Gamma-Induced Transformations in PVC-Based Nanocomposites for Tailored Optical Properties
Abstract
This study explores the effects of gamma irradiation on the properties of polyvinyl chloride (PVC):(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films. Gamma irradiation significantly enhances the optical conductivity (σopt) of the films, signifying increased light-to-current conversion efficiency. This improvement is attributed to a reduction in the bandgap and increased light absorption induced by irradiation. X-ray diffraction (XRD) analysis reveals a decrease in crystallinity due to disrupted atomic order within the nanocomposite. Williamson–Hall (W-H) plots show a dose-dependent reduction in crystallite size and a corresponding rise in lattice strain with increasing irradiation dose. Infrared (IR) spectroscopy suggests potential bond breaking of O-H groups and modifications in C=O functional groups upon irradiation. The absorption coefficient and binding energy exhibit a nonmonotonic response with increasing dose, reaching an optimum at around 100 kGy. These findings demonstrate the potential of gamma irradiation for tailoring the optoelectronic properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposites. This work paves the way for the development of next-generation optoelectronic devices with optimized functionalities.
1. Introduction
Understanding the response of multilayered nanocomposites to gamma irradiation is crucial for their successful application in various fields. This review investigates the effects of gamma radiation on polyvinyl chloride (PVC)/CeO2/TiO2 nanocomposites, a promising material with potential applications in (mention potential applications based on your research). While individual components of this nanocomposite (PVC, CeO2, and TiO2) have been studied under gamma irradiation [1–3], the combined effect on the overall structure and performance of the nanocomposite remains unclear. Existing literature provides valuable insights into potential consequences for each layer: PVC: chain scission leading to decreased mechanical strength and potentially chlorine evolution [4]; CeO2: formation of defects impacting electrical conductivity, catalytic activity, and potentially inducing radioluminescence [5, 6]; and TiO2: phase transformation affecting photocatalytic activity and bandgap energy [2]. However, a critical gap exists in our understanding of how gamma radiation affects the interfaces between these layers. Interface interactions play a crucial role in the overall performance of the nanocomposite. Potential consequences like compromised interface bonding due to structural changes in individual layers need further investigation. This review is aimed at analyzing the existing knowledge on the effects of gamma irradiation on PVC/CeO2/TiO2 nanocomposites and identifying key research gaps. By highlighting these gaps, we can pave the way for future studies to optimize the design and functionality of these nanocomposites for applications potentially involving gamma irradiation. Predicting the precise effects of gamma radiation on your PVC:(50 nm)/CeO2:(10 nm)/TiO2:(3 nm) nanocomposite at different doses (150, 100, 75, and 50 kGy) requires detailed information about the material properties and experimental conditions. Exposure to a high dose of 150 kGy gamma irradiation is expected to induce significant chain scission within the PVC polymer. This splitting process weakens the polymer chains, leading to a substantial decrease in the material’s mechanical strength. Additionally, it could potentially render the material more brittle and susceptible to fracture. Furthermore, the cleavage of the polymer backbone might promote the evolution of chlorine gas. Cerium oxide (CeO₂) nanoparticles: High-dose gamma irradiation can lead to the formation of a considerable number of oxygen vacancies within the CeO₂ nanoparticle lattice. These vacancies significantly alter the electrical conductivity and catalytic activity of the cerium oxide. Moreover, the presence of oxygen vacancies might induce radioluminescence, a phenomenon where the material emits light upon exposure to ionizing radiation [1].
2. Sol–Gel Preparation of PVC:(50 nm)/CeO2:(10 nm)/TiO2:(3 nm) Multilayered Nanocomposite
The sol–gel technique offers a versatile approach for fabricating the desired PVC:(50 nm)/CeO2:(10 nm)/TiO2:(3 nm) multilayered nanocomposite with precise control over precursor chemistry and processing parameters.
2.1. Precursor Preparation
The first step involves preparing individual sols for each layer. PVC powder is dissolved in a suitable solvent, such as tetrahydrofuran (THF) or dichloromethane (DCM), to create a clear and viscous PVC sol. The concentration of the solution is critical and should be adjusted to achieve the targeted final thickness of the PVC layer (50 nm in this case). For the CeO2 layer, a sol is prepared by dissolving cerium nitrate hexahydrate (Ce(NO3)3·6H2O) in ethanol or isopropanol. The sol’s pH is then carefully controlled using appropriate acids or bases to influence the hydrolysis and condensation rates, ultimately affecting the final particle size of CeO2 nanoparticles (targeted at around 10 nm for this specific layer). Similarly, a TiO2 sol is prepared by dissolving titanium tetraisopropoxide (Ti(OiPr)4) in ethanol or isopropanol. As with the CeO2 sol, the pH of the TiO2 sol is adjusted to achieve controlled hydrolysis and condensation, aiming for smaller TiO2 particles with a targeted size of approximately 3 nm for this layer [7].
2.2. Layer Deposition
Prior to deposition, substrate silicon wafers and glass slides are meticulously cleaned and pretreated to ensure optimal adhesion of the nanocomposite film. Spin coating is then employed to deposit the PVC sol onto the prepared substrate. By carefully controlling the spin speed and time, the desired thickness of the PVC layer (50 nm) can be precisely achieved. Subsequently, a sequential dip coating process is implemented to deposit the CeO2 and TiO2 layers onto the PVC-coated substrate. Each dipping cycle involves submerging the substrate into the respective CeO2 and TiO2 sols, followed by controlled withdrawal at an optimized speed. This technique allows for precise control over the thickness of each deposited layer (10 nm for CeO2 and 3 nm for TiO2). Depending on the specific sol properties, drying steps might be necessary between each dip coating cycle [8].
2.3. Sol–Gelation and Annealing
Following deposition, the coated substrate undergoes a gelation process. This typically involves allowing the film to gel at room temperature or under controlled humidity conditions. During gelation, the sols transition into solid gel networks through hydrolysis and condensation reactions. Subsequently, the gelled film is dried in a low-temperature oven (60°C–80°C) to remove residual solvents and solidify the individual layers. Further enhancement of the film’s mechanical and thermal stability can be achieved through a subsequent annealing step at higher temperatures (300°C–500°C). It is important to acknowledge that the annealing process can potentially influence the crystallinity of the oxide layers (CeO2 and TiO2) [9].
2.4. Characterization and Optimization
Once prepared, the multilayered nanocomposite film is thoroughly characterized using various techniques. Scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and atomic force microscopy (AFM) are valuable tools for assessing the layer thicknesses, morphology, and crystallinity of the film. The obtained characterization results can then be utilized to optimize the sol compositions, pH adjustments, deposition techniques, and annealing parameters. This optimization process is crucial for achieving the desired properties and targeted layer thicknesses tailored for the specific application. Compatibility of solvents with each other and the chosen substrate material should also be carefully considered during optimization [7]. An alternative deposition approach for the CeO2 and TiO2 layers might involve utilizing preformed colloidal suspensions of these nanoparticles instead of sols. This approach has the potential to offer finer control over particle size and distribution within the layers. Maintaining a clean and controlled environment throughout the entire preparation process is paramount to minimizing contamination and ensuring the fabrication of a high-quality nanocomposite film. This established sol–gel method provides a reliable foundation for the preparation of the targeted PVC:(50 nm)/CeO2:(10 nm)/TiO2:(3 nm) nanocomposite film with precise control over individual layer properties [10].
