ×128–1024 during the acme phase and subsequently experiencing a relatively gradual decline over 1000 days [10–12]. However, none of the participants in our study had experienced HZ in the past 9 years (Tables 4 and 5). Therefore, the elevated VZV-CF Ab titers observed in the participants in this study cannot be attributed to recent HZ. We hypothesized that the BNT162b2 vaccine induces subclinical VZV reactivation accompanied by mildly elevated VZV-CF Ab titers in certain individuals and that some of these individuals develop maculopapular eruptions as late-onset BNT162b2 vaccine-triggered cutaneous adverse reactions (Figure 5). Essentially, subclinical VZV reactivation is defined as the presence of VZV DNA in the saliva or a positive VZV immunoglobulin (Ig)M status without clinical signs or symptoms of HZ [13]. However, the period of reactivation fulfilling this definition may be relatively short. Therefore, a previous subclinical VZV reactivation cannot be confirmed by the presence of VZV DNA or VZV IgM. On the other hand, subclinical VZV reactivation-induced mild elevation of VZV-CF Ab titers lasts for a long time, although it gradually declines. Such elevated VZV-CF Ab titers would be indicative of past as well as current subclinical VZV reactivation. Therefore, we determined VZV-CF Ab titers to investigate whether maculopapular eruptions were associated with recent subclinical VZV reactivation.
Our results suggest that the BNT162b2 vaccine triggers elevated VZV-CF Ab titer-associated maculopapular eruptions as late-onset cutaneous adverse reactions in certain older individuals. Clinicians may have overlooked this condition because it had a delayed onset after the first and second doses of the vaccine compared with previously reported BNT162b2 vaccine-triggered maculopapular eruptions. Furthermore, no methods are available to confirm whether the BNT162b2 vaccine triggers this condition. Therefore, our findings may help facilitate its diagnosis.
In our study, two EPs concurrently experienced late-onset DLLRs at the injection sites along with late-onset maculopapular eruptions. DLLR, also known as COVID arm, is defined as the onset of an erythematous and edematous patch at the site of mRNA-based vaccinations ≥4 days after vaccination and is the most commonly noted cutaneous adverse event [14, 15]. These reactions usually appear without other eruptions within 19 days after vaccination [14], and no case has been reported where DLLRs developed longer than 4 weeks after the first BNT162b2 vaccine dose. Therefore, the development of late-onset maculopapular eruptions in association with DLLRs observed in this study not only strengthens the argument that these maculopapular eruptions are BNT162b2 vaccine-related events but also indicates that DLLRs can develop later than previously believed.
Previous studies have demonstrated that COVID-19 vaccines, including BNT162b2, can trigger HZ by reactivating latent VZV [16–18]. In addition, many studies have reported HZ development due to SARS-CoV-2 infection either during disease progression or after recovery, although a definite relationship between SARS-CoV-2 infection and HZ has not been established [18, 19]. Furthermore, Durson and Temiz [20] and Temiz et al. [21] suggested that SARS-CoV-2 infection and COVID-19 vaccines cause pityriasis rosea by reactivating latent human herpes virus 6 and 7. These studies suggest that SARS-CoV-2 infection and COVID-19 vaccines cause viral skin diseases, such as HZ and pityriasis rosea, by reactivating latent causative viruses. However, it is noteworthy that despite the observed reactivation of latent VZV in the EPs, BNT162b2 vaccine-triggered eruptions in these EPs were not HZ but maculopapular eruptions. Therefore, these findings indicate that VZV is unlikely the direct causative virus of the eruptions.
