Incorporation of Capecitabine Into Extended Chain of N-Acylated Chitosan Carrier
Abstract
Enhancing the hydrophobicity of chitosan through acylation enables the encapsulation of water-insoluble drugs within the polymeric carrier cores. In this study, hydrophobically modified chitosan was synthesized by reacting low-molecular-weight chitosan with acyl chloride (C18–C24) using an agitation method under mild conditions. The structure of acylated chitosan was analyzed using FTIR and 1H-NMR spectroscopy. The degree of substitution (DS) varied between 56% and 69% for different long-chain N-acylated chitosan, with N-stearoyl chitosan (ChC18) exhibiting the highest DS. The incorporation of capecitabine (CAP) into extended acylated chitosan increased particle size and decreased zeta potential. N-lignoceroyl chitosan (ChC24) exhibited the highest zeta potential value of −27 mV for 0.2 mg of CAP, indicating that the most extended acyl group was the most stable in the suspension. Transmission electron microscope images revealed that all acylated chitosan particles were spherical, with sizes ranging from 100 to 200 nm, and existed as stand-alone entities, indicating excellent stability in suspension. The loading of CAP increased in particle size but did not alter particle shape, except for ChC24, which exhibited agglomeration. SEM images revealed that the individual arrangement of particles in CAP-ChC18 made it more stable than other acylated chitosan. In contrast, the formation of clusters in CAP-ChC24 can be attributed to strong hydrophobic interactions. X-ray photoelectron spectroscopy results show that there is no nitrogen atom in ChC18, which means that the acyl group is oriented inward and bound to the stearoyl group via van der Waals forces. At different drug weight-to-carrier ratios, the encapsulation efficiency (EE) of CAP with varying acyl group lengths ranged from 85% to 97%. The drug loading (DL) capacity and EE increased as the amount of drug in the carrier increased. However, the length of the acyl group did not significantly affect DL and EE, even when the carrier-to-drug ratio was consistently maintained. Sustained release was observed in CAP-loaded ChC24, indicating a significant influence of the extended chain on chitosan. Consequently, extended N-acylated chitosan possesses enormous potential as a drug delivery system for CAP.
1. Introduction
Chitosan is a natural polymer derived from chitin through deacetylation in alkaline solutions. Chitosan has drawn the attention of scientists for various applications owing to its nontoxicity, solubility in acidic solutions, biocompatibility, and biodegradability [1–5]. It also exhibits beneficial biological properties, including antibiotic, antioxidant, anti-inflammatory, antifungal, mucoadhesive, fat binder, angiogenesis simulator, macrophage activator, adsorption enhancer, granular, and hemostatic formation agents [6–11].
Modification of chitosan by functionalizing the hydroxyl and amino groups attached to its backbone through different chemical reactions has been widely investigated. An overview of prior studies on chitosan functionalization is provided in Table 1. Various mechanisms, including amino quaternization (methylation), hydroxyl or amino group epoxidation, amino carboxylation, N-alkylation reduction, cross-linking, amino or hydroxyl group acylation, thiolation, sulfation, esterification, and etherification, have been used to alter chitosan molecules [27–31]. Functionalization reactions result in chitosan with new properties that are applicable in numerous fields such as biomedicine [32–34], electronics [35–38], biomaterials [39–41], energy production [42–44], and wastewater treatment [45–47]. In the pharmaceutical sector, employing chitosan as a carrier offers advantages over conventional drug administration, including enhanced drug stability and efficacy, improved encapsulation efficiency (EE), and controlled drug release and action at the target sites [24, 48–55].
| Author | Year | Research | Results |
|---|---|---|---|
| Sashiwa and Shigemasa [12] | 1999 | Preparation and water-soluble properties at various pHs of N-acylated and N-alkylated chitosan | N-acyl and N-alkyl derivatives of DAC-88 with carboxy or sulfate group showed solubility at basic pH |
| Hirano et al. [13] | 2000 | Preparation of N-higher-carbon-acyl chitosan fibers by treating chitosan fiber with carboxylic anhydrides in methanol | The titer of the filament is 5.21 dtex with a diameter of 33 mm. The N-acylation has little impact on the filament’s tenacity and elongation values |
| Le Tien et al. [14] | 2003 | Modification of chitosan using fatty acyl chlorides (C6–C16) for controlled drug release | Palmitoyl chitosan (C16) with higher degrees of functionalization showed longer drug release times: 30 h for 47% substitution and 90 h for 69% substitution |
| Hu et al. [15] | 2006 | Self-aggregated micelles of shell cross-linked chitosan oligosaccharide grafted with stearic acid for the controlled release of paclitaxel | Amphiphilic CSO-SA can self-assemble into nanosized micelles in an aqueous solution with a lower critical micelle concentration (CMC). The CMC and micelle size decrease with increasing degree of substitution of CSO-SA |
| Hu et al. [16] | 2008 | Investigate shell cross-linking of CSO-SA micelle on cellular uptake and cytotoxicity of doxorubicin-loaded micelles | The cross-linking of CSO-SA micelle shells with glutaraldehyde did not change their properties or cellular uptake ability, but cross-linking the CSO-SA/DOX mixture slowed drug release. CSO-SA micelles increased DOX efficacy against tumor cells, especially in previously insensitive cell lines |
| Cho et al. [17] | 2012 | Preparation of N-acyl chitosan with different chain lengths via self-aggregation for vitamin C carrier | Vitamin C in N-acyl chitosan nanoparticles is released slower with longer acyl chains at pH 1.3 and pH 7.4 |
| Agnihotri and Aminabhavi [18] | 2006 | Semi-interpenetrating network hydrogel microspheres of chitosan-poly(ethylene oxide-g-acrylamide) loaded with capecitabine were synthesized by emulsion cross-linking using glutaraldehyde | The encapsulation efficiency of capecitabine was 79%–87% in a spherical microsphere with particle sizes of 82–168 μm |
| Ameli and Alizadeh [19] | 2022 | Establishing a pH-responsive micelle-based drug delivery system using cyclodextrin and copolymer for targeted CAP drug release in colon cancer | Nano polymeric micelles based on beta-cyclodextrin can slow the release of capecitabine in acidic conditions |
| Xiao et al. [20] | 2019 | A strategy for preparing composite micelles (CM) that contain both a cisplatin (IV) (CisPt [IV]) prodrug and capecitabine using a co-assembly method | The composite micelle demonstrates superior sustained release of capecitabine at pH 5 compared to pH 7.4 |
| Nayanika [21] | 2018 | Designing, characterizing, and conducting cytotoxic experiments of polymeric micelles of cellulose-EDTA-loaded capecitabine | Formulation F2 was found to be the best, with a size of 1754 nm, 5.6 mg drug content, and an entrapment efficiency of 66.8%. The zeta potential ranged from −14 to −22.3 Mv, with a PDI of less than 0.4, and the particle size ranged from 1155 to 2752 nm |
| Marlina and Misran [22] | 2023 | Preparation stearoyl chitosan for incorporating antimetabolite | Stearoyl chitosan loaded with antimetabolites has particles ranging in size from 225 to 369 nm and have a spherical shape. SC3 showed the zeta potential values of −15 mV |
| Lee, Powers, and Baney [23] | 2004 | Preparation of N-acylation chitosan using butanoic, hexanoic, and benzoic anhydride in the presence of methanol under homogeneous conditions | The degree of substitution was 22%–38%, determined by 1H-NMR. The particle size of N-acyl chitosan ranged from 200–360 nm and was positively charged (10–20 mV) after freeze-drying in a saline solution |
| Soliman et al. [24] | 2022 | The statistically optimized formulation of ciprofloxacin-HCl (CHCl)-loaded chitosan nanoparticles (CS-NPs) enhances its antibacterial activity over pure CHCl | Prepared CS-NPs were found in the particle size range of 198–304 nm with a zeta potential of 27–42 mV. EE and drug release were in the range of 23%–45% and 36%–61%, respectively |
| Tiew and Misran [25] | 2018 | Acylated low-molecular-weight chitosan encapsulating salicylic acid for sustained topical release | The release mechanism of salicylic acid involved both diffusion and polymer swelling |
| Kousar et al. [26] | 2023 | Surface functionalization of thiolated chitosan using hyaluronic acid for targeted delivery and sustained release of oncolytic NDV in cervical cancer cells | Thiolated chitosan nanoparticles had a smooth surface and spherical features, with an average size of 290.4 nm and zeta potential of 22.3 mV. The in vitro release of NDV was continuous but sustained for up to 48 h |
| Naseer et al. [27] | 2023 | Formulation of thiolated chitosan coated with hyaluronic acid nanoparticles for encapsulating oral measles vaccine (OMV) | The best nanoformulation (NF) has a particle size of 275.6 mm, PDI of 0.372, and ±11.5 zeta potential. SEM and XRD showed a smooth spherical surface and crystalline nature, respectively, while TEM confirmed virus encapsulation within nanoparticles. The virus was continuously released from NFs for up to 48 h |
| Kousar et al. [28] | 2022 | Synthesized and characterized the nanoparticles of vincristine (VC) loaded hyaluronic acid (HA) coated thiolated chitosan (TC) | The analysis showed a particle size of 267.9 nm, PDI 0.210, and zeta potential of +15.1. Raman analysis confirmed hydrophilic groups, porous surfaces, and agglomeration. SEM/TEM showed spherical nanoparticles with a smooth surface; XRD confirmed a crystalline nature for targeted drug delivery. Drug loading/encapsulation efficiencies were 41.4% and 87%, respectively. The kinetics study showed simple diffusion by the Higuchi equation in an acidic medium, and the in vitro study showed sustained release at pH 7.4 and 6.8 up to 72 h |
Increasing the hydrophobicity of chitosan by adding an acyl group to its amino group offers novel opportunities for encapsulating hydrophobic drugs or active compounds; hence, the N-acylation of chitosan is a remarkable breakthrough in drug delivery. It has been reported that acylated chitosan produced via ring-opening reactions with various cyclic acid anhydrides, propionyl, butyryl, hexanoyl, octanoyl, benzoyl, and succinyl, unlocked the potential of chitosan as a carrier [12]. In another study, Hirano et al. [13] found that chitosan and its derivatives have the capability to undergo self-assembly in aqueous solutions. Self-assembly occurs by balancing the hydrophilic and hydrophobic properties of these compounds [56].