3. Materials
PVC serves as the base material for the nanocomposite films. Targeted functionalities are achieved through the incorporation of metal oxide nanoparticles, specifically cerium oxide (CeO2) and titanium dioxide (TiO2). The resulting nanocomposite structure is a trilayer film with a designated thickness profile: PVC (50 nm)/CeO2 (10 nm)/TiO2 (3 nm) [11]. Sol–gel processing offers a well-established technique for meticulously controlling the composition and distribution of the embedded nanoparticles within the PVC matrix. This method allows for the precise orchestration of the nanocomposite’s properties. The film assembly itself is a relatively straightforward process involving the spin coating of a nanoparticle-infused PVC solution onto a suitable substrate. This initial coating dries to form a uniform thin film, ready for further treatment. Gamma irradiation serves as the key technique for modulating the properties of the nanocomposite films. Exposing the films to controlled doses of gamma radiation can induce a cascade of microscopic alterations within the material, effectively reshaping its characteristics. XRD analysis is a valuable tool for investigating these changes. It can reveal alterations in the crystallinity of the film, potentially indicating a more ordered arrangement between the polymer chains and the nanoparticles. Such changes could translate to enhanced mechanical and thermal properties. Further insights into the material’s composition can be gleaned from energy-dispersive X-ray (EDX) analysis [12]. This technique provides a detailed picture of the elemental composition, allowing to identify subtle shifts in the relative abundance of PVC and the incorporated nanoparticles [13]. These variations can hold the key to understanding changes in electrical or magnetic properties of the nanocomposite. Fourier transform infrared (FTIR) spectroscopy offers a complementary perspective by focusing on the interactions between molecules within the composite. This analysis can reveal potential modifications in the interplay between PVC and the nanoparticles, potentially affecting the film’s flexibility and barrier properties.
For a deeper understanding of the impact of radiation on the CeO2 and TiO2 nanoparticles specifically, electron paramagnetic resonance (EPR) spectroscopy is employed. This technique delves into the realm of magnetic centers within the material, charting their evolution upon exposure to gamma radiation. Insights gained from EPR can inform the development of magnetic sensing or device applications. The most dramatic transformations induced by gamma irradiation might be observed in the realm of light absorption and interaction. UV-Vis spectroscopy is a powerful tool for investigating these changes [14]. It can reveal alterations in the optical bandgap of the irradiated films, potentially leading to modifications in transparency, color, and even electrical conductivity. The ability to fine-tune these optical properties through controlled gamma irradiation opens doors to a variety of applications, including flexible optoelectronic devices, sensors, and even photovoltaics. The potential of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films extends far beyond these initial explorations. By meticulously optimizing irradiation parameters, the nanocomposite composition, and film assembly techniques, can create materials with tailor-made functionalities for diverse applications. This opens doors to the development of radiation-resistant electronics, biocompatible medical devices, and a future brimming with possibilities, all illuminated by the transformative power of gamma irradiation [15].
4. Results and Discussion
4.1. The Light and Structure
Ultimately, the intricate interplay between defects, bandgaps, and gamma irradiation culminates in a remarkable transformation of the optical properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films. By manipulating the irradiation dose and intensity, researchers can fine-tune the bandgap of these materials, opening doors to a plethora of exciting applications. Sensors: Narrower bandgaps can render these films more sensitive to specific wavelengths of light, enabling the development of sensors for environmental monitoring or medical diagnostics. Displays: With tunable bandgaps, these materials can be utilized in dynamic displays capable of producing vibrant and adjustable colors. Solar cells: Tailoring the bandgap can optimize light absorption for efficient solar energy conversion. The possibilities, akin to the intricate dance of light and structure within these nanocomposite films, are as boundless as human imagination. By deciphering the language of defects and bandgaps, researchers can unlock the true potential of these chameleonic materials, shaping the future of technologies that interact with light in captivating and transformative ways [16].
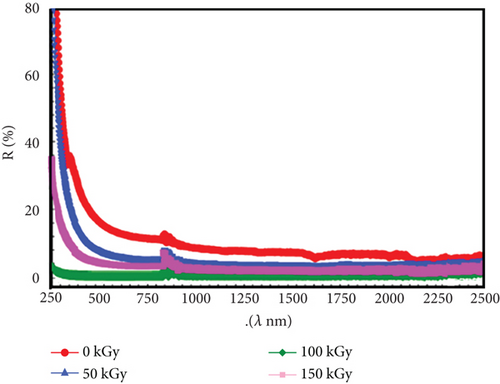
Figure 1 explains the effect of gamma rays at different doses on the compound PVC/CeO2/TiO2. Several curves are plotted on the graph, each representing the absorption spectrum for a PVC/CeO2/TiO2 nanocomposite film that received a different gamma radiation dose. The curves are labelled with the gamma radiation dose in kilogray, which is a unit of absorbed ionizing radiation dose. The different curves correspond to 0 kGy (control group, not exposed to gamma radiation), 50 kGy, 100 kGy, and 150 kGy. From the shape of the curves, we can see some general trends. Increased absorption at lower wavelengths: Across all curves, there is a trend of generally higher absorbance at lower wavelengths (towards the left side of the x-axis) especially below 500 nm. This suggests that the PVC/CeO2/TiO2 nanocomposite film absorbs light more efficiently in the UV and blue light regions of the spectrum. The effect of gamma irradiation on the absorbance is that there might be a slight increase in absorbance after exposure to higher gamma radiation doses (100 and 150 kGy) compared to the control group (0 kGy) across a range of wavelengths, particularly between 300 and 800 nm. Understanding how gamma irradiation affects the absorbance of this nanocomposite film can be crucial for various applications. For instance, depending on the specific changes, the material might become more suitable for UV protection or light-harvesting devices.
4.2. Unveiling of Defects and Bandgaps in PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites
The optical properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films exhibit remarkable tunability, akin to a chameleon adapting its color based on its environment. This fascinating interplay of light and matter unfolds at the atomic level, orchestrated by the intricate interplay between defects, bandgaps, and the influence of gamma irradiation [17]. Defects as architects of light interaction: The presence of the CeO2 phase within the nanocomposite introduces structural imperfections known as defects. These defects disrupt the otherwise ordered crystalline lattice of the material. By creating localized energy states within the band structure, defects significantly influence how light interacts with the material. Depending on their type and distribution, defects can introduce new absorption bands, modify existing ones, or even induce luminescence. In essence, defects act as the architects of color within the material’s electronic landscape. The bandgap: a gateway for photon absorption: The bandgap (Eg), a fundamental concept in semiconductor physics, represents the energy difference between the valence and conduction bands within a material. This energy gap dictates which wavelengths of light can be absorbed or emitted. A wider bandgap allows only higher energy photons, such as ultraviolet light, to be absorbed, while a narrower gap permits the absorption of lower energy photons, such as visible light. Defects, by introducing new energy levels within the bandgap, can modify its size and thereby alter the range of wavelengths the material interacts with, influencing its perceived color.
4.3. Unveiling of Light and Structure in PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites
The optical properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films exhibit remarkable tunability, akin to a chameleon adapting its color based on its environment. This fascinating interplay of light and matter unfolds at the atomic level, orchestrated by the intricate interplay between defects, bandgaps, and the influence of gamma irradiation [20].
4.3.1. Sculpting the Crystalline Landscape: Diminishing Disorder
Gamma irradiation plays a pivotal role in sculpting the optical properties of the nanocomposite. At low doses, it acts as a microscopic sculptor, meticulously reducing structural irregularities and defects within the crystalline lattice of CeO2 nanoparticles. The CeO2 lattice can be visualized as a delicate network holding atoms in specific positions. Gamma rays, like microscopic surgeons, remove imperfections and smoothen the network, reducing strain. This translates to a more ordered and relaxed atomic configuration within the material [19].
