Comparative Analysis of Water and Glycerin Emulsification: Particle Size, Stability, Engine Performance, and Emissions in Biodiesel Fuels
Abstract
Biodiesel has emerged as a promising alternative to conventional diesel fuel, offering potential reductions in greenhouse gas (CO2) emissions. However, its use in diesel engines results in higher levels of nitrogen oxides (NOx). This study investigates emulsification techniques for reducing NOx emissions from biodiesel combustion. Two techniques, glycerin and water emulsification, are examined. Approximately 10 vol. % of crude glycerin is produced during biodiesel manufacturing as a waste or by-product. The study attempts on-site purification of crude glycerin, which is then used as a phase for glycerin-biodiesel emulsions. These emulsions are compared to water emulsions in terms of emulsion stability, mean particle droplet size, microscopic fuel structure, and fuel properties. In addition, engine performance and emissions are evaluated using a small direct injection (DI) diesel engine, with both water and glycerin emulsion fuels. Results show that both emulsion fuels significantly reduce smoke emissions and further mitigate NOx emissions from biodiesel combustion. With 10% glycerin and water emulsions, smoke emissions were reduced by over 50% compared to pure biodiesel, and NOx emissions decreased by more than 15%. Emulsification techniques in the biodiesel industry could offer a viable solution for reducing both smoke and NOx emissions. Employing glycerin emulsification not only decreases NOx emissions but also transforms crude glycerin into a value-added resource. Otherwise, disposal of crude glycerin could pose significant challenges for small and remote biodiesel producers due to cost constraints.
1. Introduction
The need for cleaner and renewable fuels has become increasingly important due to rising pollution levels, climate change, and the depletion of fossil fuels. Biodiesel has emerged as a promising alternative in this search for cleaner fuels [1]. When used in diesel engines, biodiesel combustion can significantly reduce emissions of carbon monoxide (CO), hydrocarbon (HC), and particulate matter (PM) [2], while also exhibiting significantly lower life cycle carbon dioxide (CO2) emissions. In fact, studies have shown that pure biodiesel (B100) can reduce net CO2 emissions by 78.45% compared to petroleum diesel per unit of work delivered by a bus engine, with B20 (20% biodiesel + 80% diesel) blends achieving a 15.66% reduction in life-cycle CO2 emissions [3]. However, it is important to note that biodiesel tends to increase NOx emissions compared to diesel fuel [2].
Previous research by Zhang et al. [4] on a marine diesel engine demonstrated that biodiesel’s oxygen content reduces HC and CO emissions but increases NOx emissions compared to pure diesel. Similarly, Nabi et al. [5] found that biodiesel blends at various concentrations led to decreased CO, HC, and PM emissions but increased NOx emissions. Roy et al. [6] conducted experiments on a direct injection (DI) diesel engine using different biodiesel blends and observed an increase in brake-specific fuel consumption (BSFC) and NOx emissions with higher biodiesel content, while CO and HC emissions decreased. Qi et al. [7] investigated the impact of biodiesel on combustion, performance, and emissions in a diesel engine, revealing that the lower heating value of biodiesel increased BSFC but resulted in reduced CO, HC, NOx, and smoke emissions under full load. In addition, Hasan and Rahman [8] examined the engine performance and emission characteristics of biodiesel-diesel blends in compression ignition (CI) engines, finding that biodiesel-diesel blends exhibited shorter ignition delays than diesel, reduced HC, CO, and PM emissions, but increased NOx emissions. In a recent publication by Rajpoot et al. [9], the investigation involved the use of a nanoadditive, carbon nanotube (CNT), in small amounts (100 ppm) with three different biodiesels in a diesel engine. The findings revealed a slight increase in brake thermal efficiency (BTE), a decrease in smoke by approximately 6–13% but an increase in NOx by 14–18%.
Emulsions, which consist of two immiscible liquids stabilized by an emulsifying agent, have gained recognition as a promising method for fuel upgrading [10–12]. Emulsifiers play a crucial role in creating stable emulsion fuels by reducing the surface tension between oil and water [13, 14]. The stability of an emulsion relies on the hydrophilic-lipophilic balance (HLB) of the emulsifier, which determines its degree of affinity to water or oil [15]. HLB values range from 1 to 20, with lower values (1 to below 10) indicating a preference for oil and creating a stable water-in-oil emulsion, while higher values (11–20) favor water and result in a stable oil-in-water emulsion [16, 17]. Emulsions can be categorized based on particle size as microemulsions, macroemulsions, and nanoemulsions [18]. In terms of phases, emulsions can be classified as two-phase (water-in-oil and oil-in-water) or three-phase (oil-in-water-in-oil and water-in-oil-in-water) [19]. Two-phase emulsions are commonly used in the production of combustion fuels due to their smaller droplet size and higher heating value, while three-phase emulsions find applications in the food, medicine, and cosmetics industries [19, 20]. Various methods, such as electronic, mechanical, ultrasonic, and magnetic forces, can be employed to prepare emulsions [21, 22].