Le Tien et al. [14] found that chitosan N-acylated with a range of fatty acid chlorides (C6–C16) demonstrated potential as an effective excipient for controlled drug delivery. Furthermore, controlled drug release was achieved when paclitaxel [15] and doxorubicin [16] were loaded into stearic acid-grafted chitosan oligosaccharides. The self-aggregation of different N-acyl chitosan (C3–C18) was reported by Cho et al. [17], where the particle size of the N-acyl chitosan was reduced by ~40% with vitamin C loads.
Capecitabine (CAP) or fluoropyrimidine carbamate is one of the most popular chemotherapeutic drugs [57–60]. It contains a carbamate ester, which is modified by adding fluorine and a derivative of N-(penyloxy) carbonyl (Figure 1a) [61]. CAP exhibits higher solubility in lower alcohols with a linear structure than in lower alcohols with a branched structure. The solubility of CAP decreases as the carbon chain length of the alkyl alcohol increases [62]. The solubility of CAP in water is ~26 mg mL−1 [63].
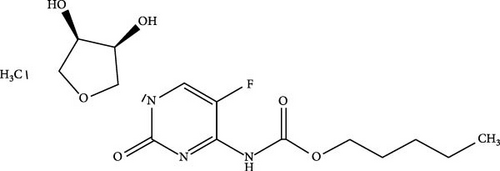
CAP is classified as an oral 5-fluorouracil (5-FU) prodrug. Once CAP enters the bloodstream, it is rapidly absorbed and transformed into 5-FU metabolite via three enzymatic processes. Commonly, patients are required to take two 2.5 g m−2 CAP dosages daily due to its short elimination half-life of 0.5–1 h [19]. Nonetheless, consuming the drug can result in significant adverse effects, including diarrhea, nausea, and vomiting. Hence, there is a requirement for a drug delivery system that can prolong the half-life of CAP and mitigate its adverse effects.
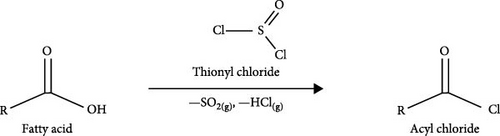
Previous studies have extensively investigated the in vitro release process of CAP-incorporated polymer-carriers and composite micelles [18, 20, 21, 64–66], as well as the physicochemical properties of the carriers. However, there is a lack of research on using long-chain acylated chitosan with carbon chains greater than 18 as a drug carrier, particularly for encapsulating CAP. Therefore, the main objective of this study was to synthesize and characterize N-acylated chitosan using several acyl chlorides ranging from C18 to C24. The acid chloride used in this study was synthesized through a reaction between fatty acids and thionyl chloride, as illustrated in Figure 1b,c. Furthermore, to assess the efficacy of the extended chain of N-acylated chitosan as a delivery system for drug administration, CAP was employed as a model drug integrated into the newly modified chitosan. The toxicity, EE, and in vitro release were investigated to assess the capabilities of this novel carrier. It was hypothesized that the hydrophobicity of chitosan can be enhanced by employing extended-chain N-acylated chitosan. This enhancement is believed to result in an increased capacity to efficiently encapsulate CAP, as well as the ability to sustain the release of CAP. Consequently, this sustained-release mechanism may enhance the therapeutic efficacy of CAP.
The significant study of extended-chain N-acylated chitosan offers a versatile platform for the development of advanced drug delivery systems with improved solubility in organic solvents and water, controlled release kinetics, increased stability in physiological environments, and targeted delivery owing to the presence of long-chain fatty acids. These advances could potentially overcome multiple challenges in drug delivery and contribute to the development of safer and more effective therapeutic interventions.
2. Materials and Methods
2.1. Materials
All chemicals used in this study were utilized without further treatment. Chitosan, with an average molecular weight of 300 kDa and a deacetylation degree of 75%–85%, was obtained from Acros Organics. Stearic, arachidic, behenic, deuterated acetic, lignoceric acids, thionyl chloride, deuterium oxide, deuterated dimethyl sulfoxide, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and phosphate-buffered saline (PBS) were purchased from Sigma–Aldrich (United States of America [USA]). Sodium hydroxide (NaOH) and sodium nitrite (NaNO2) were obtained from Fluka (Switzerland). Ethanol, hydrochloric acid (HCl), chloroform (CHCl3), acetone, tetrahydrofuran (THF), and sodium dodecyl sulfate (SDS) were purchased from Merck (Germany). All the samples and solutions in this study were prepared with deionized water with 18.2 cm−1 resistivity.
2.2. Synthesis of Acyl Chlorides
The stearoyl, arachidoyl, behenoyl, and lignoceroyl chlorides obtained in this study were synthesized by reacting various unsaturated fatty acids (stearic acid, arachidic acid, behenic acid, and lignoceric acid) with thionyl chloride. To produce stearoyl chloride (SC), stearic acid (5.69 g, 20 mmol) was dissolved in CHCl3 (50 mL) and agitated for 30 min at 30°C using a magnetic stirrer. Subsequently, thionyl chloride (2.90 mL, 40 mmol equivalents) was added dropwise to the fatty acid solution and gently stirred for 5 h at 50°C. After acylation, the mixture was vacuum distilled to eliminate excess thionyl chloride. This procedure generates HCl and SO2 gases as byproducts collected and trapped in a 1.0 M NaOH solution. Other acyl chlorides, including arachidoyl (AC), behenoyl (BC), and lignoceroyl (LC) chlorides, were synthesized using the same method.
2.3. Preparation of the Extended N-Acylated Chitosan
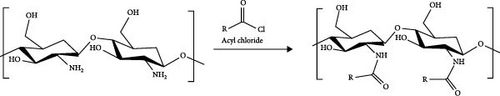
In this study, extended N-acylated chitosan was synthesized through the acylation process, in which chitosan was reacted with acyl chloride containing a C18–C24 chain. The purpose of this reaction is to enhance the hydrophobic nature of chitosan. The acylation was conducted using low-molecular-weight chitosan (LMWC). LMWC are less viscous and more soluble in water than high-molecular-weight chitosan and medium-molecular-weight chitosan (MMWC). Therefore, further reactions were more accessible to the process. To produce LMWC, MMWC was depolymerized based on the procedure outlined in a previous study with minor modifications [25, 67]. First, MMWC (1 g) was dissolved in 1% v/v acetic acid solution (100 mL) to generate an acidified chitosan solution (1% w/v). NaNO2 (7 mL) was then introduced to this chitosan solution at a 0.5 mL min−1 rate under magnetic stirring. The reaction was conducted in a glass-jacketed reactor connected to a Lauda Ecoline E100 water bath at a reaction temperature of 30°C. After stirring for 5 h, NaOH (0.05 M) and HCl (0.05 M) were added to achieve a pH of 7. An equal volume of acetone was then added to the mixture to form a suspension. The suspension was subsequently centrifuged to separate and collect LMWC.
Four extended chains of N-acylated chitosan were synthesized by mixing LMWC with various acyl chlorides: stearoyl chloride (SC), arachidoyl chloride (AC), behenoyl chloride (BC), and lignoceroyl chloride (LC), which were prepared in the first phase of this study. First, 1% (w/v) LMWC was dissolved in 1% (v/v) acetic acid to obtain an LMWC solution, which was then adjusted to pH 7 using NaOH (0.05 M) and HCl (0.05 M). Concurrently, an acyl chloride solution was prepared in THF and gradually introduced into the LMWC solution. The quantity of acyl chloride employed in this acylation procedure was determined using the molar ratio of LMWC to acyl chloride, specifically 1:5 (equivalent to N-glucosamine units), as shown in Figure 1c. The mixture was continuously stirred for 24 h at 30°C, and nitrogen gas was purged into the mixture to remove THF [68]. Subsequently, acetone was gradually added to the mixture to obtain a suspension. The resulting precipitate was then separated using a Dynamica Velocity 18R refrigerated tabletop centrifuge (Scientific Dynamica Ltd.) at 5000 rpm for 15 min. Finally, N-acylated chitosan was obtained by washing the residual mass with CHCl3 to remove the free fatty acids, followed by vacuum drying. The final products obtained were stearoyl, arachidoyl, behenoyl, and lignoceroyl chitosan, labeled as ChC18, ChC20, ChC22, and ChC24, respectively.
2.4. Synthesis of Self-Assembled Extended N-Acylated Chitosan
The self-assembly method was utilized to create acylated chitosan nanoparticles, as it can generate well-defined, stable, and scalable nanoparticles under mild conditions while preserving the biocompatibility and biodegradability of chitosan. Self-assembly is the spontaneous arrangement of particles in a structured pattern without any external direction [69]. The self-assembly of N-acylated chitosan occurs when it is dispersed in an aqueous solution owing to the presence of hydrophobic acylation groups using the nanoprecipitation method [70]. To prepare the solution, 0.4 mg of N-acylated chitosan was dispersed in 1 mL of CHCl3 for each acyl group (ChC18, ChC20, ChC22, and ChC24). The dispersion solution was gradually introduced into 10 mL of PBS while being stirred at a temperature of 60°C to facilitate the evaporation of CHCl3. N-acylated chitosan will spontaneously arrange itself into particles distributed throughout the PBS solution. Finally, the N-acylated chitosan dispersion was sonicated overnight to produce nanosized particles at a constant temperature of 30°C using a Lauda Ecoline RE207 water bath. The extended N-acylated chitosan suspension was characterized and used as a carrier for the model drug.
