Thoracoscopic Surgical Biatrial Ablation vs. Catheter Ablation in Patients with Persistent Atrial Fibrillation
Abstract
Background. Limited randomized controlled studies showed that thoracoscopic surgical left atrial ablation was not superior to catheter ablation (CA) in patients with persistent atrial fibrillation (PerAF). Right atrium might play an important role in triggering and maintaining atrial fibrillation (AF) in patients with PerAF. This study aimed to compare the efficacy of thoracoscopic surgical biatrial ablation versus CA in patients with PerAF. Methods. Patients with PerAF underwent thoracoscopic surgical biatrial ablation or CA were included in this study. Propensity score matching (1 : 2) was applied to select patients in CA group and surgical ablation (SA) group. The primary endpoint was to compare the probability of freedom from atrial tachyarrhythmias between SA and CA. Atrial tachyarrhythmia recurrence was defined as any atrial tachyarrhythmias longer than 30 s documented by 24-hour Holter monitoring after the 3-month blanking period. Results. After propensity score matching, 51 patients in surgical biatrial ablation group and 102 patients in CA group were enrolled (mean left atrial diameter: 45.8 mm). The probability of freedom from atrial tachyarrhythmias on antiarrhythmia drugs was 62.7%, 60.6%, and 60.6% in SA group and 42.0%, 39.6%, and 36.7% in CA group at 12, 24, and 36 months, respectively (p = 0.011), and off antiarrhythmia drugs were 56.9%, 52.5%, and 52.5% in SA group and 36.0%, 31.4%, and 27.5% in CA group at 12, 24, and 36 months, respectively (p = 0.007). After adjustment of age, sex, left atrial diameter, and AF duration history, multivariable Cox regression analysis suggested that SA procedure was an independent factor to reduce the risk of atrial tachyarrhythmia recurrence (HR: 0.589, 95% CI 0.370–0.937, p = 0.025). Conclusion. Compared with CA, thoracoscopic surgical biatrial ablation might achieve superior effectiveness for patients with PerAF.
1. Introduction
Previous studies have found that catheter ablation (CA) is less effective in the treatment of persistent atrial fibrillation (PerAF) in spite of the use of various ablation strategies, such as pulmonary vein isolation (PVI) plus linear ablation, PVI plus complex fractionated atrial electrogram ablation, rotor ablation, and the stepwise approach [1–3]. Thoracoscopic surgical ablation (SA) is a rapidly emerging treatment of atrial fibrillation (AF) which has promising efficacy in selected populations [4].
As two major ablation procedures, both CA and thoracoscopic SA have yet to be fully investigated on the efficacy for the treatment of PerAF [5–7]. Previous randomized controlled studies demonstrated that there was no significant difference between SA and CA in patients with PerAF [6–8], whereas the SA procedure in both trials only focused on the left atrium and did not involve the right atrium ablation. Recent studies suggested that the right atrium might play an important role in triggering and maintaining AF, and 85% of patients with PerAF had right atrium sources [9, 10]. Adding right atrial ablation to left atrial ablation might achieve better results during thoracoscopic SA procedure.
Few studies have reported the results of thoracoscopic surgical biatrial ablation for patients with PerAF [11, 12]. Furthermore, to our knowledge, there is no study comparing surgical biatrial ablation with CA for the treatment of PerAF so far. Therefore, this study aimed to compare the efficacy of thoracoscopic surgical biatrial ablation versus CA in patients with PerAF.
2. Patients and Methods
2.1. Ethical Statement
This study was approved by the Institutional Review Board of Fuwai Hospital (date of review, October 24, 2019; approval 2017-880) and informed consent was waived.
2.2. Study Population
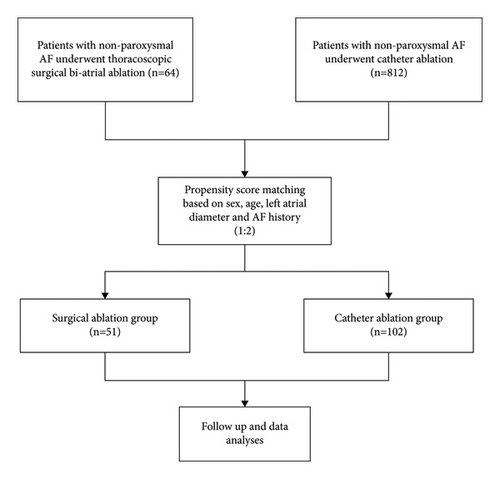
From September 2010 to November 2021, 64 patients with PerAF who underwent thoracoscopic surgical biatrial ablation were retrospectively selected in SA group at Fuwai Hospital, Beijing, China. During the similar time, there were 812 patients with PerAF who underwent CA in our institution. Propensity score matching (1 : 2) was applied to enroll patients in SA and CA groups (Figure 1).