This study raises the following two critical questions: (1) the mechanism of BNT162b2 vaccine-triggered VZV reactivation and (2) the mechanism of BNT162b2 vaccine-triggered maculopapular eruptions as well as the etiological role of VZV reactivation in the mechanism. Regarding the first question, one possible mechanism is that BNT162b2 vaccine-induced humoral and cellular adaptive immune responses and increased amount of plasma cytokines may cause immune dysregulation, as postulated by Drago et al. [22]. This dysregulation results in the reactivation of latent VZV, particularly through the impairment of VZV-specific CD8+ T cells. The second question is difficult to explain. It has been considered that most drug-induced maculopapular eruptions represent T cell-mediated delayed-hypersensitivity reactions [8]. Plausibly, these reactions are also involved in the mechanism underlying BNT162b2 vaccine-triggered maculopapular eruptions. This notion may be supported by the fact that some EPs experienced maculopapular eruptions concurrently with DLLRs, which are delayed-type hypersensitivity reactions to the spike protein or different components of the vaccine [14, 15]. Cebeci Kahraman et al. [7] identified several risk factors, such as age, sex, and comorbidities, associated with the development of COVID-19 vaccines-induced acute cutaneous adverse reactions (within 20 days of the first dose), including maculopapular eruptions, depending on the vaccine and type of cutaneous adverse reactions. In this study, we did not examine the risk factors associated with BNT162b2 vaccine-triggered late-onset maculopapular eruptions. Notably, although BNT162b2 vaccinations were administered to people of all ages, except children aged ≤12 years, all 15 EPs were aged ≥57 years. This finding suggests that older individuals are already at risk of BNT162b2 vaccine-triggered late-onset maculopapular eruptions. Therefore, we consider that BNT162b2 vaccine-induced immune dysregulation, combined with aging, played a crucial role in the development of BNT162b2 vaccine-triggered elevated VZV-CF Ab titer-associated late-onset maculopapular eruptions. In addition to these critical questions on BNT162b2 vaccine-triggered late-onset maculopapular eruptions described above, several questions on the mechanisms of COVID-19 vaccine-triggered early cutaneous adverse reactions have been proposed [23]. Providing clear answers to these questions will be instrumental in investigating not only BNT162b2 vaccine-induced cutaneous adverse reactions but also the diverse adverse events caused by the vaccine [24–27].
Our study had some limitations. First, because of the nature of cross-sectional studies, causality among BNT162b2 vaccination, maculopapular eruptions, and elevated VZV-CF Ab titers cannot be inferred. Second, the statistical power of our analyses was compromised by the small sample size and not fully meeting the Mann–Whitney U test’s assumption of similarity of distribution between groups. This limitation may affect the robustness and interpretability of our findings. Third, since BNT162b2 vaccination-induced elevated VZV-CF Ab titers undergo a relatively gradual decline over time, the difference in time points for determining VZV-CF Ab titers between EPs and CPs may have caused the discrepancy in VZV-CF Ab titer results between the groups. Finally, our study population might not fully represent the general population since this study was conducted at a single center, and a single dermatologist assessed skin eruptions. Based on these limitations, we consider that it is desirable to conduct a prospective, large-scale study targeting not only individuals planning to receive the BNT162b2 vaccine but also those who did not receive the vaccine to further strengthen our hypothesis that the BNT162b2 vaccine triggers elevated VZV-CF Ab titer-associated maculopapular eruptions as late-onset cutaneous adverse reactions in older adults.
5. Conclusions
Our results suggest that the BNT162b2 vaccine triggers elevated VZV-CF Ab titer-associated maculopapular eruptions as late-onset cutaneous adverse reactions in the older adult population. Therefore, these findings may contribute to the improved diagnosis of this condition and facilitate further investigation of diverse BNT162b2 vaccine-induced adverse events.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors’ Contributions
Masahiro Oka was responsible for conceptualization, study design, data collection, data curation, and manuscript writing. Yosuke Fujii performed statistical analysis. Sae Murakami reviewed and edited the manuscript.
Acknowledgments
The authors thank Editage for their translation services. This study was funded by the Medical Research Fund of Hyogo Medical Association (grant no. MRF-R-4-22).
Open Research
Data Availability
The data that support the findings in this study are available from the corresponding author upon request. The data are not publicly available due to privacy/ethical reasons.