2.5. Preparation of CAP-Loaded N-Acylated Chitosan
Another batch of extended N-acylated chitosan was produced according to the aforementioned procedure. A range of CAP weights (0.2–1 mg) was dissolved in 10 mL of PBS (pH 7.4) before being added to the extended N-acylated chitosan suspension. The mixture was then incubated overnight at 30°C on a GyroMaxTM 721 orbital shaker at 200 rpm. CAP-loaded N-acylated chitosan at different weight ratios were then prepared with stearoyl (ChC18), arachidoyl (ChC20), behenoyl (ChC22), and lignoceroyl (ChC24) chitosan. The ratio of the drug to N-acylated chitosan ranged from 0.2:1 to 1:1. After incorporating the drug into the N-acylated chitosan solution using an orbital shaker, the resulting mixture was kept in a refrigerator at 8°C. The sample was then analyzed to determine the EE using centrifugation and UV absorption techniques as well as for the in vitro release study.
2.6. Characterization of N-Acylated Chitosan
2.6.1. Fourier-Transform Infrared (FTIR) Analysis
The structures of the prepared LMWC and N-acylated chitosan were determined using an attenuated total reflectance (ATR) (GladiATR) FTIR spectroscope (Pike Technologies, USA). FTIR analysis revealed the presence of functional groups, such as carbonyl, hydroxyl, amine, amide, and other vibrations in the extended N-acylated chitosan and LMWC. Prior to FTIR measurement, the chitosan samples underwent a 24-h drying process in a vacuum oven to minimize the effect of water adsorption on chitosan and consequently prevent inaccuracies in the degree of substitution (DS). Approximately 5 mg of each sample was placed on diamond and germanium interchangeable crystal ATR plates with the tip gently depressed against the specimen for measurement. A total of 32 scans were performed at a resolution of 4 cm−1 within the 4000 and 450 cm−1 wavenumber range. The assessments were performed in triplicate.
2.6.2. 1H NMR Analysis
1H NMR analysis provided detailed information on the arrangement and number of hydrogen atoms and the presence of substituents in the modified chitosan. The proton numbers of LMWC, ChC18, ChC20, ChC22, and ChC24 were evaluated using AVANCE III 400 MHz (AVN 400) Bruker proton nuclear magnetic resonance (1H NMR). Approximately 20 mg of LMWC was dissolved in deuterated acetic acid (CD3COOD) and deuterium oxide (D2O) before the evaluation. In contrast, extended N-acylated chitosan was analyzed after dissolution in deuterated dimethyl sulfoxide (d6-DMSO).
2.6.3. Average Molecular Weight Determination
The static light scattering method was employed to determine the average molecular weight of the substance. This method is preferred because it is nondestructive and does not modify or damage the sample under analysis. In addition, it is a fast measurement technique that can provide results within a short period [71]. A Zetasizer Nano ZS (Malvern Instruments, UK) equipped with a 633 nm laser and a single-angle 173° backscatter system was used to evaluate the average molecular weights of the LMWC and N-acylated chitosan produced in this study. First, stock solutions were diluted to obtain samples with different concentrations. A mildly acidic solution (pH 6) was added to the LMWC, and the acylated chitosan was mixed with THF. The toluene reference and solvents employed in the current study were filtered twice with 0.22 μm PTFE syringe filters. Subsequently, static light scattering was used to determine the average molecular weight of the samples.
2.6.4. X-Ray Diffraction (XRD) Analysis
2.6.5. Particle Size and Zeta Potential Evaluation
2.6.6. Morphology Assessment
This study employed a LEO Libra-120 (Carl Zeiss, Germany) transmission electron microscope (TEM) at an accelerating voltage of 120 kV to examine the morphology of the unloaded drug and drug-loaded extended N-acylated chitosan. The specimens were placed on a carbon-coated copper grid and negatively stained with 1% (w/v) phosphotungstic acid (PTA).
The surface morphologies of the LMWC and acylated chitosan were analyzed using a field-emission scanning electron microscope (FESEM) (SU8220, Hitachi) at 22,000× magnification. The samples in this study were spray-dried for 24 h prior to analysis.
The LMWC and stearoyl chitosan prepared in the present study were evaluated by X-ray photoelectron spectroscopy (XPS) (Kratos Shimadzu Axis Ultra). Aluminum monochromatic radiation was used as the X-ray source to examine the surfaces of the specimens under a vacuum. The sample was irradiated before the energy of the electrons that escaped from the surface was measured. Subsequently, the kinetic energy of the electrons was compared with the number of electrons observed in each energy interval to produce a spectrum. Additionally, peak height, area, and chemical compound identification provided quantitative data.
2.7. Cytotoxicity Evaluation
2.7.1. Cell Culture
In the current study, two cancer cell lines were cultured before the MTT assay. The A549 (ATCC CCL-185) human lung cancer and HeLa (ATCC-CCL-2) cervical cell lines were obtained from Dr. Lee Poh Foong and Dr. Teoh Boon Yew of the Lee Kong Chian Faculty of Engineering and Science, Universiti Tunku Abdul Rahman (UTAR), who purchased the cell lines from the American Type Culture Collection (ATCC) [72] Human lung carcinoma (A549) and cervical adenocarcinoma (HeLa) cells were cultured in DMEM supplemented with 10% FBS and maintained at 37°C in a 5% carbon dioxide (CO2) atmosphere. Prior to the study, the cell culture was not subjected to mycoplasma, bacterial, or fungal screening. In order to maintain uniformity and precision, A549 and HeLa cells were subjected to three cycles of passaging after thawing before commencing the experiments.
2.7.2. The MTT Assay
A total of 5000 A549 and HeLa cells per well were seeded in a 96-well cell culture plate and incubated in DMEM supplemented with 10% FBS for 24 h. The cells were then treated with acylated chitosan dispersion in DMEM at various concentrations (0.004–0.4 mg mL−1) for 24, 48, and 72 h. At the end of the incubation period, 5 mg mL−1 of MTT solution was added to each well and incubated again for 4 h at 37°C.
2.8. Drug EE and Loading Capacity
CAP with varying weights (0.2–1 mg) was dissolved in PBS (0.01 M, pH 7.4) before being added to the prepared acylated chitosan suspension. An orbital shaker was then used to agitate the mixtures overnight at room temperature to obtain homogenous solutions. Subsequently, 1 mL aliquots were transferred to a Vivaspin 6 centrifugal concentrator (MWCO 10 kDa).
The percentages indicated in the measurement of the EE demonstrated the ability of the carrier system to successfully maintain and protect the encapsulated drug. Higher percentages generally indicate superior efficiency, as a greater amount of drug is effectively encapsulated rather than lost throughout the process.
2.9. In Vitro Release
This study employed a Franz glass diffusion cell system (Hanson Research Co., USA) comprising six vertical diffusion cells with a 4 mL receptor chamber to evaluate the in vitro drug release of the samples at 37 ± 0.1°C of the samples assessed in the current study. A regenerated cellulose membrane with a molecular weight cutoff of 5 kDa (63 mm, Nest Group Inc.) was rinsed with deionized water and immersed in a PBS solution for 12 h. The membrane was then placed in a glass diffusion cell with an effective diffusion area of 0.6362 cm2. A similar weight-to-carrier drug ratio employed in the EE procedure was used to produce drug-loaded acylated chitosan solutions, which were then gently placed in the donor chamber of the diffusion cell system for the release study.
The drug diffused across the membrane to the receptor chamber containing PBS solution (0.01 M) owing to the gradient concentration. Sampling was performed at 1/2, 1, 2, 4, 6, 8, 12, 16, 20, and 24-h intervals with 1 mL aliquots of automated transfer from the receptor chamber to the vials (Agilent). During the analysis, the solutions in the receptor chambers were gradually replaced with fresh PBS to maintain a consistent volume (0.01 M). Subsequently, drug content was quantified using a Varian Cary 50 UV–Vis spectrophotometer at 304 nm. All assessments in this study were performed in triplicate.
2.10. Statistical Analysis
All data obtained in the present study were analyzed using analysis of variance (ANOVA) in the Origin Software. Data analysis was performed using one-way ANOVA followed by Tukey’s test. Statistical significance was set at p < 0.05.
3. Results and Discussions
3.1. FTIR
Substituting hydrogen atoms in the amino groups with extended acyl groups altered the chemical structure of the prepared chitosan (Figure 2). The broad peak at 3436 cm−1 in the LMWC spectra was attributed to O─H stretching, which overlapped with N─H stretching. In addition, the three weak bands observed at 1644, 1567, and 1031 cm−1 were assigned to C═O stretching vibration (amide I), N─H bending vibration (amide II) [14], and C─O─C (glycosidic linkage) stretching vibrations, respectively [73]. The amide bonds and glycosidic linkages that appeared in the LMWC were derived from chitosan.