Despite biodiesel typically producing around 10% more NOx emissions than diesel fuel [2], the use of emulsification techniques can help reduce NOx emissions [23]. Several studies have investigated the impact of emulsions on NOx emissions. Debnath et al. [24] explored the role of water-diesel emulsion in reducing emissions and found that it increased engine brake power and brake-specific fuel consumption (BSFC) while aiding in the reduction of NOx, smoke, and hydrocarbon (HC) emissions. Attia and Kulchitskiy [25] examined the performance of water-in-diesel fuel emulsion and observed that larger water droplets led to reduced NOx emissions, while finer droplets reduced smoke, HC, and improved engine efficiency. Alahmer et al. [26] studied the performance of an engine using pure diesel and emulsified fuel and discovered that an increase in water content resulted in increased BSFC at high engine speeds, while brake thermal efficiency (BTE), exhaust gas temperature, and NOx decreased due to the higher water content. Kumar and Jaikumar [27] investigated the emissions of waste cooking oil obtained from palm oil (WCO) and its emulsion fuel in a diesel engine, finding that the WCO emulsion significantly reduced smoke, HC, and CO compared to neat WCO and neat diesel. Zaid et al. [28] tested water-in-diesel emulsion on a diesel engine to evaluate the effects of water emulsification on exhaust gas temperature and engine performance and concluded that an increase in water content resulted in increased BSFC and BTE, while decreasing exhaust gas temperature (EGT).
Lin et al. [23] conducted research on biodiesel emulsions and investigated their emulsification and fuel properties. They found that an emulsifier with an HLB of 13 exhibited the highest emulsification stability, and neat biodiesel had lower kinematic viscosity and carbon residue compared to biodiesel emulsions. Furthermore, they observed that two-phase biodiesel emulsions had smaller mean droplet sizes compared to three-phase biodiesel emulsions.
Crude glycerin, a by-product of biodiesel production, has been explored as a potential alternative fuel [29, 30]. Its utilization in electricity and heat generation in boilers or furnaces can lead to reduced emissions and improved sustainability in biodiesel production [31]. Ayoub and Abdulla [32] studied the formation and utilization of crude glycerin and proposed its use as an additive in various fuel formulations. Bohon et al. [33] investigated the combustion properties and emissions of glycerin in a prototype refractory burner and furnace, observing complete glycerin combustion. Roy and Da Silva [34] examined the use of crude glycerin as an alternative fuel in the Canadian wood pellet industry and found reduced CO2 and NOx emissions when glycerin-soaked pellets were burned compared to pure wood pellets. Noureddini et al. [35] explored glycerin ethers as compatible additives for diesel and biodiesel fuels, noting a reduction in cloud point and viscosity. The transportation sector also holds potential for glycerin as a renewable and biodegradable fuel, which can reduce fossil fuel dependency and transportation costs [36]. Researchers are actively investigating glycerin’s viability as an alternative fuel. Oprescu et al. [37] studied glycerin derivatives as additives in a diesel engine, with methyl hexanoate showing promise in reducing HC, CO, and smoke emissions but slightly increasing NOx emissions. Beatrice et al. [38] examined glycerin-derived ethers’ effects on a CI engine and observed a decrease in PM emissions but a slight increase in NOx emissions. Steinmetz et al. [39] analyzed the combustion of crude glycerin and identified comparable volatile organic compound emissions to natural gas combustion. Eaton et al. [40] investigated glycerin-diesel emulsions and their impact on stability, combustion, and emissions, finding that glycerin addition reduced NOx and PM emissions while increasing brake thermal efficiency and brake-specific fuel consumption. Despite some studies on glycerin emulsions with diesel, few studies have investigated glycerin emulsions with biodiesel and their effects on diesel engine performance and emissions [41]. In addition, microscopic studies on biodiesel blend structure and particle size distribution are limited. Recently, Raman and Roy [42] conducted a microscopic study on EPS-infused biodiesel with DEE.
In this study, a clear research gap in biodiesel emulsification is addressed. Not only is regular water emulsification explored but also glycerin emulsification is introduced as a novel approach. The authors compare the droplet size distribution, microscopic structure, stability, and fuel properties of glycerin-emulsified diesel-biodiesel blends with those of water-emulsified diesel-biodiesel blends. Furthermore, the potential for NOx reduction through biodiesel-emulsification processes is evaluated. In addition, other emissions and the engine’s thermal efficiency using different blends of diesel-biodiesel and their glycerin and water emulsions are studied. This research aims to provide insights into the properties and performance of glycerin-emulsified biodiesel blends and to compare them with water-emulsified biodiesel blends.
2. Methods and Procedure
2.1. Transesterification
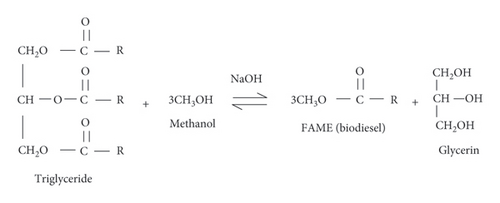
Transesterification is a vital process in biodiesel production, widely adopted for its efficiency and cost-effectiveness in the industry [43, 44]. This method involves the combination of fat or oil with alcohol and a catalyst to produce biodiesel, along with crude glycerin as a by-product. Methanol is commonly used to produce biodiesel due to its rapid reaction rate and affordability, making the process straightforward and accessible [45, 46]. Figure 1 illustrates the transesterification reaction, where triglycerides react with methanol in the presence of a catalyst, such as sodium hydroxide, resulting in the production of biodiesel, specifically fatty acid methyl ester (FAME), and glycerin.