4.3.2. The Bandgap: A Conductor for the Color
4.3.3. Beyond the Bandgap: A Chorus of Contributing Factors
4.3.4. Tuning the Light: Unveiling the Potential Applications
By understanding the intricate interplay between defects, bandgaps, and gamma irradiation, researchers can tailor the irradiation conditions to achieve the desired optical properties in PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films. This knowledge opens doors to a plethora of exciting applications across diverse fields. Photovoltaics: Tailoring the bandgap can optimize light absorption for efficient solar energy conversion in photovoltaic devices. LEDs: By precisely controlling the bandgap, these nanocomposites could be developed into LEDs capable of emitting specific colors. Sensors: Narrower bandgaps can render these films more sensitive to specific wavelengths of light, enabling the development of sensors for environmental monitoring or medical diagnostics [22]. Biocompatible materials: By carefully controlling the irradiation process, these materials could potentially be engineered for biocompatible applications. The relationship between gamma irradiation dose (D) and bandgap energy (Eg) can be modeled using fitting equations (Eg = f(D)). However, the specific function (f) depends on the material properties and irradiation conditions. Further research is needed to fully characterize these dependencies and optimize the irradiation process for targeted applications. It is important to note that this fitting function likely incorporates complex interactions between defect states, bond formation, and strain, requiring further investigation to establish a robust predictive model.
4.4. Unveiling the Light-Harvesting Potential: Sculpting the Optical Properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites With Gamma Irradiation
The optical properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films exhibit remarkable tunability under gamma irradiation, akin to a hidden masterpiece being unveiled. This fascinating phenomenon unfolds at the atomic level, orchestrated by the intricate interplay between defects, bandgaps, and the influence of gamma rays [23].
4.4.1. Defect Removal: A Brighter Stage for Light Absorption
4.4.2. Photons: Bandgap Modulation and Enhanced Absorption
4.4.3. The Light: Unveiling a Spectrum of Potential Applications
4.5. Unveiling the Light-Matter Ballet: Orchestrating Refractive Index and Extinction Coefficient in PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites With Gamma Irradiation
The interaction of light with PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films unfolds like a captivating ballet, meticulously choreographed by the interplay between the material’s structure and the invisible conductor of gamma irradiation. In this intricate dance, two key parameters take center stage, the refractive index (n) and the extinction coefficient (k), governing how light bends and disappears within the material [26].
4.5.1. Refractive Index: Light Bending
The refractive index dictates the degree to which light bends within the material. Imagine a ballerina gracefully changing direction; the refractive index governs the light’s equivalent maneuver. In our nanocomposite films, gamma irradiation orchestrates a fascinating response in the refractive index. At lower doses (up to 100 kGy), n unexpectedly dips. This seemingly paradoxical behavior arises from a combination of factors: the formation of active sites that reduce the film’s anisotropic nature and the relaxation of spinel lattice strain caused by the diminishing disorder within the α-CeO2 phase. However, at higher doses (150 kGy), n rebounds due to crosslinking, bond breaking, and steric strain within the material [27].
4.5.2. Extinction Coefficient: A Light Absorption
While the refractive index guides light’s path, the extinction coefficient (k) quantifies the material’s avidity for absorption. In our nanocomposite films, k’s response to gamma irradiation paints a distinct picture. As the irradiation dose increases up to 150 kGy, k steadily rises, signifying enhanced light absorption. This behavior stems from the combined effects of a narrowed optical bandgap and the presence of the CeO2 phase. However, beyond 150 kGy, k embarks on a downward trajectory, indicating a reduction in light absorption at higher doses.
4.5.3. Unveiling the Atomic: Oscillator and Dispersion Energies
To gain deeper insights into the light-matter ballet, we can delve into the realm of quantum mechanics. The oscillator energy (Eo) and dispersion energy (Ed), calculated using the Wemple–DiDomenico relationship, reflect the electronic transitions within the material. These parameters offer a glimpse into the atomic-level tango between light and electrons. Both Eo and Ed exhibit a dip with increasing irradiation doses up to 100 kGy, followed by a resurgence at 150 kGy. This behavior suggests a modulation of electronic transitions within the material, influenced by the aforementioned structural changes induced by gamma irradiation [28].
4.5.4. A Light Control: Unveiling a Spectrum of Applications
4.6. Unveiling the Light-Matter Duet: Orchestrating Optical Properties in PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites With Gamma Irradiation
The interaction of light with PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films unfolds like a well-rehearsed duet, meticulously choreographed by the invisible conductor of gamma irradiation. In this intricate performance, a harmonious interplay between material structure and light governs the flow of photons. Key parameters like the refractive index (n), extinction coefficient (k), and dielectric constants (ε) dictate this interaction. By deciphering the language of these parameters and their response to irradiation, researchers can fine-tune the optical properties of these films, unlocking a spectrum of potential applications [29, 30].
4.6.1. Refractive Index and Extinction Coefficient
The refractive index (n) dictates the degree of light bending within the material, similar to how a ballerina gracefully changes direction. In our nanocomposite films, gamma irradiation orchestrates a fascinating response in n. At lower doses (up to 100 kGy), n unexpectedly dips. This behavior arises from a combination of factors: the formation of active sites that reduce the film’s anisotropic nature and the relaxation of spinel lattice strain caused by the diminishing disorder within the α-CeO2 phase. However, at higher doses (150 kGy), n rebounds due to crosslinking, bond breaking, and steric strain within the material. The extinction coefficient (k) quantifies the material’s avidity for light absorption. In our nanocomposite films, k’s response to gamma irradiation paints a distinct picture. As the irradiation dose increases up to 150 kGy, k steadily rises, signifying enhanced light absorption. This behavior stems from the combined effects of a narrowed optical bandgap and the presence of the CeO2 phase. However, beyond 150 kGy, k embarks on a downward trajectory, indicating a reduction in light absorption at higher doses. This interplay between n and k orchestrates the overall light propagation and absorption within the films, laying the foundation for their tunable optical properties.
4.6.2. Dielectric Constants
The dielectric constants, εr (real part) and εi (imaginary part), provide valuable insights into the material’s overall response to electromagnetic fields. In these nanocomposite films, bothεrandεiexhibit photon energy dependence—they increase with increasing photon energy, indicating a stronger interaction between light and the material at higher energies. This aligns with the observed trends innandk, highlighting the collective response of the material to light across the spectrum— and nonmonotonic response to irradiation—they mirror the behavior of n and k, decreasing up to 100 kGy and increasing at 150 kGy. This suggests a complex interplay between structural changes and optical properties, reinforcing the notion of gamma irradiation as a conductor delicately influencing the material’s interaction with light at the atomic level [31].
4.6.3. Static Parameters: The Material’s Foundation
The static refractive index (n0), static dielectric constant (εs), and optical oscillator strengths (f) offer a glimpse into the material’s fundamental optical properties in the absence of external fields. In these nanocomposite films, their behavior under gamma irradiation aligns with the previously observed trends, decreasing up to 100 kGy and increasing at 150 kGy. This consistency underscores the interconnectedness of the material’s intrinsic and irradiation-induced optical characteristics. Gamma irradiation wields a remarkable influence over the optical properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films. By carefully choreographing the refractive index, extinction coefficient, and various energy parameters, it crafts a delicate duet between light and matter. This interplay reveals the potential for tailored optical responses in diverse applications, ranging from miniaturized waveguides to tunable filters and enhanced photovoltaics. Further exploration of these intricate relationships promises to compose a symphony of innovations, illuminating the future of light-matter interactions at the nanoscale [32].
4.7. Unveiling the Static Landscape: Gamma Irradiation’s Influence on Fundamental Optical Properties in PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites
Beyond the dynamic interplay of refractive index and extinction coefficient lies a hidden realm within PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films: the influence of gamma irradiation on their fundamental optical properties. Here, we explore the effects of irradiation on the static refractive index (n0) and static dielectric constant (εs), which are intrinsic constants defining the material’s response to light in the absence of external fields [33].