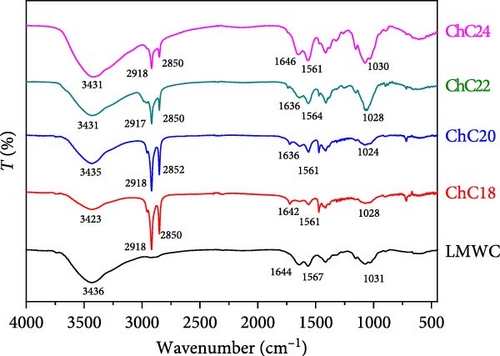
The acylated chitosan also exhibited peaks similar to those of LMWC, corresponding to O─H, C═O (amide I), N─H (amide II), and glycosidic linkages. Nevertheless, owing to the modification process, the peak intensities varied with slightly shifted wavenumbers. The two prominent peaks at 2918 and 2850 cm−1, attributed to the asymmetric and symmetric CH2 stretching vibrations [22], respectively, confirm the presence of the ChC18 structure. Comparable peaks were observed for ChC20, ChC22, and ChC24, but with lower intensity and slight shifts.
These results demonstrated the successful acylation of chitosan with acyl chains of different lengths. The chitosan acylation mechanism is more selective for amine groups than hydroxyl groups because of the high reactivity of the amine [2]. The reduced intensity of the N─H band (amide II) in acylated chitosan was due to the acylation of amino groups, particularly in ChC18.
The lignoceroyl (ChC24) chitosan prepared in this study exhibited a very broad band at 3431 cm−1 compared to stearoyl (ChC18), arachidoyl (ChC20), and behenoyl (ChC22). The band broadening in ChC24 was mostly attributed to the robust hydrogen bonds formed by O─H and N─H groups. These interactions hinder the bonding between many lignoceroyl groups and the N atoms in chitosan. This implies that ChC24 contains a higher concentration of free amino groups than other acylated chitosan compounds.
The IR spectrum obtained in the present study was used to determine the DS of the procured acylated chitosan (Table 2). Approximately 60% of the acyl groups were grafted onto the LMWC backbone via reaction with an amino group. Furthermore, the yield of various acylated chitosan within the 74%–88% range indicated successful acylation.
| Type of chitosan | Molecular weight (kDa) | DS (%) | Yield (%) | Crystallinity index (%) |
|---|---|---|---|---|
| LMWC | 17 ± 0.2 | — | 85 | — |
| ChC18 | 38 ± 0.7 | 69.7 | 88 | 29.8 |
| ChC20 | 20 ± 0.3 | 56.4 | 87 | 24.7 |
| ChC22 | 37 ± 0.4 | 61.6 | 85 | 23.1 |
| ChC24 | 35 ± 0.4 | 60.3 | 74 | 15.6 |
The acylation process involves the modification of the amino group in the LMWC by attaching a long-chain acyl group to nitrogen. The DS was determined based on the ratio of the amide I band to the O─H band. The amide I band overlapped with the O─H band of water at a frequency of 1644 cm−1, indicating the presence of both chitosan hydroxyl groups and bound water [69]. As shown in Table 2, the highest DS was observed for ChC18 at 69.7%, whereas ChC20 exhibited the lowest DS (56.7%) among the four extended-chain N-acylated chitosan. This finding demonstrates that there is no correlation between DS and the length of the acyl group, despite previous research suggesting an increase in DS owing to longer acyl chains [30].
3.2. The 1H-NMR Analysis
The LMWC and ChC18 1H-NMR spectra are illustrated in Figure 3. The intense deuterium oxide and deuterated acetic acid solvent protons were documented at 4.67 and 2.36–2.28 ppm for LMWC, respectively, while the d6-DMSO peak appeared at 2.48 ppm.
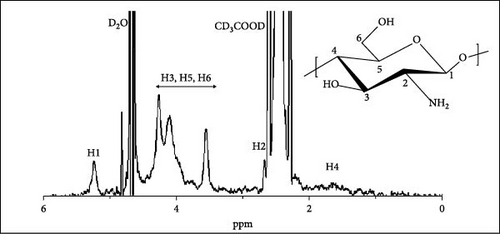
The peak at 1.81 ppm corresponded to the proton attached to carbon 4 (─CHCHCH─) in the LMWC (Figure 3a), and the proton attached to carbon 2 (─CHCH(NH2)CH─) was detected at 2.97 ppm. The peaks recorded at 3.57–4.63 ppm were assigned to the protons of carbons 3 (─CH2CH(OH)CH─), 5 (CH2CH(CH2)O─), and 6 (─CHCH2OH) [25]. On the other hand, the protons attached to carbon 1, which was connected to glycosidic linkages (─C─O─C─), resulted in a 5.23 ppm peak [20].
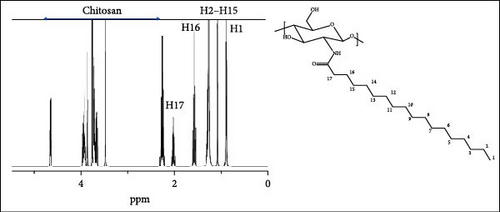
Figure 3b demonstrates the 1H-NMR data of ChC18 prepared in this study. Additional peaks at 0.88, 1.25–1.29, 1.57–1.59, and 2.2 ppm were observed, which were attributable to the protons attached to carbons 1 (HNCO (CH2)16CH3), 2–15 (HNCO (CH2)2 (CH2)14CH3), 16 (HNCOCH2CH2 (CH2)14CH3), and 17 (HNCOCH2CH2 (CH2)14CH3), respectively. Similar peaks were observed for ChC20, ChC22, and ChC24, but with slight chemical shifts owing to acylation. All these peaks correspond to those in a previous study on N-acylated C6–C16 chitosan [14].
3.3. Average Molecular Weight Determination
The average molecular weight was determined using the static light scattering method because of its nondestructive, high sensitivity, versatility, fast measuring capabilities, accuracy, precision, and compatibility with diverse solvents. Table 2 displays the average molecular weights of LMWC, ChC18, ChC20, ChC22, and ChC24 produced in this study. On average, LMWC had a molecular weight of 17 kDa after depolymerization. Conversely, the average molecular weights of ChC18, ChC20, and ChC24 were significantly higher than those of LMWC after incorporation of an acyl group. Nevertheless, the increase in average molecular weight was not very pronounced in ChC20. This finding could be attributed to the small number of arachidoyl groups embedded in the chitosan. Consequently, the average molecular weight did not increase proportionally with the acyl length.
As shown in Table 2, ChC20 exhibited molecular weight and DS values that deviated from the established pattern. Unlike other acyl groups, the length of the acyl group is directly proportional to both molecular weight and DS. The observed discrepancy can be attributed to the comparatively lower efficiency of the functionalization reaction with the arachidoyl group than with other acyl chlorides, resulting in a reduced DS.
3.4. XRD Analysis
XRD analyses were performed to obtain the diffraction patterns of the prepared LMWC and acylated chitosan, as the intensity and width of the XRD patterns corresponded to the physical form of the polymers, such as amorphous and crystalline. The diffractograms of LMWC and acylated chitosan obtained in this study are illustrated in Figure 4.
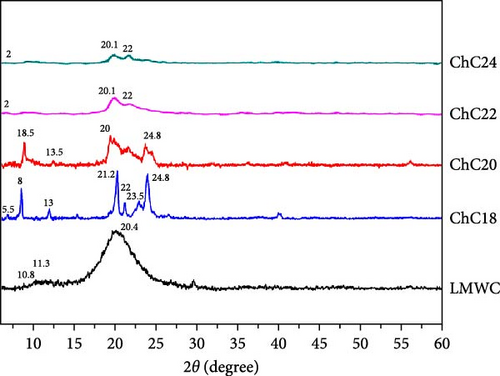
Two peaks (2ϴ) and a very broad peak were observed at 10.8°, 11.3°, and 20.4° for LMWC, confirming its amorphous structure [74]. Although LMWC is a linear polysaccharide, its XRD pattern shows very broad peaks, most likely owing to the amorphous nature of the polymer [75]. In contrast, the diffractogram of extended N-acylated chitosan showed several peaks. ChC18 exhibited three significant reflections 2ϴ at 8°, 21.2°, and 24.8°, and four weak peaks at 5.5°, 13°, 22°, and 23.5° [76]. An analogous peak was also observed for ChC20, albeit with a broader peak width. Compared with other acylated chitosan, ChC18 exhibited a higher and narrower peak intensity. Each peak is associated with a specific set of lattice planes within the crystal structure. This is in accordance with the ChC18 crystallinity index, which was the most prominent at 29.8%, as shown in Table 2. This suggests that ChC18 likely has a structure with well-organized stearoyl groups in its backbone.
ChC22 and ChC24 exhibit weak and broad peaks at 2°, 20.1°, and 22°, respectively. This finding contrasts with the research conducted by Tien et al. [14], who observed that longer acyl chains (C8–C14) resulted in sharper and more crystalline peaks. The observed pattern could be attributed to the presence of more robust hydrophobic intermolecular bonds in ChC22 and ChC24, particularly in the behenoyl and lignoceroyl groups of chitosan, leading to the formation of aggregates [14]. This considerably influences the physicochemical attributes, including thermal properties, transparency, and mechanical strength.