2.2. Biodiesel Production Process
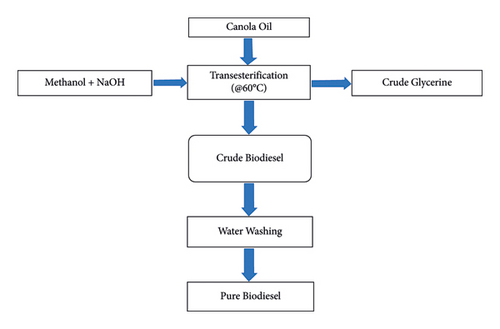
The biodiesel production process followed in this study is based on the procedure outlined in [47]. Canola oil was used as the feedstock, obtained from a local store, and the necessary chemicals were sourced from the chemistry lab at Lakehead University. The catalyst (3.5 grams) was dissolved in methanol (200 ml) through vigorous stirring in a small flask. A magnetic ceramic stirring hot plate provided by Thermo Scientific, Model No. SP 131325, was used for heating and stirring. Once the catalyst was fully dissolved in the methanol, the methoxide solution was prepared and ready to be added to the oil. Preheating the oil prior to mixing can accelerate the reaction rate, thereby reducing the reaction time. The oil was heated to a temperature a little below the boiling point of methanol (64.5°C). One litre heated canola oil (at 60°C) was transferred to the biodiesel batch reactor, followed by the addition of the catalyst/alcohol mixture to the oil. The resulting mixture was vigorously stirred for 30 minutes using a blender under ambient pressure. Successful transesterification led to the formation of two liquid phases: fatty acid methyl ester (FAME), which is the biodiesel, and crude glycerin. Crude glycerin, being the denser liquid, settled at the bottom after several hours. Phase separation typically occurred within 10 minutes and could take up to 2 hours to complete. Full settling of the phases might require up to 20 hours. After 24 hours of settling, the crude biodiesel was separated from the glycerin layer. However, the crude biodiesel contained residual catalyst, water, unreacted alcohol, free glycerin, and soaps generated during the transesterification process. Purification of biodiesel is commonly achieved through three main methods: water washing, dry washing, and membrane extraction. Since both glycerol and alcohol are highly soluble in water, water washing is an effective approach for removing contaminants, including residual sodium salts and soaps. In this study, water washing (performed twice) was employed to eliminate impurities from the crude biodiesel. After washing, the finished biodiesel was obtained, with a biodiesel collection efficiency of approximately 90% of the initial oil used. Figure 2 depicts the steps involved in biodiesel production. The quality of the biodiesel was tested according to industry standard (ASTM D6751), which is outlined in Table 1.
| Test names | Test method | ASTM limit | Results |
|---|---|---|---|
| Free glycerine (mass %) | ASTM D6584 | Max. 0.02 | 0 |
| Total glycerine (mass %) | ASTM D6584 | Max. 0.24 | 0.112 |
| Flash point, closed cup (°C) | ASTM D93 | Min. 130 | 169 |
| Water and sediment (vol. %) | ASTM D2709 | Max. 0.050 | 0 |
| TAN (mg KOH/g) | ASTM D664 | Max. 0.5 | 0.14 |
| Sim. Dist, 50% recovery (°C) | AST M D2887 | N/A | 359.8 |
| Cetane index | ASTM D976 (2 variable formula) | N/A | 50 |
| Copper corrosion 3 h @ 50°C (rating) | ASTM D130 | Max. 3a | 1a |
2.3. Crude Glycerin
During the biodiesel production process, it was observed that approximately one-tenth of the total production consists of crude glycerin, which contains impurities such as salts, water, and residual alcohol. Due to the high cost associated with purification, crude glycerin is often utilized in its crude form for various applications, including anaerobic digestion and composting [40, 48, 49]. However, crude glycerin possesses a low autoignition quality and heating value, and its combustion can generate carcinogenic acrolein and lead to ash-related issues. This highlights the necessity for the purification of crude glycerin to improve its quality and ensure its safe utilization.
2.4. Crude Glycerin Purification
In order to prepare the emulsion fuel, purified glycerin was used instead of crude glycerin, which contains impurities such as salts, water, and alkaline catalysts that can destabilize the biodiesel glycerin emulsion within a short period of time [50]. Purification of crude glycerin helps to reduce ash-related issues [51]. The purification process involved acidification and desalting which is described in [41]. Figure 3 is a flowchart of crude glycerin purification.
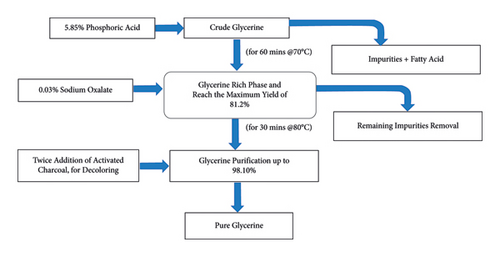
The process was started with a 5.85% phosphoric acid (H3PO4) solution in addition to the crude glycerin until the pH reached approximately 5-6. The solution was kept at 70°C for 60 minutes to obtain the phase separation of the impurities from the glycerin-rich phase, resulting in a yield of 81.2%. For further removal of the residual impurities from the semiglycerin product, the reaction of glycerin with 0.03% sodium oxalate solution at 80°C was carried out for 30 minutes. The purified glycerin (98.10%) was obtained through distillation, with the temperature maintained between 164°C and 200°C. Decolorization was carried out using 2% activated charcoal at 80°C, and the process was repeated twice. This purification process was also conducted in [52] and is known as the orthogonal test method.