4.7.1. A Unified Response: Reflecting Change Across Parameters
Intriguingly, the behavior of n0 and εs under gamma irradiation mirrors the trends observed for other optical parameters. Similar to the dynamic refractive index, both constants exhibit a decrease with increasing doses up to 100 kGy. This suggests a reduction in the material’s inherent ability to interact with light. However, reminiscent of the extinction coefficient, n0 and εs rebound at 150 kGy, showcasing a complex interplay between structural alterations and fundamental optical properties. This unified response across diverse optical parameters underscores the profound influence of gamma irradiation at the material’s core.
4.7.2. Untangling the Web of Influences: A Multifaceted Effect
This enigmatic response can be attributed to a confluence of factors [34]. Optical bandgap modulation: Gamma irradiation narrows the bandgap, facilitating easier light penetration and contributing to the initial decrease in n0 and εs. α-CeO2 phase influence: The presence of the secondary α-CeO2 phase introduces additional scattering centers, hindering light interaction and further reducing n0 and εs. Degradation and crosslinking: At higher doses, these competing processes become dominant. While degradation weakens the material’s response, crosslinking introduces new polarizable bonds, leading to the observed increase in n0 and εs.
4.7.3. Tailoring Optics Through Irradiation
This intricate dance between gamma irradiation and fundamental optical properties holds immense potential for material engineering. By manipulating the irradiation dose and intensity, researchers can fine-tune n0, εs, and other static parameters, crafting materials with tailored optical responses for diverse applications [35]. Enhanced transparency: Materials with lower n0 and εs could exhibit increased transparency, ideal for window coatings or optical sensing devices. Tunable nonlinear optical response: Materials with controlled n0 and εs could exhibit tailored nonlinear optical effects, finding applications in laser frequency conversion or optical switching technologies.
4.8. Unveiling the Electron’s Journey: Decoding Volume Energy Loss Function (VELF) and Surface Energy Loss Function (SELF) in PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites
Both VELF and SELF are inextricably linked to the material’s complex dielectric function, a nuanced entity capturing its response to an electric field. The real part (ε1), analogous to a savings account, stores energy, while the imaginary part (ε2), akin to a leaky faucet, represents energy lost. These components orchestrate a captivating dance, ultimately giving rise to the energy loss functions. VELF: Proportionate to ε2 and inversely proportional to the sum of ε1 2 and ε2 2, the VELF paints a vivid picture of bulk energy dissipation, governed by the intricate interplay between stored and lost energy. SELF: Integrating the difference between ε1 and 1, weighted by the exponential decay of the electric field within the material, the SELF unveils the energy specifically lost at the surface, revealing the complex interplay between surface properties and electron interactions.
4.8.1. Gamma Irradiation: A Conductor of Energy Flow
This seemingly static scenario undergoes a dramatic transformation under the influence of gamma irradiation. Up to 100 kGy, both VELF and SELF experience a noticeable increase, signifying enhanced energy depletion for electrons. This phenomenon can be attributed to two key factors. Changes in bandgap: The irradiation-induced narrowing of the optical bandgap facilitates easier electron excitations, leading to increased energy loss. Presence of α-CeO2 phase: The introduction of this secondary phase introduces additional scattering centers, hindering electron movement and further contributing to energy dissipation [36]. However, at 150 kGy, the trend reverses. Both VELF and SELF embark on a downward trajectory, suggesting a reduction in energy loss. This intriguing response likely arises from a complex interplay of competing processes. Degradation: Weakening the material’s structure can offer smoother passage for electrons, reducing their overall energy loss. Crosslinking: This process introduces new bonds, potentially creating alternative pathways for energy exchange and alleviating the burden on bulk and surface interactions.
4.8.2. From Understanding to Tailored Materials
Deciphering the language of VELF and SELF under gamma irradiation holds immense potential for material design. By manipulating irradiation doses and exploring different material compositions, researchers can engineer materials with tailored optical properties—fine-tuning the interplay between VELF and SELF can lead to materials with enhanced transparency or controlled nonlinear optical responses for diverse applications—optimize charge transport—materials with reduced energy loss can exhibit improved conductivity, finding applications in solar cells, sensors, and optoelectronic devices— and develop radiation-resistant materials—understanding the complex response of VELF and SELF to gamma irradiation can inform the design of materials with enhanced radiation resistance for critical applications.
4.9. Unveiling Opportunities: Tailoring Light Interactions in Gamma-Irradiated Nanocomposites
The intricate interplay between electron transport, energy loss, and gamma irradiation within PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposites presents a compelling opportunity to manipulate light interactions. This interplay is governed by the VELF and SELF, quantifying energy dissipated by electrons traversing the bulk and surface regions, respectively. By understanding and manipulating these functions through controlled gamma irradiation, researchers can unlock a treasure trove of exciting possibilities [37].
4.9.1. Tailored Light Absorption for Enhanced Solar Cells
Fine-tuning the imaginary component of the dielectric constant within both the bulk (VELF) and surface (SELF) regions can optimize light absorption across the desired spectrum. This targeted manipulation can significantly improve the efficiency of photovoltaic materials, leading to enhanced solar energy harvesting.
4.9.2. Engineering Surface Plasmon Resonances
Precise control of SELF, particularly the imaginary part, in metallic nanocomposites allows for the design of materials with specific light-scattering properties. This opens doors for novel applications in plasmonic sensors, waveguides, and metamaterials, where controlled manipulation of surface electromagnetic waves is crucial [38].
4.9.3. Developing Advanced Sensors Through Unveiling Energy Loss Functions
Unraveling the intricate relationship between gamma irradiation, VELF, and SELF paves the way for the development of highly sensitive and selective sensors. By monitoring changes in energy loss functions under various stimuli, researchers can design materials capable of detecting specific analytes with unprecedented precision.
4.9.4. A Nuanced Perspective: Bulk and Surface in Harmony
It is crucial to recognize that the energetic journey of electrons within these nanocomposites is influenced by both bulk and surface interactions. VELF captures the intricate energy dissipation within the material’s core, while SELF unveils the dynamic energy exchange at the material-environment interface. This interplay necessitates a nuanced understanding of how gamma irradiation affects both VELF and SELF to achieve targeted manipulation of light interactions.
4.9.5. Pushing the Boundaries
By delving with the ability to fine-tune VELF and SELF through gamma irradiation, we can design materials with unprecedented control over light absorption, scattering, and propagation. This opens doors for a plethora of groundbreaking applications in solar energy, sensing, optics, and beyond. The captivating world of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposites awaits further exploration, promising to illuminate the path towards a future brimming with exciting discoveries [39].
4.10. Unveiling the Light-Charge Ballet: Optical Conductivity (σopt) in PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites
4.10.1. Gamma Irradiation: A Conductor of Light-to-Current Conversion
Gamma irradiation has a remarkable effect on σopt in these nanocomposite films. As the irradiation dose increases, σopt increases as well, signifying an enhanced ease of light-to-current conversion. This transformation arises from a complex interplay of factors. Decreased bandgap: Under irradiation, the optical bandgap, the energy required for electron excitation, narrows. This facilitates light-induced electron transitions, promoting their contribution to conductivity. Enhanced light absorption: Gamma irradiation can introduce defects or modify the electronic structure, leading to increased absorption of light across a broader spectral range. This translates to a larger pool of excited electrons, further contributing to the current flow.