3.5. Particle Size and Zeta Potential
Dispersion of molecules in water-based solutions results in hydrophobic effects. The hydrophobic moiety rearranges itself to minimize interactions with polar groups, which is energetically favorable and leads to micelle formation. The present study observed substantial differences in size between unloaded and loaded LMWC and extended-chain N-acylated chitosan. It is shown in Figure 5a that the particle sizes of LMWC, ChC18, ChC20, ChC22, and ChC24 without DL were 117, 120, 164, 201, and 253 nm, respectively. This indicated that the size of the extended-chain N-acylated chitosan particles was greatly affected by the length of the acyl group. The observed correlation between the length of the acyl group and the increase in particle size was primarily attributed to hydrophobic interactions. Acyl chains with greater lengths exhibited higher hydrophobicity than acyl chains with shorter lengths. In aqueous solutions, these long hydrophobic chains tend to form clusters, thus minimizing their contact with water. This aggregation process causes the formation of larger micelles or vesicles, which in turn leads to an increase in particle size. In contrast to previous studies, hydrophobic interactions played a vital role in determining the particle size of acylated guava gum. Specifically, the longer the acyl group, the smaller the particle size [77].
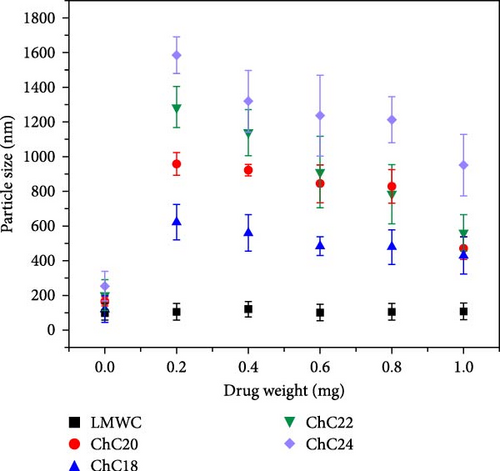
The incorporation of CAP into an extended chain of N-acylated chitosan significantly affected the particle size. Figure 5a shows that by loading 0.2 mg CAP, the particle size of the four acylated chitosan increased drastically. An increase in the particle size was observed in ChC24 after CAP loading, with the size increasing from 253 to 1584 nm. This suggests that the size of CAP itself can influence the overall particle size, as it occupies more space on the surface or inside the particle, thereby leading to an increase in the particle size. The entrapment of CAP within the acylated chitosan core was facilitated by van der Waals interactions between the hydrophobic groups in CAP and the acyl groups in chitosan.
By increasing the amount of CAP incorporated into ChC24, the attractive forces in the core became more prominent than the repulsive forces, resulting in a progressive reduction in particle size. A similar trend was observed for the three extended N-acylated chitosan. This phenomenon can be explained by incorporating more CAP into the acylated chitosan core, which promoted tighter packing and a more efficient micellization process, resulting in a smaller particle size. In addition, the inclusion of more CAP in the acylated chitosan core can potentially alter the interfacial tension between the hydrophobic core and the surrounding aqueous environment. This alteration can affect the curvature of micelles and reduce their particle size [76].
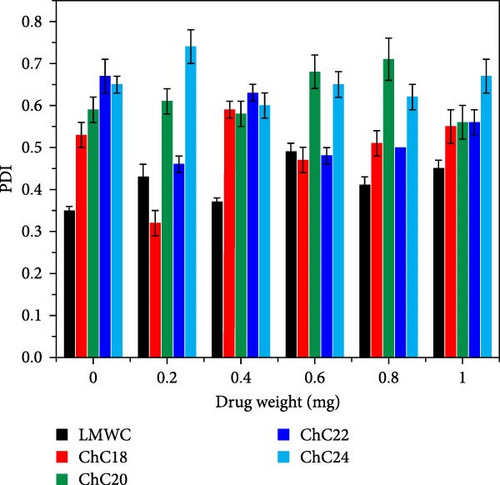
The particle size distribution of each extended N-acylated chitosan is shown in Figure 5b. All extended N-acylated chitosan samples have a polydispersity index (PDI) value greater than 0.4, indicating the presence of a mixture of particle sizes ranging from small to large in the system. This high value indicates particle size non-uniformity, likely due to the aggregation or Ostwald ripening of N-acylated chitosan during synthesis or storage. Aggregation occurs when nanoparticles collide and adhere to one another, resulting in the formation of larger aggregates characterized by a wider range of sizes. Ostwald ripening involves the growth of larger particles at the expense of smaller particles, leading to a shift toward larger particle sizes over time [78].
Zeta potential measurements of extended N-acylated chitosan fabricated in this study were performed in PBS (pH 7.4). The stability of the extended N-acylated chitosan carrier was observed for 28 days through zeta potential measurements, as shown in Figure 6a. These extended N-acylated chitosan were stored for 28 days at −20°C to prevent clumping, which could alter the properties and effectiveness of the nanoparticles. This storage condition also prevented chemical degradation and ensured the structural integrity of the nanoparticles over time. The zeta potential values of the four extended acylated chitosan were measured immediately after preparation. Among them, ChC22 exhibited the highest value of −22 mV, followed by ChC24. In contrast, ChC18 and ChC20 showed lower zeta potentials of −11.3 and −12.2 mV, respectively. These values are comparatively lower than those reported in previous research. For most nanoparticles, a zeta potential magnitude greater than ±30 mV indicates good stability because the repulsive forces between the particles prevent aggregation or sedimentation [79]. Fluctuations in the zeta potential of all extended N-acylated chitosan were observed over 28 days. Based on these results, it can be inferred that the stability of extended N-acylated chitosan increased as the length of the acyl group increased. However, it is worth noting that the difference in the zeta potential between ChC22 and ChC24 was not substantial.
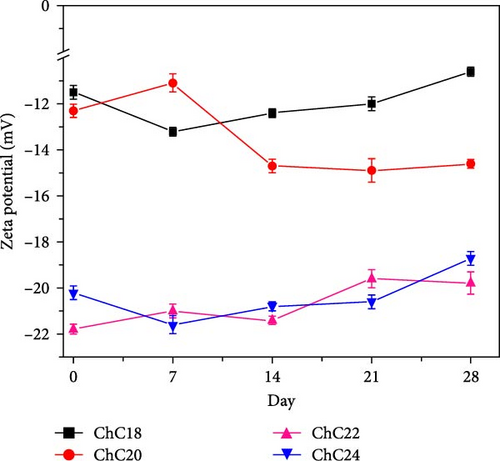
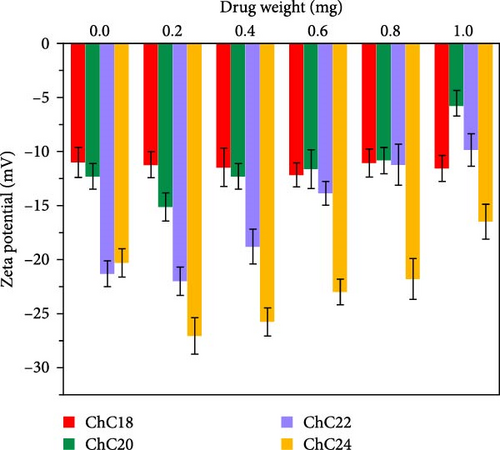
Figure 6b illustrates the zeta potential values of extended N-acylated chitosan with various drug weights. It is shown that the highest zeta potential value was recorded for ChC24, indicating ChC24 particles are more stable in suspension than other extended acylated chitosan due to its zeta potential value above −15 mV for a drug range of 0.2–1 mg, with the maximum value of −27 mV for 0.2 mg of CAP. This value is similar to that of the incorporation of thymoquinone into N-acyl chitosan nanoparticles, as reported by Prajapati et al. [80]. This suggests that the addition of a small amount of CAP to ChC24 stabilizes the drug carrier. The high zeta potential value observed for ChC24 suggests that the repulsive force between particles is significantly greater than the attractive force exerted by the lignoceroyl group. This finding corresponds with previous research conducted by Gosh and Dey, in which the presence of long and large acyl groups prevented hydrogen bonding and aggregation [81].
Nonetheless, the zeta potential of ChC24 gradually decreased with the addition of more CAP. A similar trend is observed for ChC20 and ChC22. This phenomenon may be caused by the hydrophobic interaction between the acyl and alkyl groups of CAP, resulting in the formation of agglomerates and decreased stability of acylated chitosan. Moreover, as the amount of CAP increased, the intermolecular interaction between CAP and ChC24 strengthened. This overcomes the repulsive force between the acyl groups and causes the two molecules to come closer together and form a clump. As a result, the acylated chitosan became less stable. In contrast, ChC18 exhibited a different pattern, as it maintained relatively consistent zeta potential values across different CAP weights. This result is consistent with a prior study that investigated the addition of raltitrexed to stearoyl chitosan [22].
The standard deviation of particle size and zeta potential increased for all extended N-acylated chitosan. This phenomenon arises from the presence of a wide variety of particle sizes, encompassing both large and small particles with different surface charges. Moreover, variations in the sample, such as the presence of aggregates or particle clusters, might lead to increased standard deviations, as they can potentially impact the accuracy of measurements.
3.6. Morphology Analysis
Transmission electron microscopy images provided essential insights into the physical form of extended N-acylated chitosan, including its size, shape, and distribution. This information is crucial for understanding how acylated chitosan interacts with other materials and for designing new drug delivery systems based on acylated chitosan. According to the findings presented in Figure 7a–d, the morphology of all the extended N-acylated chitosan particles was predominantly spherical with a varying size distribution. These results agree with the data obtained from dynamic light scattering experiments, which indicated that the particle size ranged from 100 to 250 nm. As shown in Figure 7a,b,d, as the length of the acyl group increased, the ChC18, ChC20, and ChC24 particles tended to stand individually. This can be attributed to the bulky and long acyl groups that impede hydrogen bonding, consequently preventing particle aggregation [23]. However, the ChC22 particles exhibited a distinct behavior in which they tended to aggregate. This is likely due to van der Waals forces, which attract particles to form clusters. This phenomenon is associated with the DS shown in Table 2, where the DS of ChC22 is marginally greater than that of ChC24. This indicates that there are more behenoyl groups attached to chitosan than lignoceroyl groups.