2.5. Emulsion Fuel Preparation
A two-phase emulsion was preferred for the production of the emulsion fuel.
2.6. Particle Size Distribution and Microscopic Fuel Structure
The particle size distribution experiment was conducted using a Malvern Mastersizer 2000 instrument, which utilizes a laser diffraction technique. The fuel sample was mixed with distilled water and placed in a glass container. A medium with the appropriate refractive index was selected for the experiment. The mixture was stirred and then pumped into the particle size measurement equipment using the Hydro 2000 S system designed for liquid samples. The measurement, interpretation, and representation of data were carried out using the software provided by the equipment manufacturer. The Mastersizer software generated particle size distribution graphs. The system’s measurement range spanned from 0.2 to 2000 μm. Figure 4 illustrates the particle size measuring setup.

The fuel structure was examined at a resolution of 10× using a Polaris IX 51 inverted microscope, and digital images were captured using software. Figure 5 displays the Polaris microscope utilized in this study. The microscopic analysis provided insights into the changes in the fuel structure resulting from the addition of water and glycerin. The results of the particle size distribution and microscopic fuel structure analysis are presented in the forthcoming “Results and Discussion” section.

3. Results and Discussion
3.1. Emulsion Fuel Droplet Size Distribution and Microscopic Structure
A total of eighteen diesel-biodiesel emulsion blends were tested, prepared with varying concentrations of 5% and 10% glycerin and water. Figures 6 and 7 are sourced from [41], and they show particle size distribution and microscopic fuel structure of the glycerin emulsion fuels and water emulsion fuels, respectively. Both glycerin and water emulsion fuel samples exhibited a bimodal distribution in particle size, indicating the presence of two distinct droplet sizes. The bimodal distribution suggests that heavier oil emulsions tend to have larger droplet sizes [54]. The graphs also revealed the presence of smaller droplets below 5 µm in diameter, which can be attributed to shear stress causing droplet fragmentation and subsequent oil-water phase separation. Figure 6(a) illustrates that the droplet size increased with an increase in biodiesel percentage, and with the increase in glycerin concentration in the emulsion fuels, the reason of which can be attributed to the fact that the addition of the heavier particle will result in enlarging of the droplet size [40]. A similar trend was also observed for water emulsion fuels, as depicted in Figure 7(a), which shows particle size distribution of the water emulsion fuels. The trend observed for the emulsion fuels indicates that higher levels of glycerin and water, as well as heavier oil molecules, lead to larger droplet sizes. The mean particle size distributions for B0W5%, B0W10%, B0G5%, and B0G10% were measured to be 4.3 μm, 6.3 μm, 4.6 μm, and 6.8 μm, respectively. For B20, B50, and B100 emulsion fuels, the particle size distributions ranged from 0.45 μm to 50 μm, as observed in Figures 6(a) and 7(a). Emulsion fuels with lower water and biodiesel concentrations exhibited smaller mean particle sizes, with a higher proportion of smaller particles ranging between 0.45 μm and 6 μm.
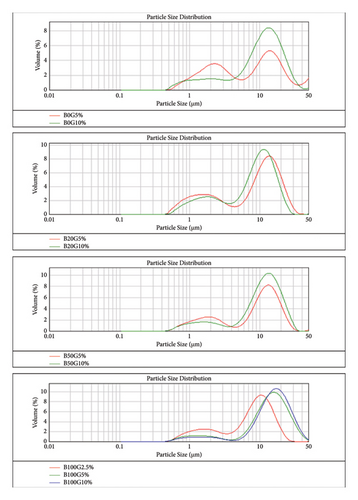

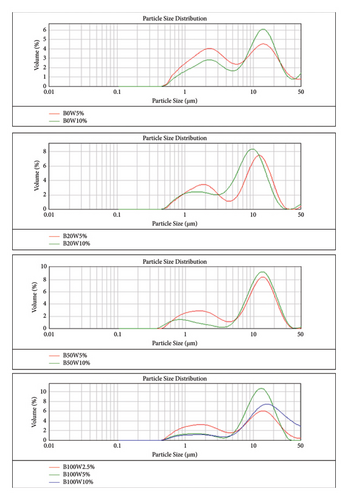

The photographs of glycerin and water emulsion fuels, as shown in Figures 6(b) and 7(b), visually confirm the presence of droplets of different sizes within the emulsions, similar to that in [55]. The application of shear force during emulsion preparation resulted in a reduction in droplet size, while an increase in water concentration led to the formation of larger droplets. The droplet size increased continuously with an increase in biodiesel percentage, as well as with an increase in glycerin and water concentrations in this study. The droplet size of glycerin particles was observed to be larger than that of water particles. Consequently, the stability of glycerin emulsions was found to be lower than that of water emulsions, as indicated by the distinct and smaller droplets in the size distribution of water emulsions, which are indicative of better emulsion stability according to [56]. Coalescence was also observed in the photographs of emulsions with higher concentrations of glycerin and water, leading to the formation of larger droplets with increased average diameters. Coalescence occurs due to weak repulsive forces between droplets, resulting in droplet collision, reduction of the interfacial film separating them, and eventually merging into larger droplets [57].