4.10.2. A Unified Narrative: Volume, Surface, and σopt
Interestingly, the story of σopt finds a parallel narrative in the behavior of VELF and SELF. As discussed previously, these functions quantify the energy lost by electrons traversing the bulk and surface regions, respectively. Both VELF and SELF exhibit a similar upward trend with increasing irradiation doses up to 100 kGy, suggesting a higher probability of energy exchange between electrons and the material. This seemingly counterintuitive trend, however, holds the key to understanding the rise in σopt. Imagine an energetic electron, excited by a photon. As it travels through the material, it loses energy through interactions with surrounding atoms and defects. However, under gamma irradiation, these interactions can sometimes lead to the transfer of energy back to the electron, propelling it further and contributing to the overall current flow. This subtle interplay between energy loss and gain, reflected in both VELF/SELF and σopt, unveils the intricate dance orchestrated by gamma irradiation within these nanocomposite films [41].
4.10.3. Tailoring Light With σopt
Deciphering the language of σopt and its response to gamma irradiation opens doors to a plethora of possibilities. By manipulating irradiation doses and exploring different material compositions, researchers can craft efficient solar cells—tailoringσoptcan optimize light absorption and enhance charge generation within the device, leading to improved solar cell efficiency—design sensitive photodetectors—Engineeredσoptcan create materials with heightened response to specific wavelengths of light, paving the way for advanced light detection technologies— and develop vibrant LEDs—tailoringσoptproperties can optimize light emission and create efficient, customizable LEDs with desired spectral characteristics. The captivating story of σopt in PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films illuminates the transformative power of gamma irradiation in sculpting light-matter interactions. By wielding this tool with precision, researchers can design materials that dance with light and electricity, shaping the future of optoelectronic technologies and beyond [42].
4.11. Unveiling the Material’s Fingerprint: Combined XRD and EDX Analyses
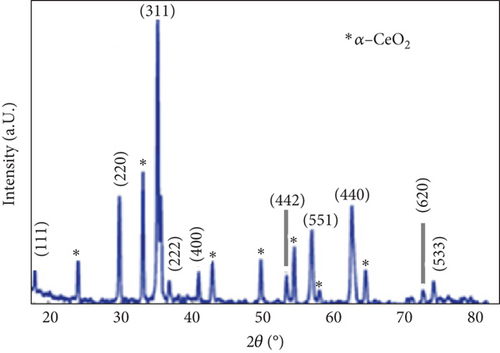
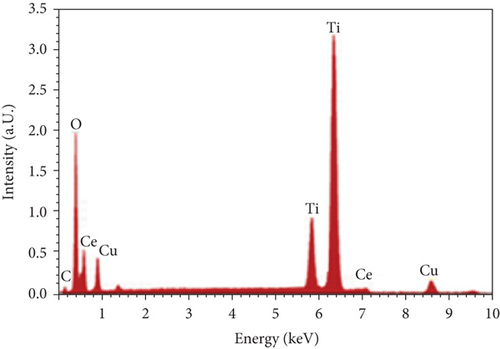
Figure 2 presents a combined characterization of a PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite using XRD and EDX spectroscopy. XRD analysis: The XRD pattern (Figure 2(a)) reveals sharp peaks, indicative of substantial crystallinity within the material. This suggests a well-defined long-range order in the atomic arrangement. However, a relatively broad peak around 20° hints at the possible presence of an amorphous phase alongside the crystalline domains. Further investigation into the influence of this amorphous phase on the material’s properties, particularly its mechanical behavior, would be of significant value. EDX analysis: The EDX spectra (Figure 2(b)) confirm the presence of the expected elements: carbon (C), oxygen (O), cerium (Ce), and titanium (Ti). These findings provide a crucial initial insight into the material’s elemental composition. However, the relative intensities of the peaks deviate from the expected stoichiometry for the targeted PVC/CeO2/TiO2 composition. This discrepancy could arise from various factors. Inhomogeneous elemental distribution: The elements might not be uniformly distributed throughout the material, leading to localized variations in stoichiometry. Presence of undetected elements: The EDX spectra may not capture all elements present in the sample, particularly those with low abundance. Chemical bonding and oxidation states: The oxidation states of elements like Ce or Ti can affect their peak intensities in the EDX spectra. Addressing these questions through further analysis using techniques such as high-resolution TEM (HRTEM) and X-ray photoelectron spectroscopy (XPS) will provide a deeper understanding of the material’s structure, elemental distribution, and potential interactions between components. Overall, the combined XRD and EDX data offer a valuable initial characterization of the PVC:(50 nm)/CeO2:(10 nm)/TiO2 composite, laying the groundwork for further exploration of its properties.
4.12. Unveiling the Crystalline Landscape: XRD Analysis of Pristine and Irradiated Nanocomposites
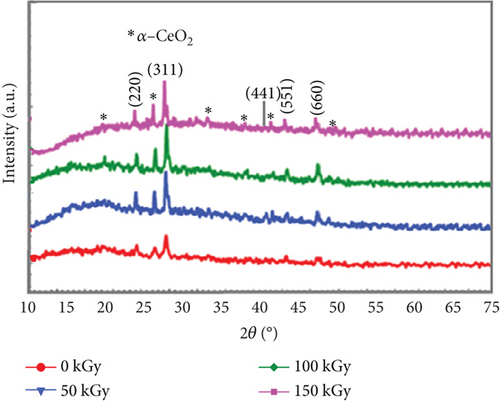
Figure 3 explores the XRD morphological characteristics of pristine and gamma-irradiated PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films. Pristine film (Figure 3): Sharp, well-defined peaks in the pristine film indicate a high degree of crystallinity, signifying a highly ordered, repeating pattern of atomic arrangement. The positions of these peaks correspond to specific interplanar distances within the crystal lattice of PVC, CeO2, and TiO2. By analyzing these peak positions, researchers can identify the crystal structures present. Additionally, the relative peak intensities reflect the relative abundance of the different crystalline phases within the film. Irradiated films (50 and 100 kGy): Compared to the pristine film, the peaks in the irradiated films exhibit broadening, suggesting a decrease in crystallinity due to the gamma irradiation. This signifies a disruption of the ordered atomic arrangement. In some cases, the peaks might also shift slightly in position after irradiation, potentially due to changes in the crystal lattice parameters. The relative intensities of the peaks might change as well, indicating alterations in the abundance of crystalline phases or the formation of new phases induced by irradiation. Overall observations: Gamma irradiation has a noticeable effect on the crystallinity of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films. The observed decrease in crystallinity suggests that gamma rays disrupt the ordered atomic arrangement within the material. The extent of this disruption appears to be dose-dependent. As shown in Figure 3, the broadening of peaks and changes in peak intensities are more pronounced at the higher irradiation dose (100 kGy) compared to the lower dose (50 kGy).
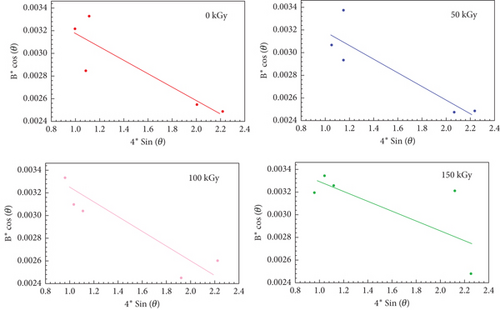
4.13. Unveiling the Nanoscale: Williamson–Hall (W-H) Analysis of Crystallite Size and Strain
Figure 4 presents W-H plots for PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films, elucidating the evolution of crystallite size and strain under varying gamma irradiation doses. W-H plots depict the full width at half maximum (FWHM) of XRD peaks (β) against their corresponding cos(θ) values. The slope and y-intercept of the resulting linear relationship provide estimates for crystallite size and strain, respectively. Analysis of the plots reveals intriguing trends. Crystallite size: The y-intercept of the lines progressively decreases with increasing irradiation dose. This signifies a dose-dependent reduction in average crystallite size, potentially attributable to gamma-induced fragmentation of larger crystals into smaller units. Strain: Conversely, the slope of the lines exhibits a gradual increase with higher doses, indicating a corresponding rise in lattice strain within the material. Gamma irradiation can introduce various defects into the crystal lattice, such as vacancies or interstitials. These defects distort the regular atomic arrangement and lead to increased strain. These observed changes in crystallite size and strain are expected to significantly impact the properties of the nanocomposite films. Potential consequences include alterations in mechanical strength, electrical conductivity, and thermal stability. However, it is crucial to acknowledge the limitations of the W-H model. It is a simplified approach, and additional factors besides gamma irradiation might contribute to the observed phenomena. Moreover, the accuracy of the extracted parameters depends on the quality of the underlying XRD data and the assumptions made during the analysis. Overall, the W-H plots offer valuable insights into the dose-dependent modifications in crystallite size and strain experienced by the PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films under gamma irradiation. Further investigations are warranted to explore the precise mechanistic effects of irradiation and translate these observations into a comprehensive understanding of the material’s altered properties.