2.3. Thoracoscopic Surgical Biatrial Ablation Procedure
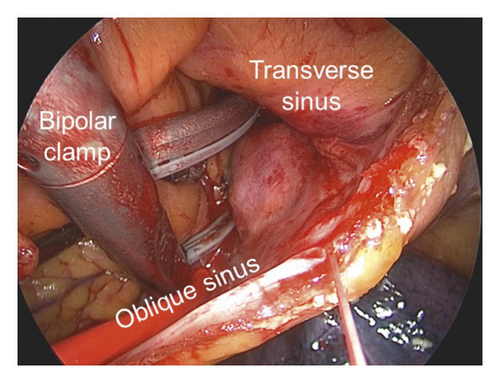
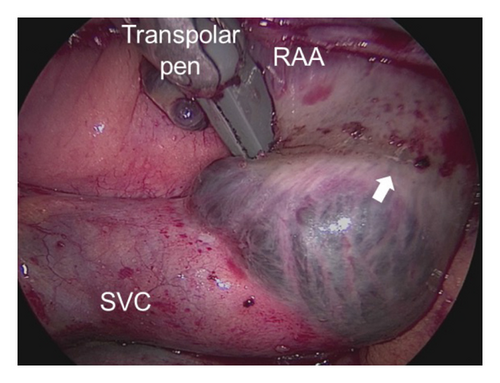
The detailed procedure had been introduced in our previous studies [11, 13], as described briefly in the following. The access to the pericardial space was achieved through bilateral transthoracic approach. Two working ports were positioned in the posterior-axillary fourth intercostal space and the midclavicular second intercostal space. A camera port was positioned in the anterior-axillary third intercostal space. The right-side ports were positioned more anteriorly. Generally, left-side ablation was performed first. The left atrial appendage (LAA) was excised by linear stapler (EZ 60; Ethicon Endosurgery, Cincinnati, Ohio, USA). The ligament of Marshall was divided by ultrasonic scalpel. The bipolar radiofrequency clamp (Isolator, AtriCure, Mason, Ohio) was used to create left pulmonary veins (PVs) isolation, left-side roof line and inferior line, left trigone line, and ganglion plex ablation at the Waterston groove fat pad. The transpolar radiofrequency pen was used to complement the left trigone line. The linear lesion connecting the left superior PV to the LAA and the linear lesion connecting the left inferior PV (LIPV) to the great cardiac vein were ablated with the transpolar radiofrequency pen. On the right side, the lesion set included right PVs isolation, right-side roof line and floor line, superior vena cava (SVC)-inferior vena cava (IVC) line, right atrial appendage (RAA) line (the apex to the base at the level of the root of the aorta), and the line connecting the tip of the RAA to SVC-IVC line (Figure 2).
2.4. CA Procedure
CA procedure had been detailed described by a previous study [14]. Lasso NAV SAS or Pentaray catheter was used to construct the 3-dimensional surface of the left atrium and PVs with guidance of the CARTO 3 or EnSite NavX system. PVI was completed in all patients at first. If AF persisted, the following steps were completed in sequence. (1) Further stepwise linear ablation in anterior wall, roof, mitral isthmus (including the linear lesions connecting LIPV and right inferior PV (RIPV) to posterior mitral valve annulus), coronary sinus (CS), and cavotricuspid isthmus (CTI) would also be conducted. (2) If AF continued, intravenous ibutilide was administered, followed by elimination of atrial tachycardia under activation and voltage mapping if atrial tachycardia occurred. (3) If AF still continued, direct-current cardioversion would be applied. If sinus rhythm (SR) was restored after any steps above, the contact force monitoring (10–20 g) would be used to confirm the durable transmural lesions of PVI, mitral isthmus line, and CTI line with intensive pacing (10 mA, pulse width 2 ms) (Figure 3).