3.2. Emulsion Fuel Stability
Emulsion stability refers to the ability of emulsions to maintain their emulsifying layer over time when kept stationary at room temperature (25°C). Several factors influence emulsion stability, including stirring rpm, surfactant type and concentration, techniques employed, and viscosity of the continuous phase. In this study, two emulsifiers (Tween 80 and Span 80) were utilized to achieve the desired stability. It was observed that the stability of emulsions decreased as the concentrations of biodiesel, water, and glycerin increased, resulting in larger droplet sizes. Among the tested samples of glycerin emulsions, the emulsion with a 5% glycerin concentration (B0G 5%) exhibited the highest stability, maintaining its emulsifying layer for up to 70 hours. For water emulsions, the greatest stability was observed in B0W 5%, which retained its emulsion state for up to 58 days. However, the stability of B50 and B100 emulsions with 10% glycerin and water concentrations was considerably lower, possibly due to coalescence. Table 2 provides an overview of the fuel samples used in the testing, including their fuel properties. The labels B, W, and G represent biodiesel, water, and glycerin, respectively. The table also presents the stability and mean droplet diameter of the water- and glycerin-emulsified fuels. The best stability among diesel-biodiesel blend emulsions is obtained with B20W5, which remains stable for 46 days.
| Fuel | Heating value (kJ/kg) | Density (kg/m3) | Viscosity (cSt) | ||
|---|---|---|---|---|---|
| B0 (100% diesel) | 45,124 | 838 | 1.87 | ||
| B20 (20% biodiesel + 80% diesel) | 44,219 | 845 | 2.29 | ||
| B50 (50% biodiesel + 50% diesel) | 42,860 | 856 | 2.87 | ||
| B100 (100% biodiesel) | 40,597 | 875 | 4.23 | ||
| Water-emulsified fuels | Heating value (kJ/kg) | Density (kg/m3) | Viscosity (cSt) | Stability (days/hrs) | Mean diameter (μm) |
| B0W 5% (5% water in diesel) | 41,965 | 850 | 2.22 | 58 days | 4.3 |
| B0W 10% (10% water in diesel) | 39,709 | 856 | 2.59 | 43 days | 6.3 |
| B20W 5% (5% water in B20) | 41,123 | 857 | 2.62 | 46 days | 7.9 |
| B20W 10% (10% water in B20) | 38,912 | 864 | 3.1 | 37 days | 9 |
| B50W 5% (5% water in B50) | 39,860 | 867 | 3.63 | 73 hrs | 11.2 |
| B50W 10% (10% water in B50) | 37,717 | 874 | 4.14 | 44 hrs | 13.6 |
| B100W 2.5% (2.5% water in B100) | 38,770 | 881 | 4.69 | 23 hrs | 9 |
| B100W 5% (5% water in B100) | 37,755 | 884 | 4.81 | 20 hrs | 13.3 |
| B100W 10% (10% water in B100) | 35,725 | 890 | 5.66 | 11.5 hrs | 15.5 |
| Glycerin-emulsified fuels | Heating value (kJ/kg) | Density (kg/m3) | Viscosity (cSt) | Stability (hrs) | Mean diameter (μm) |
| B0G 5% (5% glycerin in diesel) | 42,948 | 858 | 2.43 | 70 | 4.6 |
| B0G 10% (10% glycerin in diesel) | 41,675 | 874 | 2.71 | 58 | 6.8 |
| B20G 5% (5% glycerin in B20) | 42,106 | 864 | 3.01 | 59 | 8.1 |
| B20G 10% (10% glycerin in B20) | 40,878 | 880 | 3.33 | 41 | 10.1 |
| B50G 5% (5% glycerin in B50) | 40,843 | 875 | 3.77 | 29 | 12.4 |
| B50G 10% (10% glycerin in B50) | 39,683 | 890 | 4.45 | 25 | 16.1 |
| B100G2.5% (2.5% glycerin in B100) | 39,262 | 886 | 4.84 | 26.5 | 9.5 |
| B100G 5% (5% glycerin in B100) | 38,738 | 893 | 5.22 | 18 | 16.2 |
| B100G 10% (10% glycerin in B100) | 37,691 | 907 | 5.86 | 11 | 19.2 |
3.3. Engine Experimental Setup
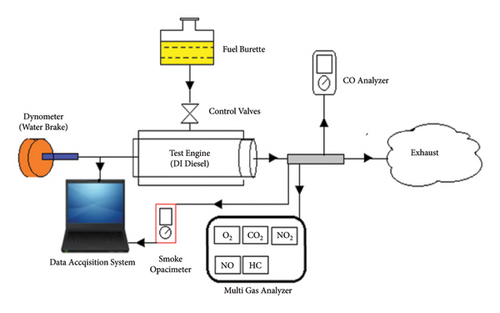
The engine experimental setup is depicted in Figure 8. The experiment employed a light-duty, DI diesel engine. The specifications of the engine are presented in Table 3. Load tests were conducted using a water dynamometer and Dyno 2010 software. The engine experiments were carried out at 3000 rpm under full load conditions. The Dyno 2010 software enabled the measurement of key parameters, including torque, brake power, and speed. To detect regulated emissions such as CO, NOx, and HC, specific equipment was utilized. NOx and HC were measured using a Nova Gas 7466 K analyzer, while CO levels were detected using a Dwyer 1205A instrument. Smoke opacity was determined using a Smart 2000 smoke opacimeter connected to a computer. The technical specifications and accuracies of the emission measurement devices are detailed in Table 4.