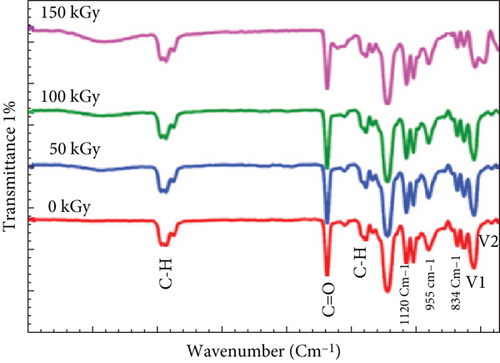
4.14. Unveiling the Chemical Landscape: IR Spectroscopy Analysis
Figure 5 presents the IR spectra of pristine and gamma-irradiated PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films, providing insights into their chemical structure and modifications induced by irradiation. Pristine film: O-H stretching (3300 cm-1): A broad peak around 3300 cm−1 corresponds to O-H stretching vibrations, primarily attributed to hydroxyl groups within the PVC component. C=O stretching (1750 and 1275 cm−1): Sharper peaks at 1750 and 1275 cm−1 originate from the C=O stretching vibrations of carbonyl groups in PVC and TiO2, respectively. These peaks indicate the presence of ester and Ti-O bonds within the material. Irradiated film: Reduced O-H intensity: Compared to the pristine film, the irradiated film exhibits a decrease in the intensity of the O-H stretching peak. This observation suggests bond breaking of O-H groups, potentially within the PVC chains or at the interface with CeO2/TiO2 nanoparticles. Enhanced C=O intensity: An increase in the C=O stretching peak intensity at 1750 cm−1 is observed in the irradiated film. This could be attributed to several factors: C=O formation due to O-H bond cleavage: Cleaved O-H groups might generate additional carbonyl groups via secondary reactions. Increased conjugation or chain scission: Gamma irradiation might promote conjugation in the PVC chains or induce chain scission, leading to exposed carbonyl groups at the chain ends. Irradiation effects: crosslinking: Besides bond breaking, gamma radiation might also induce crosslinking between PVC and the CeO2/TiO2 nanoparticles. This possibility requires further investigation using complementary techniques. Changes in material properties: The modifications in the chemical structure are expected to influence the properties of the nanocomposite films, potentially affecting their mechanical strength, electrical conductivity, and thermal stability. Overall significance: IR spectroscopy proves valuable for analyzing the chemical structure evolution of these nanocomposite films under gamma irradiation. The observed changes provide clues about potential bond rearrangements, functional group modifications, and potential crosslinking. Further investigations are necessary to fully understand the interplay between irradiation and the material’s chemical landscape.
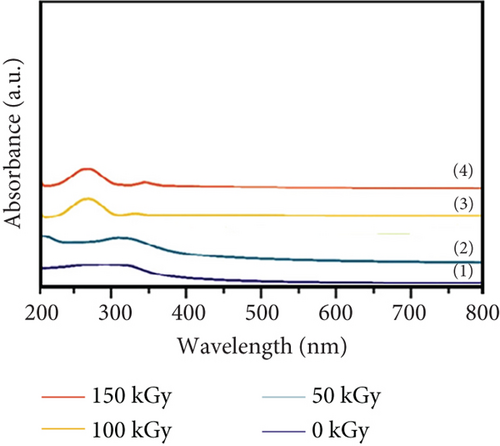
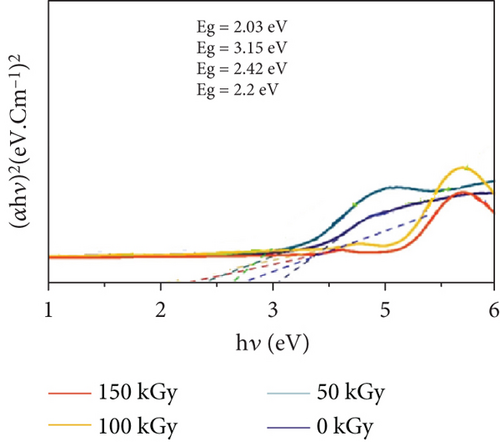
4.15. Unveiling the Light-Matter Dance: Optical Properties Under Gamma Irradiation
Figure 6 explores the influence of gamma irradiation on the optical properties of PVC/CeO2/TiO2 nanocomposites. Absorption spectra (Figure 6(a)): The figure presents the absorption spectra of the nanocomposite at different irradiation doses (0–150 kGy). The horizontal axis represents the wavelength of light (nanometer), and the vertical axis represents the absorption coefficient (a.u.). Irradiation effects: As the irradiation dose increases from 0 to 100 kGy, the absorption coefficient increases across the entire wavelength range. This signifies enhanced light absorption by the material across the spectrum with increasing irradiation. Peak behavior: Interestingly, the absorption coefficient exhibits a nonmonotonic trend with increasing dose. It reaches a maximum value at 75 kGy and then decreases slightly at 100 kGy before dropping significantly at 150 kGy. This behavior suggests competing factors influencing the absorption properties under irradiation. Possible explanations: The observed increase in absorption at lower doses could be attributed to the formation of new electronic states or defects within the material induced by gamma rays. These defects can act as traps for light, leading to increased absorption. However, at higher doses, these defects might recombine or cluster, reducing their effectiveness as light traps and leading to a decrease in absorption. Additionally, high-energy gamma rays at 150 kGy might initiate degradation of the material itself, further reducing its light absorption capacity. Binding energy (Figure 6(b)): This figure depicts the relationship between the binding energy of the PVC/CeO2/TiO2 nanocomposite and the gamma irradiation dose. The horizontal axis represents the photon energy (hγ, electronvolt), and the vertical axis represents a term related to the density of electronic states ((αhv)2, eV2 cm−2). Irradiation effects: Similar to the absorption spectra, the binding energy also increases with increasing irradiation dose up to 100 kGy. This suggests that gamma rays modify the electronic structure of the material, leading to the formation of stronger bonds within the nanocomposite. Peak behavior: Again, the binding energy reaches a maximum value at 100 kGy and then decreases at 150 kGy, mirroring the behavior observed in the absorption spectra. This suggests a connection between the electronic structure modifications and the light absorption properties of the material. Overall significance: These findings demonstrate that gamma irradiation can significantly alter the optical properties of PVC/CeO2/TiO2 nanocomposites by modifying their electronic structure and inducing defect generation. The optimal irradiation dose for achieving the highest light absorption and binding energy appears to be around 100 kGy.