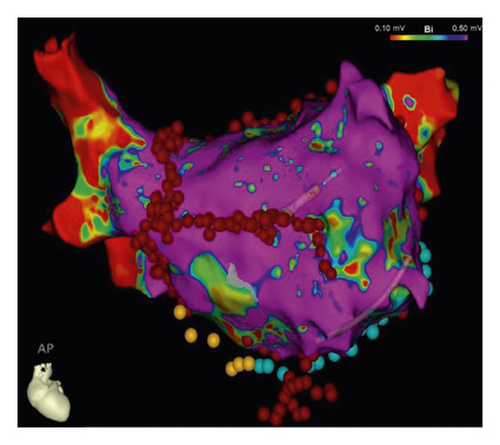
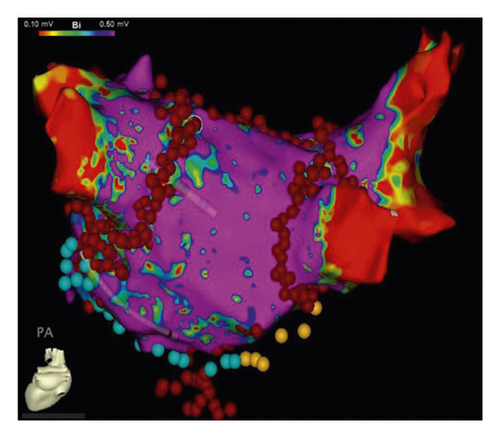
2.5. Postoperative Drug Treatment
In SA group, Class III antiarrhythmia drugs (AADs) with preference to sotalol would be routinely applied after ablation until three months. Anticoagulants were initiated as soon as bleeding risk was acceptable. AADs were advised to continue or CA was recommended if atrial tachyarrhythmia occurred after postoperative three months, and continuation of oral anticoagulants relied on the risk of stroke according to CHA2DS2-VASc score or LAA stump. In CA group, AADs and oral anticoagulants were routinely applied after ablation until three months. Continuation of oral anticoagulants and AADs was suggested if atrial tachyarrhythmia occurred. Repeated CA was also suggested in patients with AF recurrence or atrial flutter.
2.6. Follow-Up Visits
Patients were followed up at 3, 6, and 12 months after the procedure and annually thereafter and underwent 24-hour Holter monitoring. Any arrhythmia symptoms or suspicious atrial tachyarrhythmia recurrence following ablation were deemed deserving 12-lead electrocardiograms. All electrocardiograms were analyzed during follow-up. The questionnaire about ischemic stroke, death, and hospitalization for heart failure was completed via telephone.
2.7. Endpoints
The primary efficacy endpoint was defined as freedom from any atrial tachyarrhythmias in the absence of AADs, which was no evidence of AF, atrial flutter, or other atrial tachyarrhythmias with a duration longer than 30 s as documented by 24-hour Holter monitoring [15]. The first 3 months after operation was considered as a blanking period. Permanent pacemaker implantations perioperatively and during follow-up were also reported, and the indications for permanent pacemaker implantation included sinus node dysfunction, third degree atrioventricular block, and severe second degree type II atrioventricular block. Event free survival of ischemic stroke, death, and hospitalization for heart failure were also reported.
2.8. Statistical Analysis
Clinical characteristics were described as frequency (percentage) for categorical variables and mean (standard deviation, SD) for continuous variables. Student’s t-test or Mann–Whitney’s U-test was used in comparison of intergroup differences for continuous variables, while Chi-squared tests or Fisher’s exact test for categorical variables. To account for potential baseline differences between the two groups, propensity score matching (1 : 2) with nearest neighbor method was conducted. Propensity scores were estimated by a logistic model with the R MatchIt package and were matched without replacement between groups within a caliper of 0.2 propensity score SD units. Matching covariates consisted of variables that were significantly different in the baseline characteristics of the two groups and variables that previous studies had reported to influence AF recurrence. For each baseline covariate included in the propensity score model, balance between groups was assessed using absolute standardized differences (Supplementary Figure 1). The Kaplan–Meier analysis and log-rank test were used to evaluate the time to atrial tachyarrhythmia recurrence. Univariable and multivariable Cox proportional hazard models were applied to uncover the independent factors related to the primary endpoint. Variables with p < 0.15 in univariable model or variables associated with outcomes (sex, age, AF history, and left atrial diameter (LAD)) were selected into the multivariable model. Only 2-sided p value < 0.05 was considered to be significant. Analysis was performed using R version 4.1.1 software (R Foundation for Statistical Computing, Vienna, Austria) [16].