| Engine make and model | Hatz 2G40 |
|---|---|
| Engine type | Four-stroke, air-cooled |
| Number of cylinders | 2 |
| Bore/stroke | 92 mm/75 mm |
| Displacement | 997 cc |
| Compression ratio | 20.5 : 1 |
| Fuel injection timing | 8°BTDC (≤2250 rpm); 10°BTDC (≥2300 rpm) |
| Fuel injection pressure | 26 MPa |
| Continuous max. rated power | 13.7 kW @ 3000 rpm |
| Maximum rated power | 17 kW @ 3600 rpm |
| Method of detection | Species | Measured unit | Range | Resolution | Accuracy (%) |
|---|---|---|---|---|---|
| Nova gas 7466 PK | |||||
| Infrared detector | CO2 | % | 0–20% | 0.10% | ±1 |
| Electro chemical | NO | ppm | 0–5000 ppm | 1 ppm | ±1 |
| Electro chemical | NO2 | ppm | 0–800 ppm | 1 ppm | ±1 |
| Electro chemical | O2 | % | 0–25% | 0.10% | ±1 |
| Infrared detector | HC | ppm x 10 | 0–20000 ppm | 10 ppm | ±1 |
| Dwyer 1205 A electrochemical | CO | ppm | 0–2000 ppm | 1 ppm | ±5 |
| Smart 2000 | Opacity | % | 0–100% | 0.10% | ±0.5 |
| Extech EA10 | Temperature | 0.1°C | (−)200°C–1360°C | 0.1°C | ±0.3 |
Many instrumental, physical, and human limitations can lead to measurement errors. Replicating experiments multiple times, performing error analysis, and calibrating instruments can significantly reduce these errors. For this research, we used up-to-date instrument calibration, repeated experiments, and error measurement techniques. The experimental error or uncertainty is best described by the standard deviation or standard error of the dataset. In this study, the error is presented using the standard error. For each test case, data were collected from three repetitions, and the results are displayed graphically with the ± standard error of the average value.
3.4. Engine Performance and Emissions
Performance and emission tests were conducted on a small DI diesel engine. The engine used was an air-cooled, 2-cylinder, 4-stroke engine. Figure 9 illustrates the BSFC of water- and glycerin-emulsified diesel-biodiesel blends. All blends exhibited higher BSFC values compared to B0. Furthermore, water-emulsified diesel-biodiesel blends demonstrated higher BSFC values primarily due to their lower heat content than glycerin-emulsified blends. Among the blends, the B100W10 blend exhibited the highest BSFC, which was approximately 6% higher than that of B0.

Figure 10 displays the brake thermal efficiency (BTE) of different blends. All blends demonstrated higher BTE values than B0. Blends with higher biodiesel content exhibited higher BTE values due to improved combustion resulting from the increased oxygen content. Water-emulsified blends showcased higher BTE values compared to glycerin-emulsified blends. This increased BTE with water emulsions can be attributed to the microexplosion of water-emulsified fuel, which causes better air-fuel mixing. More importantly, the evaporation phenomenon of water during combustion contributes an additional enhancing effect on air-fuel mixing in water-emulsified fuels, thereby improving combustion efficiency. The highest BTE achieved in this study was observed in the B100W10 blend, which exhibited a BTE approximately 6 points higher than B0. The B100G10 blend demonstrated approximately 4 points higher efficiency than B0. Among diesel-biodiesel blend emulsions, B50W10 exhibited the highest BTE, which is about 4 points higher than that of B50.
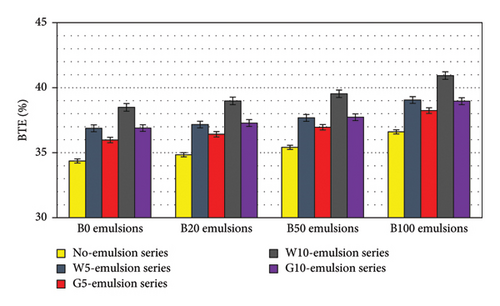
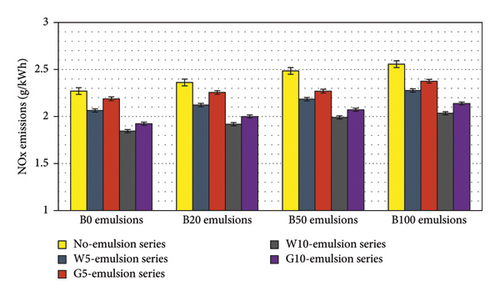
Figure 11 presents a comparison of NOx emissions between water- and glycerin-emulsified fuel blends. The results indicate that the addition of biodiesel in the blends leads to an increase in NOx emissions, with B100 exhibiting approximately 13% higher NOx compared to B0. However, the incorporation of water and glycerin in diesel-biodiesel blends results in a reduction of NOx emissions. Specifically, the addition of 10% water in B100 leads to a reduction of NOx emissions by approximately 20% compared to B100, and in fact, B100W10 demonstrates even lower NOx emissions than B0. Similarly, B100G10 also exhibits lower NOx emissions than pure diesel. It is worth noting that the in-cylinder temperature significantly impacts NOx emissions, with lower temperatures leading to reduced NOx emissions. Among diesel-biodiesel blend emulsions, B20W10 showed the lowest NOx emissions, which were more than 20% lower than those of B20.