4.16. Unveiling the Nanolandscape: Morphology of CeO2 and TiO2 Nanoparticles
Figure 7 presents high-resolution SEM images of the individual CeO2 and TiO2 nanoparticles employed in the nanocomposite fabrication. Particle size and shape: The CeO2 nanoparticles appear as small, dark, roughly spherical particles, with diameters predominantly below 10 nm. In contrast, the TiO2 nanoparticles exhibit a larger size, lighter contrast, and more angular morphology, with many particles in the 20-nm diameter range. These disparities in size and shape could stem from several factors, including the specific synthesis methods used for each material or inherent properties of the materials themselves. Particle distribution: The nanoparticles in both images are not uniformly distributed. They appear clustered in certain regions and sparser in others. This inhomogeneity likely arises from the sample preparation process for SEM analysis. Surface texture: At the presented magnification, the surfaces of both CeO2 and TiO2 nanoparticles appear smooth and devoid of discernible features. However, employing higher magnification SEM imaging could reveal finer details about the surface texture. Overall, Figure 7 provides valuable insights into the morphology of the constituent CeO2 and TiO2 nanoparticles. The observed differences in size, shape, and distribution between the two materials could significantly influence their properties and potential applications in the developed nanocomposite.

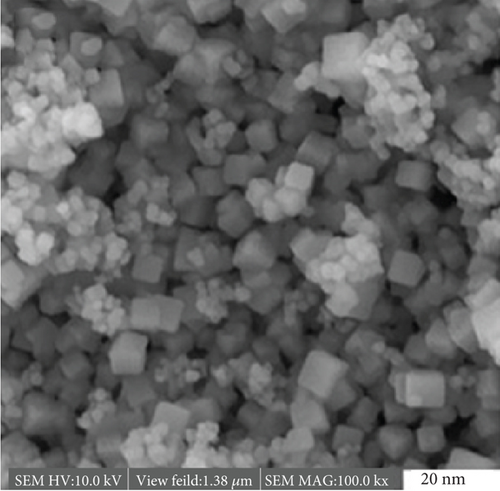
4.17. Structural and Crystalline Induced by Gamma Irradiation
Polymer nanocomposites, where conventional polymers like PVC are reinforced with nanoparticles like CeO2 and TiO2, offer a unique combination of properties including biodegradability, light-responsive functionalities, and potential magnetic characteristics [43]. However, optimizing these functionalities often requires precise control over the material’s structure and properties. Gamma irradiation presents a powerful tool for manipulating these characteristics at the atomic level. Solution casting serves as the initial step in nanocomposite preparation, meticulously incorporating CeO2 and TiO2 nanoparticles into the PVC matrix [44]. This technique lays the foundation for subsequent irradiation-induced modifications. XRD analysis reveals crucial changes in the crystalline structure of the nanocomposite films following gamma irradiation. A narrowing of diffraction peaks signifies a reduction in crystalline imperfections, analogous to smoothing wrinkles in fabric. Additionally, the growth of sharper and more intense peaks suggests an increase in crystal size and a decrease in lattice strain within the material [45]. Interestingly, XRD analysis also indicates a gradual decrease in the unit cell size with increasing radiation dose. This phenomenon can be likened to a sculptor meticulously removing excess material to refine the final form. These intricate atomic rearrangements can potentially influence the ionic hopping distance within the nanocomposite, a critical parameter governing electrical conductivity and magnetism [46]. Determining the exact hopping distance necessitates complex calculations involving lattice parameters and ionic radii. By elucidating how irradiation affects this crucial distance, gain valuable insights into the electrical and magnetic properties of the nanocomposite. The impact of gamma irradiation extends beyond structural transformations. Understanding the changes in crystallinity, defect concentration, and phase composition within the irradiated nanocomposite is akin to deciphering a hidden code. This code holds the key to tailoring the material’s optical properties, electrical conductivity, and even magnetic behavior [47]. By unlocking these previously hidden secrets, gamma irradiation empowers to design PVC-based nanocomposites with specific functionalities for diverse applications.
4.18. Tailoring Functionalities Through Gamma Irradiation
Polymer nanocomposites, exemplified by PVC reinforced with CeO2 and TiO2 nanoparticles, offer a captivating blend of properties such as biodegradability, light-sensitive functionalities, and potential magnetic characteristics [48]. However, harnessing these functionalities to their full potential necessitates precise control over the material’s response to external stimuli. Gamma irradiation emerges as a powerful tool for sculpting these materials at the atomic level, enabling the fine-tuning of their performance. The initial step involves meticulously incorporating CeO2 and TiO2 nanoparticles into the PVC matrix through solution casting, laying the groundwork for subsequent irradiation-induced modifications [44]. XRD analysis serves as a vital tool for investigating the structural transformations triggered by gamma irradiation. A noticeable sharpening of diffraction peaks is observed, indicating a reduction in crystalline imperfections within the nanocomposite. This phenomenon can be likened to the fading of wrinkles, signifying the elimination of structural defects [49]. These seemingly subtle changes translate to significant improvements in the material’s properties. The growth of sharper and more intense peaks suggests an increase in crystal size and a decrease in lattice strain, essentially signifying a more robust and ordered crystalline structure. Additionally, XRD analysis reveals a gradual decrease in the unit cell size with increasing radiation dose. This phenomenon can be compared to a sculptor meticulously removing excess material to refine the final form.
4.19. Unveiling the Functional Consequences of Gamma Irradiation
Polymer nanocomposites, such as PVC reinforced with CeO2 and TiO2 nanoparticles, offer a promising combination of properties including biodegradability, light-sensitive functionalities, and potential magnetic characteristics [48]. However, optimizing these functionalities necessitates a deeper understanding of the interplay between the material’s structure and its response to external stimuli like gamma irradiation. XRD analysis provides valuable insights into the structural transformations induced by gamma irradiation. A noticeable sharpening of diffraction peaks is observed, indicating a reduction in crystalline imperfections within the nanocomposite. This phenomenon can be attributed to the elimination of structural defects and signifies an improvement in the material’s crystallinity [49]. The observed growth of sharper and more intense peaks suggests an increase in crystal size and a decrease in lattice strain, essentially translating to a more robust and ordered crystalline structure. Additionally, XRD analysis reveals a gradual decrease in the unit cell size with increasing radiation dose. The intricate atomic rearrangements observed through XRD can potentially influence the electrical and magnetic properties of the nanocomposite. Ionic hopping distance, a critical parameter governing these properties, is likely affected by the irradiation-induced structural changes [46]. Determining the exact hopping distance requires complex calculations that incorporate lattice parameters and ionic radii obtained from XRD data. By elucidating how irradiation affects this crucial distance, gain valuable insights into the electrical and magnetic behavior of the nanocomposite. The impact of gamma irradiation extends beyond structural modifications. A comprehensive understanding of the changes in crystallinity, defect concentration, and phase composition within the irradiated nanocomposite is crucial for deciphering the complex interplay between the radiation and the various components of the material. This knowledge unlocks the potential for tailoring the material’s optical properties, electrical conductivity, and even magnetic behavior [50].
At lower irradiation doses, the repulsive interactions between the PVC chains and the nanoparticles can promote a more ordered arrangement of the polymer chains [54]. Additionally, radiation can strengthen the interfacial interactions between the nanocomposite and the polymer matrix through the formation of more robust hydrogen bonds. However, beyond a critical dose, the balance shifts. Gamma irradiation can generate free radicals within the material, which act like disruptive agents within the ordered structure [55]. These free radicals can also initiate crosslinking reactions within the PVC matrix, leading to the formation of new covalent bonds between polymer chains. While crosslinking can enhance some mechanical properties, it can also lead to a weakening of the individual polymer chains if excessive. Unraveling this intricate interplay between chain scission, crosslinking, and the resulting changes in material properties is crucial for optimizing the performance of the nanocomposite for specific applications. FTIR spectroscopy offers complementary insights into the radiation-induced changes within the nanocomposite. As the irradiation dose increases from 0 to 100 kGy, the intensity of certain characteristic peaks in the FTIR spectra increases. This can be attributed to an improvement in the overall order and crystallinity of the polymer chains [56]. However, at higher doses (150 kGy), the intensity of these peaks diminishes. This signifies a disruption in the ordered chain alignment, likely due to the dominance of crosslinking reactions at these higher radiation levels [57]. Understanding this interplay between degradation, crosslinking, and the resulting changes in chain conformation is essential for tailoring the properties of PVC-based nanocomposites for specific applications. By deciphering the complex dance between these processes can design materials with properties like biodegradability, light sensitivity, and potential magnetic functionalities [58]. This knowledge can be extended to other polymer systems, providing valuable insights into the general response of polymers to gamma irradiation.