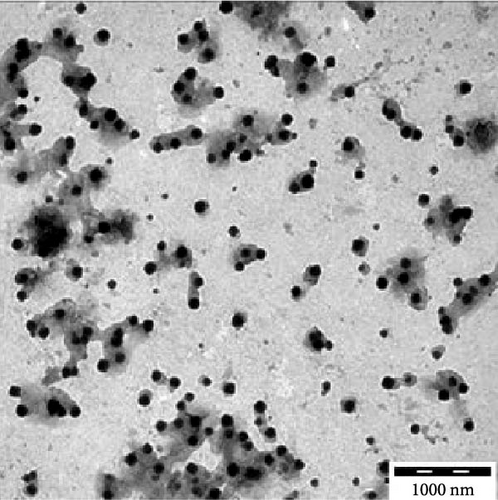
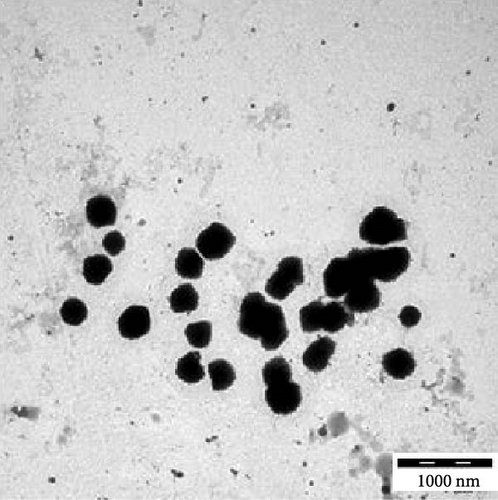
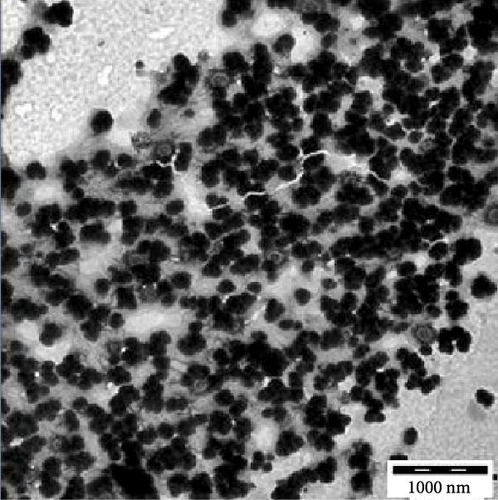
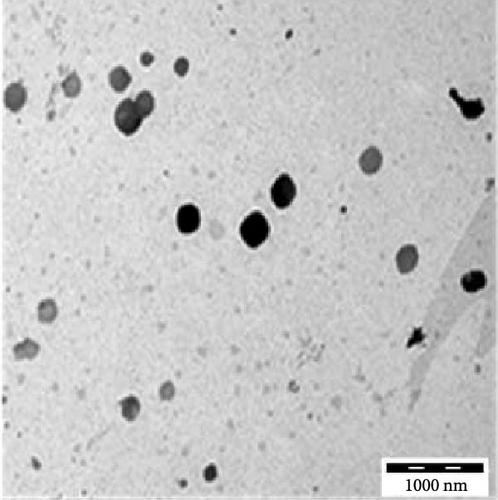
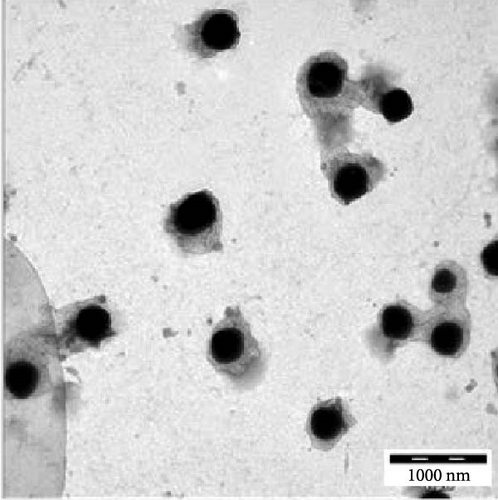
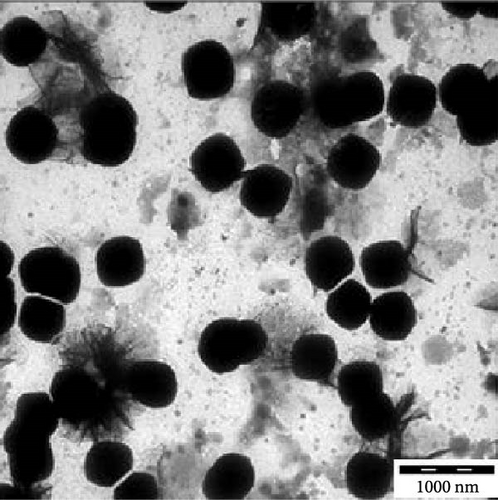
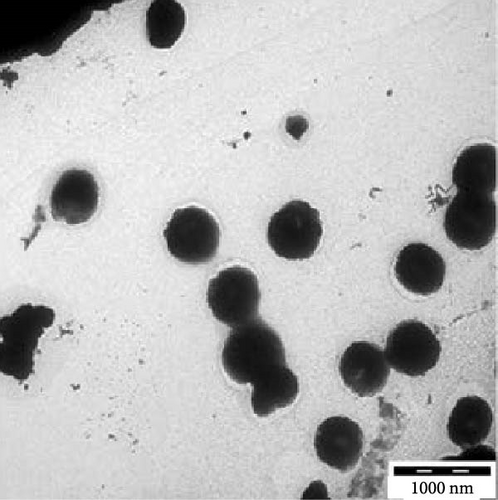
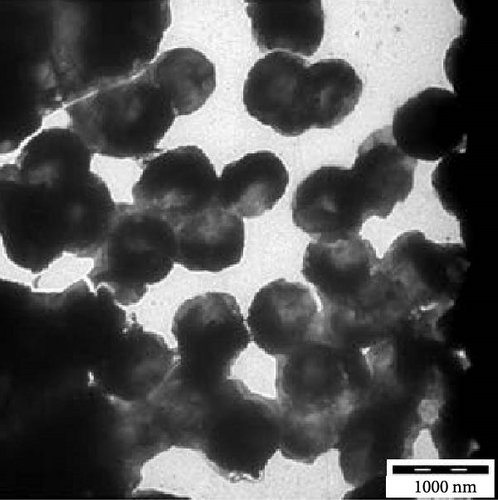
Moreover, as shown in Figure 7e–h, the extended N-acylated chitosan particles maintained their shape following CAP loading. However, their sizes were more pronounced, ranging from 500 to 1000 nm. It is noteworthy that ChC24 particles loaded with CAP had a higher tendency to agglomerate compared to other N-acylated chitosan. This phenomenon arises from van der Waals forces, similar to the results of a previous study conducted by Yanat and Schroën [82]. ChC24 demonstrates a fluctuating distribution of electrons, causing lignoceroyl and hydrophobic groups of CAP to attract each other and form agglomerations. This occurs when attractive forces overpower repulsive forces [83].
The SEM images of the LMWC indicate a compact, irregularly fragmented, and nonporous powder-like structure (Figure 8a). The acylation modified the orientation of the initially flat and horizontal LMWC, allowing them to tilt unevenly from the hydrophobic interactions between the acyl groups in chitosan. The incorporation of CAP into acylated chitosan altered the morphology of its surfaces. After spray-drying, the morphology of CAP-loaded acylated chitosan was observed as particles with a round-like shape. This indicates that extended acylated chitosan is also capable of encapsulating drugs and can serve as a carrier because of its pores.

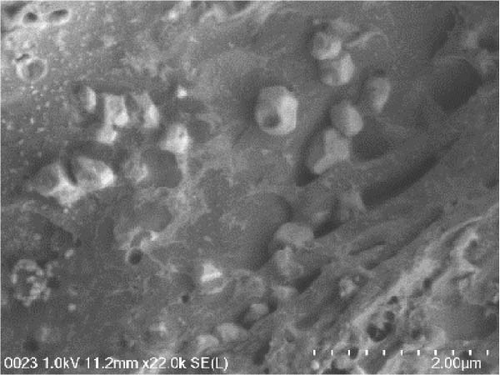



As shown in Figure 8b–d, the particles were not uniform in size, with an average diameter of less than 1 μm for CAP-loaded N-acylated chitosan, which is consistent with the dynamic light scattering measurements. The difference in size could be associated with the solvent effect, where the samples were dehydrated during SEM examination. SEM images revealed that the individual arrangement of particles in CAP-ChC18 made it more stable than other acylated chitosan. In contrast, the formation of clusters in CAP-ChC24 can be attributed to the presence of strong hydrophobic interactions, as shown in Figure 8e. During the freeze-drying process, the particles exhibit reduced mobility, resulting in collisions and subsequent gradual settling, leading to the formation of agglomeration clusters [84].