Measurements of exhaust gas temperature (not shown here) reveal that the addition of 10% glycerin and 10% water in B100 compared to pure biodiesel resulted in a temperature reduction of approximately 5.8–7.3%. The higher end of the range corresponds to water-emulsified B100. Furthermore, the temperature reduction with 10% glycerin- emulsified and 10% water-emulsified diesel blends compared to pure diesel was approximately 4.4–6.5%. Notably, the water emulsions exhibited slightly higher temperature reduction and NOx reduction compared to glycerin emulsions.
The results depicted in Figure 12 illustrate the CO emissions for blends of diesel and biodiesel emulsified with water and glycerin. The figure clearly indicates that as the biodiesel content increases in the blends, the CO emissions decrease. This reduction can be attributed to the higher oxygen content present in the diesel-biodiesel blends, which enhances engine efficiency, as demonstrated in Figure 10. In particular, the use of B100 resulted in an approximate 20% reduction in CO emissions compared to pure diesel fuel. However, when 5% and 10% water and glycerin were added, CO emissions for all emulsion fuels increased due to the lower in-cylinder temperatures. Interestingly, glycerin emulsions exhibited a greater reduction in CO emissions compared to water emulsions, despite having lower exhaust gas temperatures. This can be attributed to the higher oxygen content present in glycerin-emulsified fuels. Nonetheless, even with the addition of 5% water and glycerin to B100, lower CO emissions were still observed compared to pure diesel fuel. Specifically, B100G5 demonstrated a 7% reduction in CO emissions relative to diesel fuel.
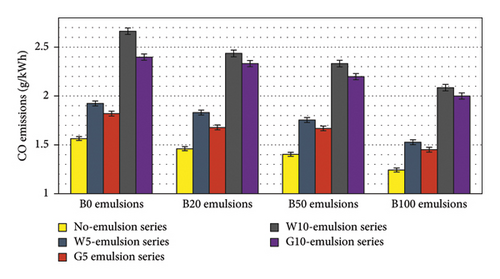
Figure 13 showcases the HC emissions of water- and glycerin-emulsified diesel-biodiesel blends. The results indicate a significant reduction in HC emissions as the biodiesel concentration in the blends increases. This reduction can be attributed to the higher oxygen content and in-cylinder temperature of the blends, which promote more complete combustion. Remarkably, B100 exhibits a notable 55% reduction in HC emissions compared to pure diesel fuel. However, it is worth noting that emulsified fuels demonstrate higher HC emissions, with water emulsions exhibiting lower HC emissions compared to glycerin emulsions. This difference may be attributed to the presence of the hydrocarbon chain in glycerin (C3H8O3), which contributes to increased HC formation. Nevertheless, it is important to highlight that a 5% water and glycerin emulsion with B100 resulted in an approximate 22% reduction in HC emissions compared to pure diesel fuel.
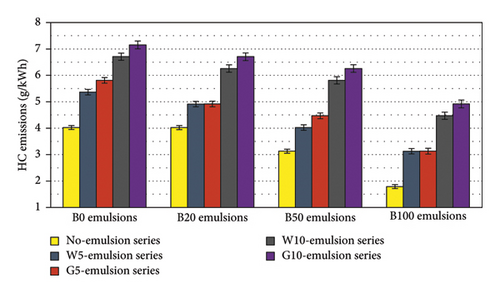
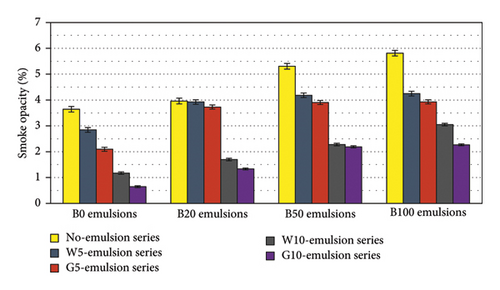
Figure 14 depicts the smoke opacity levels of diesel-biodiesel blends that have been emulsified with either water or glycerin. As the concentration of biodiesel increases in the blends, there is a corresponding increase in smoke opacity in the small research engine. These findings align with the results of a previous study [58], suggesting that the elevated viscosity of biodiesel and its tendency to adhere to engine walls contribute to this outcome. To address smoke opacity issues when utilizing biodiesel in small engines, it is advisable to optimize combustion chamber geometry, such as incorporating a deeper bowl on the piston, and adjust injection parameters, such as employing a higher number of sprays and wider injection angles between the sprays. However, the introduction of 10% water or glycerin emulsion leads to a significant reduction in smoke opacity. Water emulsion fuels exhibit reductions of over 50%, while glycerin emulsion fuels achieve reductions exceeding 60%. The use of glycerin emulsion proves more effective in minimizing smoke due to its high oxygen content of approximately 52%. Remarkably, the B100G10 blend demonstrates a 40% reduction in smoke opacity compared to pure diesel, while the B100W10 blend exhibits a 16% reduction. Among diesel-biodiesel blend emulsions, B20G10 emitted the least smoke, achieving a reduction of more than 60% compared to B20. Consequently, the application of water or glycerin emulsion shows promise as a technique to enhance engine efficiency and mitigate smoke emissions.