4.20. Unveiling the Crystalline Evolution of PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites Under Gamma Irradiation
XRD analysis serves as a powerful tool for investigating the influence of gamma irradiation on the crystalline structure of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposites. In pristine films, the initial XRD patterns establish a baseline level of crystallinity, with characteristic peaks corresponding to the ordered arrangements of atoms within the PVC and CeO2 phases. However, upon exposure to low gamma irradiation doses (up to 100 kGy), a significant transformation unfolds. A decrease in the basal width of diffraction peaks is observed, signifying a reduction in lattice defects within the crystallites [59]. These defects can be likened to imperfections or disruptions within the crystal lattice, hindering its overall order. Gamma irradiation, at these low doses, acts to rectify these imperfections, akin to a meticulous weaver restoring order to the crystalline tapestry. Further insights are gleaned from the observed decrease in strain within the CeO2 spinel lattice. The XRD analysis suggests a relaxation of the atomic network, analogous to loosening tension on a delicate net. This phenomenon implies that low-dose gamma irradiation promotes a more stable and optimized configuration within the CeO2 nanoparticles. However, as the irradiation intensity increases beyond 100 kGy, the XRD narrative takes an unexpected turn. The initial decrease in peak linewidth gives way to a gradual broadening. This seemingly contradictory behavior can be attributed to two competing processes. PVC degradation: At higher doses, gamma rays can trigger the scission of polymer chains within the PVC matrix, a process known as degradation [60]. This degradation can lead to the separation of the desired PVC:(50 nm)/CeO2:(10 nm)/TiO2 phase from an undesired secondary phase, α-MnFe₂O₄, which can often be identified through XRD analysis. Interestingly, this separation paradoxically promotes a more ordered arrangement of the remaining PVC:(50 nm)/CeO2:(10 nm)/TiO2 crystallites within the intact composite. This observation aligns with findings reported by [61] in their investigation of similar nanocomposites. Free radical formation: Beyond a critical dose (around 150 kGy), intense gamma irradiation triggers the generation of numerous highly reactive free radicals within the PVC matrix. These free radicals attack and disrupt the polymer chains, counteracting the order-promoting effects observed at lower doses. Essentially, the onslaught of free radicals unravels the meticulously woven tapestry, introducing new defects and disrupting the previously enhanced order. Therefore, the XRD analysis of gamma-irradiated PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposites reveals a captivating interplay between order and disorder within the crystalline structure. At low doses, gamma irradiation acts as a weaver, mending defects and promoting crystallinity [62]. However, at higher doses, the destructive forces of free radicals dominate, unraveling the meticulously crafted order and introducing chaos. Elucidating this intricate dance can harness gamma irradiation to tailor the crystalline structure and properties of these nanocomposites for specific applications.
4.21. Unveiling the Light-Matter Dance: Gamma Irradiation and the Optical Properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites
4.22. Unveiling the Bandgap Modulation in PVC:(50 nm)/CeO2:(10 nm)/TiO2 Nanocomposites
Gamma irradiation exerts a significant influence on the optical properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films. This transformation unfolds at the atomic level through the interplay between defects, bandgaps, and the irradiation process.
4.22.1. Sculpting the Crystalline Landscape: Diminishing Disorder
Gamma irradiation acts as a sculptor at the nanoscale, meticulously reducing defects and irregularities within the crystalline lattice of CeO2 nanoparticles. The CeO2 lattice can be visualized as a delicate network holding atoms in specific positions. Gamma rays, like microscopic surgeons, remove imperfections and smoothen the network, reducing strain. This translates to a more ordered and relaxed atomic configuration within the material [66].
4.22.2. Bandgap Modulation
The consequences of this orchestrated order are profound for the material’s optical bandgap, the energy difference between its valence and conduction bands. As shown in the Tauc plots (Figure 1), the bandgap exhibits a narrowing trend with increasing irradiation dose (up to 100 kGy). This phenomenon can be attributed to the reduced strain and disorder within the material, essentially creating a smoother energy landscape for photons. In this optimized landscape, photons can transition between energy levels more readily, leading to a smaller bandgap [67].
4.22.3. Contributing Factors
However, the bandgap narrowing is not solely a consequence of a tidier lattice. Several other factors contribute to this captivating dance. Chemical bond formation: Gamma rays can promote the formation of covalent bonds between PVC chains and the CeO2 nanoparticles. These newly formed bonds create localized energy states within the bandgap, acting as stepping stones for photons. This further facilitates their movement and contributes to the observed narrowing effect. Transmittance changes: As disorder diminishes due to irradiation, the film’s transmittance of light increases. This enhanced transmittance leads to a decrease in the apparent bandgap, contributing to the observed narrowing effect. Dislocation density: The interplay between gamma irradiation and the crystal lattice can affect the density of dislocations and imperfections within the crystalline structure. A decrease in dislocation density with irradiation can contribute to the observed bandgap narrowing.
5. Conclusion
This study investigated the multifaceted impact of gamma irradiation on the structural, morphological, and optoelectronic properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films. The key findings are summarized as follows. Enhanced light-to-current conversion: Gamma irradiation significantly increases the σopt of the nanocomposite films, indicating a more efficient conversion of absorbed light into electrical current. This enhancement is attributed to a narrowed bandgap and increased light absorption upon irradiation. Crystallinity and strain modulation: Gamma irradiation disrupts the ordered atomic arrangement within the nanocomposite, leading to a decrease in crystallinity as observed by XRD analysis. W-H plots revealed a dose-dependent reduction in crystallite size and a corresponding increase in lattice strain with increasing irradiation dose. Chemical structure modifications: IR spectroscopy analysis suggests potential bond breaking of O-H groups and alterations in the C=O functional groups within the nanocomposite upon irradiation. Further investigations are necessary to fully elucidate the interplay between irradiation and the material’s chemical landscape. Tailoring optical properties: Gamma irradiation offers a powerful tool to manipulate the optical properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposites. The absorption coefficient and binding energy exhibit a nonmonotonic response with increasing dose, reaching an optimum value around 100 kGy. This finding highlights the potential for fine-tuning the material’s light absorption and electronic structure for optoelectronic applications. In conclusion, gamma irradiation emerges as a versatile strategy for tailoring the properties of PVC:(50 nm)/CeO2:(10 nm)/TiO2 nanocomposite films. By manipulating the irradiation dose, we can achieve enhanced light-to-current conversion, control crystallinity and strain, and engineer the material’s chemical makeup and optical response. These findings pave the way for the development of next-generation optoelectronic devices with optimized functionalities. Future work will focus on elucidating the precise mechanisms underlying the observed effects and exploring the potential applications of these irradiated nanocomposites in solar cells, photodetectors, and LEDs.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for funding this work through Large Groups Project under grant number L.R.G.P2/278/45.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for funding this work through Large Groups Project under grant number L.R.G.P2/278/45.
Open Research
Data Availability Statement
Data can be found at 10.6084/m9.figshare.25069160. There are no code analyses in the manuscript.