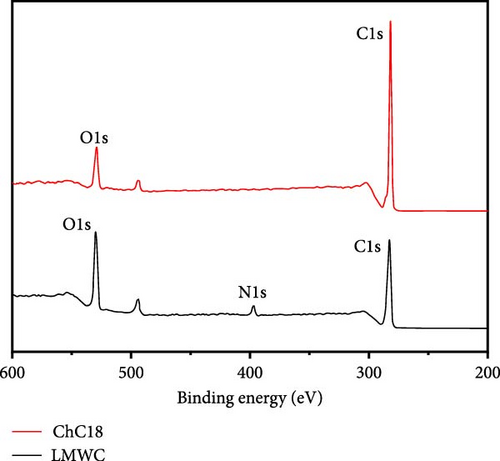
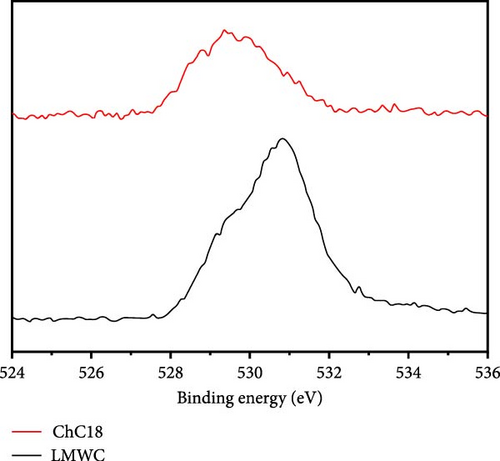
The XPS spectra of the LMWC and ChC18 are shown in Figure 9. Based on these results, the surface of the LMWC was composed of carbon (C) (55.7%), oxygen (O) (42.3%), and nitrogen (N) (1.9%), as indicated by binding energies of 283, 530, and 390 eV, respectively. The C (82.0%) and O (17.9%) atoms in ChC18 also showed marginally altered bond energies of 282 and 529 eV, respectively. Following the modification, the binding energies of the carbon and oxygen atoms in the two samples exhibited slight modifications, indicating alterations in their chemical surroundings. However, N was not detected on the ChC18 surface, suggesting that it was facing inward and bound to the stearoyl groups via van der Waals forces.

3.7. Cytotoxicity Analysis
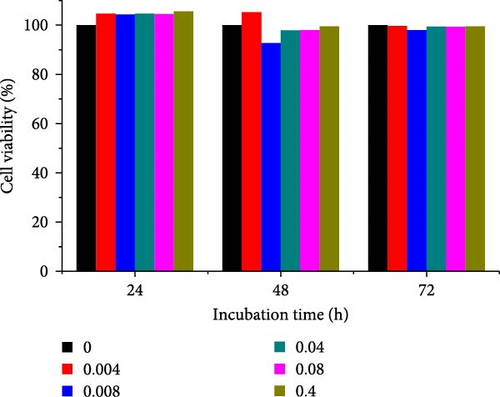
Different concentrations of the N-acylated chitosan were evaluated in A549 (human lung carcinoma) and HeLa (cervical) cell lines to determine the cytotoxicity of the carriers. The extended chain of N-acylated chitosan had a prominent effect on both the cell lines, particularly after 24 h of incubation (Figure 10). The HeLa cells recorded a 2.07–2.21 mg mL−1 of IC50 value after 24, 48, and 72 h, while the A549 cells recorded values within the 0.2–2.6 mg mL−1 range over the same period. No clear trend was observed when different acylated chitosan concentrations were employed, which might be attributable to their varying solubilities in DMEM.
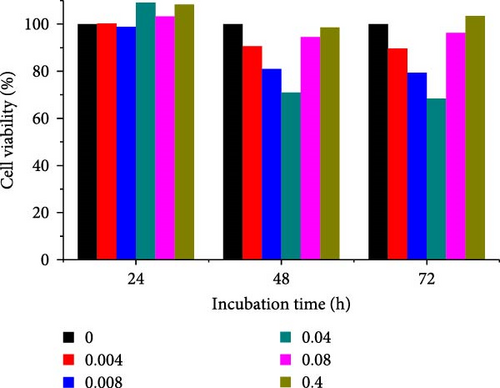
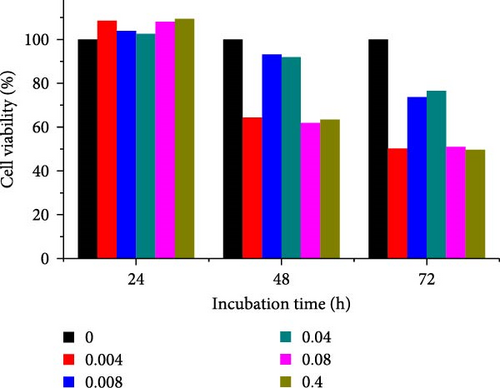
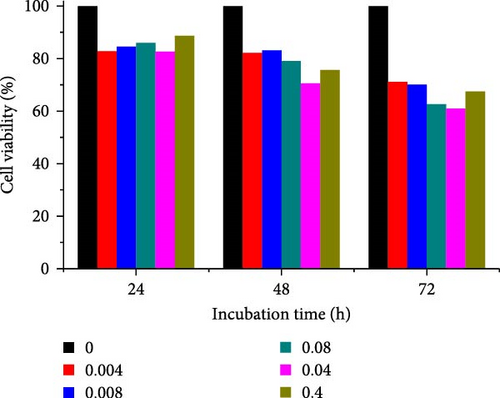
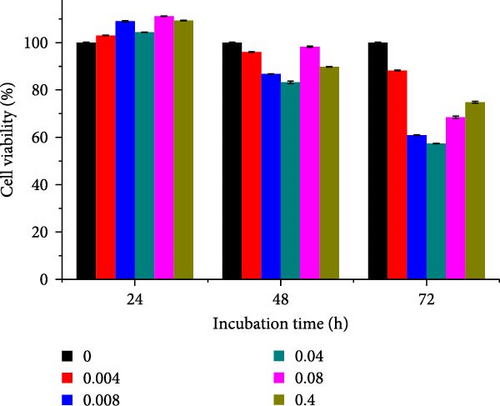
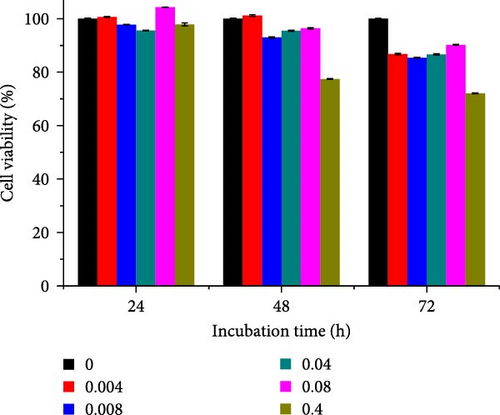
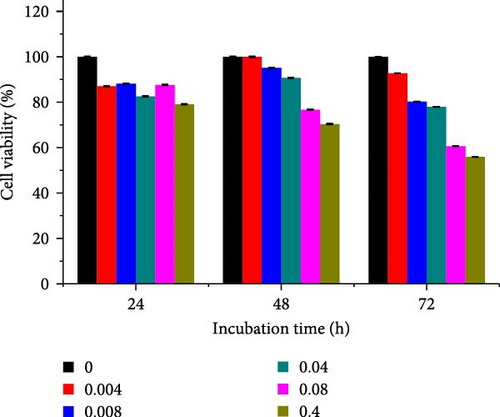
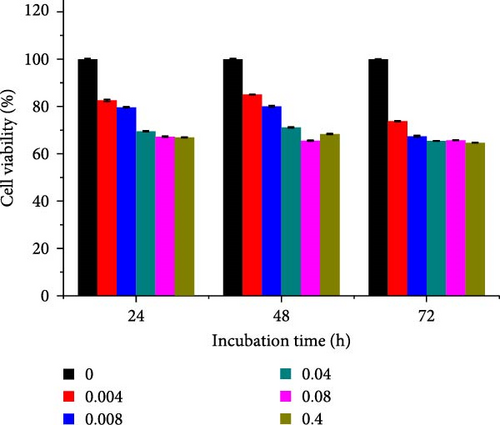
Generally, the extended chain of N-acylated chitosan prepared in the present study produced insignificant effects on the viability of HeLa and A549 cells, as indicated by over 50% viability observed after 24, 48, and 72 h of treatment. Figure 10a–d displays the viability of A549 cells after treatment with extended N-acylated chitosan, including ChC18, ChC20, ChC22, and ChC24, respectively. Different concentrations of ChC20 did not affect the A549 cell line compared to the other three N-acylated chitosans, as illustrated in Figure 10b. The ChC20 chain may exhibit less efficient penetration of the cell membrane compared to other extended N-acylated chitosan, thus resulting in unaltered cell viability in A549 cells.
N-acylated chitosan, such as ChC18, ChC20, ChC22, and ChC24, significantly affected the availability of HeLa cells, as shown in Figure 10e–h, respectively. After 24, 48, and 72 h of incubation, ChC20-treated cells showed minimal effect on HeLa cell viability among the four N-acylated chitosans (Figure 10f). As the concentration of ChC24 increased, the cell availability decreased after treatment. Nevertheless, Hela cells survived at elevated concentrations, possessing cell viability exceeding 50%. Extended N-acylation of chitosan led to minimal cytotoxicity toward A549 and HeLa cells, resulting in a concentration-dependent decrease in cell viability. However, both cell types had survival rates exceeding 50%. The low toxicity of the extended chain of N-acylated chitosan indicates its potential as a carrier.