4. Conclusions
- (1)
Glycerin-emulsified blends exhibited higher heating values, density, viscosity, and mean particle diameter compared to water-emulsified blends. However, water emulsions showed greater stability than glycerin emulsions.
- (2)
Emulsion fuels, particularly those with higher water and glycerin content, improved the engine’s brake thermal efficiency (BTE). Notably, a 10% water emulsion in B100 fuel demonstrated the highest BTE, with a significant increase compared to pure diesel (B0).
- (3)
Emulsion fuels with 10% water or glycerin content significantly reduced smoke opacity, with emissions reduced by 50–60% compared to pure B100 fuel.
- (4)
B100 blends exhibited higher NOx emissions than B0, but the addition of 10% water or glycerin emulsion effectively reduced NOx emissions. Water emulsions demonstrated greater NOx reduction compared to glycerin emulsions.
- (5)
B100 blends showed a substantial reduction in hydrocarbon (HC) emissions, approximately 55% lower than pure diesel. Emulsified fuels exhibited higher HC emissions, with water emulsions showing lower levels compared to glycerin emulsions. However, even with 5% water or glycerin emulsion in pure biodiesel, lower HC emissions were observed compared to pure diesel.
- (6)
The addition of biodiesel in the blends resulted in lower carbon monoxide (CO) emissions. Water and glycerin emulsions led to higher CO emissions compared to nonemulsified fuels. However, blends with 5% water or glycerin in pure biodiesel exhibited lower CO emissions than pure diesel.
As a recommendation, more research is needed to increase the stability of emulsified fuels, especially glycerin-emulsified biodiesel fuels. Furthermore, the crude glycerin purification method needs improvement to save both money and time. In addition, more research should focus on glycerin-emulsified biodiesel to further validate reduced NOx emissions and improved engine efficiency in diesel engines. Lastly, EGR can be tested with glycerin-emulsified biodiesels to examine further NOx reduction and its effects on other engine performance parameters and emissions.
Nomenclature
-
- BSFC:
-
- Brake-specific fuel consumption
-
- BTDC:
-
- Before top dead center
-
- BTE:
-
- Brake thermal efficiency
-
- C3H8O3:
-
- Glycerin
-
- CI:
-
- Compression ignition
-
- CO:
-
- Carbon monoxide
-
- CO2:
-
- Carbon dioxide
-
- DEE:
-
- Diethyl ether
-
- DI:
-
- Direct injection
-
- EGT:
-
- Exhaust gas temperature
-
- FAME:
-
- Fatty acid methyl esters
-
- HC:
-
- Hydrocarbon
-
- HLB:
-
- Hydrophilic-lipophilic balance
-
- H3PO4:
-
- Phosphoric acid
-
- NO:
-
- Nitric oxide
-
- NO2:
-
- Nitrogen dioxide
-
- NOx:
-
- Oxides of nitrogen
-
- NREL:
-
- National Renewable Energy Laboratory
-
- O2:
-
- Oxygen
-
- PM:
-
- Particulate matter
-
- Span 80:
-
- Sorbitan monooleate
-
- Tween 80:
-
- Polyoxyethylene sorbitan monooleate
-
- USEPA:
-
- United States Environmental Protection Agency
-
- WCO:
-
- Waste cooking oil (obtained from palm oil).
Additional Points
Highlights. (1) Investigation and comparison of glycerin- and water-emulsified biodiesel blends; (2) analysis of stability, particle size distribution, and microscopic structure of emulsified fuel blends; (3) examination of the impact of emulsion fuel on diesel engine performance and emissions; and (4) attainment of higher brake thermal efficiency (BTE) and reduced NOx and smoke emissions from both emulsion fuel blends.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Manpreet Singh Sidhu was responsible for resources by managing materials, laboratory samples, instrumentation, and other analytical tools; investigated the study by conducting research and investigation, specifically performing experiments, and data collection; and was more involved in glycerin emulsification and continued Osama’s water emulsification investigation. Dr. Murari Mohon Roy conceptualized the study by developing ideas, research goals, and aims; supervised the study by taking leadership responsibility for the research activity, including planning and execution; wrote the original draft, preparing and presenting the published work. Osama Ahmed Elsanusi was responsible for resources by managing materials, laboratory samples, instrumentation, and other analytical tools; investigated the study by conducting research and investigation, specifically performing experiments and data collection; and was involved in the initial water emulsification investigation.
Acknowledgments
Most of the materials were uploaded on Lakehead University Knowledge Common in a Thesis (https://knowledgecommons.lakeheadu.ca/handle/2453/4218) which is referenced in the paper as ref. [41]. The authors would like to extend their sincere appreciation to Greg Kekpa, technician in the Instrumentation Lab, Joe Ripku, technician in the Mechanical Engineering Department, and Debbie Puumala, technician in the Chemistry Department, for their valuable assistance throughout the course of this research. Their expertise and support were instrumental in the successful completion of this study. The employer is Lakehead University.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request, following the acceptance of the paper.