3.8. EE and DL
EE refers to the proportion of the drug that was successfully encapsulated within the chitosan particles, whereas DL was defined as the amount of drug encapsulated per unit weight of the chitosan particles. These values are fundamental for determining the effectiveness and performance of extended N-acylated chitosan as a drug carrier in this study. Table 3 summarizes the EE and DL of the extended N-acylated chitosan manufactured in this study. The DL capacity of extended N-acylated chitosan improved with increasing amounts of CAP. Nevertheless, a maximum loading capacity of ~90% was observed for the 1:1 weight ratio of the drug to extended N-acylated chitosan. Similarly, the EEs of ChC18, ChC20, and ChC22 increased from ~70% to 90% as the amount of CAP increased, whereas the EE of ChC24 ranged from 85% to 94%.
| Weight ratio (Ch:CAP) | DL (%) | EE (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| ChC18 | ChC20 | ChC22 | ChC24 | ChC18 | ChC20 | ChC22 | ChC24 | |
| 1:0.2 | 14.7 ± 0.6 | 14.3 ± 0.1 | 14.9 ± 0.2 | 17.1 ± 0.3 | 73.5 ± 0.3 | 71.5 ± 0.4 | 74.6 ± 0.3 | 85.5 ± 0.1 |
| 1:0.4 | 35.3 ± 0.5 | 34.0 ± 0.6 | 34.9 ± 0.1 | 35.2 ± 0.4 | 88.3 ± 0.1 | 84.9 ± 0.9 | 87.3 ± 0.8 | 87.9 ± 0.1 |
| 1:0.6 | 55.8 ± 0.5 | 53.9 ± 0.1 | 54.9 ± 0.4 | 55.1 ± 0.2 | 93.0 ± 0.8 | 89.9 ± 0.6 | 91.5 ± 0.3 | 91.8 ± 0.1 |
| 1:0.8 | 74.8 ± 0.6 | 73.9 ± 0.1 | 74.8 ± 0.2 | 74.5 ± 0.1 | 93.5 ± 0.6 | 92.4 ± 0.6 | 93.4 ± 0.4 | 93.1 ± 0.3 |
| 1:1 | 94.9 ± 0.5 | 93.9 ± 0.2 | 94.9 ± 0.3 | 94.2 ± 0.4 | 95.0 ± 0.5 | 93.9 ± 0.6 | 94.9 ± 0.2 | 94.2 ± 0.1 |
The hydrophobic interactions between CAP and N-acylated chitosan could be triggered by the acyl groups facing inward toward the core owing to the addition of the drug. Consequently, a greater probability of drug encapsulation by acylated chitosan results from a higher drug load. These findings were consistent with the TEM images, which revealed a significant increase in the size of CAP-loaded chitosan. This phenomenon was also observed in salicylic acid encapsulated in acylated LMWC, in which salicylic acid was entrapped in the hydrophobic core of the polymer [85].
3.9. In Vitro Drug Release
The cumulative drug release of the different N-acylated chitosans prepared in this study is illustrated in Figure 11. Free CAP was released rapidly within the first 6 h, which agreed with the first-order kinetic model. According to this model, the release rate is proportional to the drug concentration. Furthermore, free CAP was completely released into the medium after 8 h, owing to the concentration gradient. Statistical analysis was conducted using one-way ANOVA to compare the amount of CAP released without the carrier and the amount of CAP loaded in extended N-acylated chitosan. The results showed no significant difference in the mean exam scores between the groups (F [4, 45] = [1.008], p = 0.4132). However, Tukey’s HSD Test for multiple comparisons revealed that there was a significant difference in the mean scores between free CAP and CAP-ChC18 (p = 0) and between free CAP and other extended N-acylated chitosan-CAP.
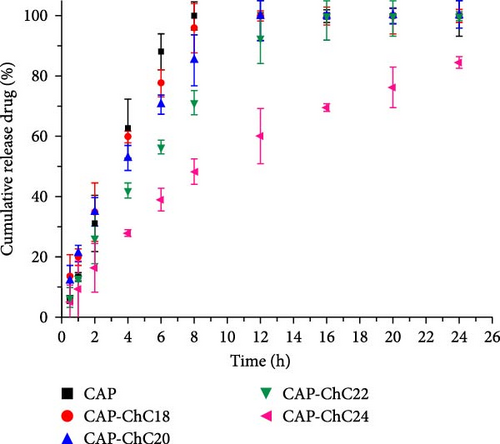
The extended N-acylated chitosan produced in the current study profoundly affected the cumulative drug release. The encapsulated drug diffused into the initial phase before swelling in the polymer matrix. ChC18-, ChC20-, and ChC22-encapsulated CAP were released at ~30% of the CAP within the initial burst phase (the first 2 h). Nonetheless, CAP loaded in ChC24 demonstrated sustained release, as evidenced by less than 20% of CAP recorded within the same period. These observations could be attributed to the stronger intermolecular interactions of the lignoceroyl group with CAP than with stearoyl (ChC18), arachidoyl (ChC20), and behenoyl (ChC22). The mechanism of sustained release could be attributed to the interaction between the drug and the polymer matrix. The ChC24 matrix may exhibit a slower swelling rate than those of ChC18, ChC20, and ChC22. Slower swelling of the matrix can lead to more controlled diffusion of the drug, resulting in sustained drug release over a longer period. In addition, the interaction between CAP and extended N-acylated chitosan may influence the kinetics of drug release. Stronger interactions, such as hydrogen bonds or electrostatic interactions between the drug and the polymer matrix, can hinder drug molecule diffusion, thus causing sustained release.
Table 4 summarizes the kinetic modeling of CAP release in the zero- and first-order models and Higuchi and Korsmeyer–Peppas. In this study, the cumulative drug release followed the Korsmeyer-Peppas model, in which the CAP fraction release was proportional to time in an exponential order. The release exponent (n) of the extended chain of N-acylated chitosan-encapsulated CAP was 0.5 < n < 1, indicating non-Fickian diffusion. This can lead to swelling and stress, which can affect drug diffusion. Consequently, CAP was more likely to swell in the N-acylated chitosan matrix before diffusion into the medium, resulting in a slow release. These findings demonstrated the impact of the length of the acyl group attached to chitosan on the release of drugs.
| CAP loaded different N-acylated chitosan | Zero-order | First order | Higuchi | Kosmeyer–Peppas |
|---|---|---|---|---|
| CAP-ChC18 | R2 = 0.9680 k0 = 8.097 | R2 = 0.9368 k1 = 0.282 | R2 = 0.9799 kH = 33.113 | R2 = 0.9905 kKP = 26.948 n = 0.578 |
| CAP-ChC20 | R2 = 0.8339 k0 = 9.855 | R2 = 0.9847 k1 = 0.217 | R2 = 0.9712 kH = 28.214 | R2 = 0.9923 kKP = 22.826 n = 0.610 |
| CAP-ChC22 | R2 = 0.9101 k0 = 6.562 | R2 = 0.8599 k1 = 0.176 | R2 = 0.9597kH = 26.460 | R2 = 0.9954 kKP = 17.126 n = 0.664 |
| CAP-ChC24 | R2 = 0.8600 k0 = 4.117 | R2 = 0.9907 k1 = 0.078 | R2 = 0.9710kH = 16.738 | R2 = 0.9966 kKP = 12.418 n = 0.613 |
4. Conclusion
The effective modification of chitosan by including long-chain carbon (C18–C24) in its backbone led to the formation of novel N-acylated chitosan. This was verified using a range of analytical techniques such as FTIR, XRD, and 1H-NMR. The introduction of CAP resulted in a notable increase in the particle size, particularly in ChC24, suggesting an enhancement in the hydrophobic characteristics of N-acylated chitosan. The stability of ChC24 in suspension, as indicated by its zeta potential with a maximum value of −27 mV for 0.2 mg of CAP, supports this conclusion, implying that carriers with longer carbon chains are more likely to maintain stability than those with shorter chains. The results demonstrated that the four newly modified chitosan variants displayed less toxicity toward A549 and HeLa cells, suggesting possible benefits as carriers for chemotherapeutic drugs. Kinetic studies indicated that the sustained release of CAP over time was notably more pronounced in N-lignoceroyl chitosan (ChC24) than in other elongated chains. Further studies are recommended to employ a model drug with greater lipophilicity to comprehensively assess the efficacy of this drug carrier. In general, the use of carbon chains exceeding 18 in the acylation of chitosan holds considerable potential as a novel avenue for the encapsulation of drugs with amphiphilic characteristics. This study highlights that achieving uniformity and reproducibility is challenging because of the large particle size and polydispersity, but these characteristics make it possible to accommodate a diverse range of drug molecules with varying sizes and properties. Additionally, low zeta potential can be advantageous for drug carriers because it can facilitate the formation of stable complexes with oppositely charged drug molecules or other excipients, thus improving EE and controlled release properties.
Further investigations should focus on improving the formulation of extended N-acylated chitosan for more effective drug delivery. Moreover, further research should investigate the cellular absorption mechanisms, intracellular movement, and interactions with biological barriers, such as the gastrointestinal mucosa or blood-brain barrier. By investigating this approach, scientists can gain a deeper understanding of the capabilities of N-acylated chitosan as a method for delivering CAP and potentially make advancements in cancer treatment.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Anita Marlina contributed to the conceptualization, data curation, writing—original draft, writing—review and editing; Misni Misran contributed to the conceptualization, funding acquisition, project administration, writing—review and editing; Witta Kartika Restu contributed to the writing—review and editing. All the authors declare that we approve the final version of the manuscript to be published.
Funding
This study was funded by the Malaysian Ministry of Higher Education (FRGS/1/2018/STG07/UM/01/1).
Acknowledgments
This study was funded by the Malaysian Ministry of Higher Education (FRGS/1/2018/STG07/UM/01/1). The authors would like to thank Dr. Lee Poh Foong and Dr. Teoh Boon Yew from the Lee Kong Chian Faculty of Engineering and Science at the University Tunku Abdul Rahman (UTAR) for providing the A549 and HeLa cell lines. In addition, I would like to express my gratitude to Ms. Afiqah Alyaa for her assistance with cytotoxicity assays.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