3. Results
3.1. Patients’ Characteristics
Before propensity score matching, there were 64 patients in SA group and 812 patients in CA group. Patients in SA group had larger LAD and longer history of AF (Supplementary Table 1). After propensity score matching, 51 patients in SA group and 102 patients in CA group were chosen for following analyses. There was no significant difference in baseline characteristics between the two groups. Overall, the LAD was 45.7 ± 4.6 mm and 45.8 ± 4.4 mm in SA and CA group, respectively. Table 1 summarized the baseline characteristics of the patients.
| Overall (N = 153) | SA group (n = 51) | CA group (n = 102) | P value | SMD | |
|---|---|---|---|---|---|
| Age, mean (SD) (y) | 55.3 (10.2) | 55.8 (9.9) | 55.1 (10.4) | 0.660 | 0.076 |
| Sex, male, n (%) | 134 (87.6) | 43 (84.3) | 91 (89.2) | 0.544 | 0.145 |
| Diabetes mellitus, n (%) | 24 (15.7) | 9 (17.6) | 15 (14.7) | 0.814 | 0.080 |
| Coronary artery disease, n (%) | 23 (15.0) | 6 (11.8) | 17 (16.7) | 0.576 | 0.141 |
| Previous stroke, n (%) | 18 (11.8) | 5 (9.8) | 13 (12.7) | 0.790 | 0.093 |
| Hyperlipidemia, n (%) | 42 (27.5) | 12 (23.5) | 30 (29.4) | 0.564 | 0.134 |
| Hypertension, n (%) | 67 (43.8) | 23 (45.1) | 44 (43.1) | 0.954 | 0.039 |
| Previous CA, n (%) | 26 (17.0) | 7 (13.7) | 19 (18.6) | 0.594 | 0.133 |
| AF duration, mean (SD) (y) | 4.9 (4.6) | 5.5 (3.9) | 4.7 (4.9) | 0.310 | 0.181 |
| CHA2DS2-VASc score, mean (SD) | 1.3 (1.3) | 1.2 (1.2) | 1.3 (1.4) | 0.697 | 0.069 |
| LAD, mean (SD) (mm) | 45.8 (4.5) | 45.7 (4.6) | 45.8 (4.4) | 0.828 | 0.037 |
| LVEF, mean (SD) (%) | 60.9 (6.6) | 61.7 (4.7) | 60.5 (7.4) | 0.288 | 0.195 |
- AF, atrial fibrillation; CA, catheter ablation; SA, surgical ablation; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; SD, standard deviation; SMD, standard mean difference.
3.2. Procedural Characteristics
In CA group, all patients underwent PVI. Roof line, anterior line, LIPV to mitral annulus line, RIPV to mitral annulus line, CTI line, and CS ablation were completed in nearly 95% of patients. After ablation, 47 patients (46.1%) restored SR, some conversions occurred after intravenous ibulitol usage. AF converted to atrial flutter in 15 patients (14.7%) and 40 patients (39.2%) maintained AF rhythm. The detailed procedural parameters are demonstrated in Table 2.
| Parameters | CA (n = 102) |
|---|---|
| Ablation routes | |
| PVI | 102 (100%) |
| Roof line | 98 (96.1%) |
| Anterior line | 93 (91.2%) |
| LIPV to mitral annulus | 97 (95.1%) |
| RIPV to mitral annulus | 97 (95.1%) |
| CTI line | 96 (94.1%) |
| CS | 96 (94.1%) |
| SVC | 2 (2.0%) |
| Left atrial ridge | 28 (27.5%) |
| Rhythm after ablation | |
| AF | 40 (39.2%) |
| AFL | 15 (14.7%) |
| SR | 47 (46.1%) |
- AF, atrial fibrillation; AFL, atrial flutter; CA, catheter ablation; CS, coronary sinus; CTI, cavotricuspid isthmus; LIPV, left inferior pulmonary vein; PVI, pulmonary vein isolation; RAA, right atrial appendage; RIPV, right superior pulmonary vein; SVC, superior vena cava; SR, sinus rhythm.
In SA group, 4 patients (7.8%) restored SR when thoracoscopic epicardial ablation was performed at the right atrium, and 5 patients (9.8%) restored SR after left atrial ablation. AF converted to atrial flutter in 3 patients (5.9%) after thoracoscopic epicardial ablation and 39 patients (76.5%) who maintained AF underwent electrical conversion after ablation, of which 35 patients were converted to SR after electrical conversion.
3.3. Procedural Complications
Four patients (7.8%) in SA group and three patients (2.9%) in CA group had perioperative complications (p = 0.223) (Table 3). There was no death and permanent pacemaker implantation within 30 days after procedure in SA group; however, two patients in SA group underwent thoracotomy due to PV bleeding. Two patients (3.9%) in SA group were diagnosed with nonsymptomatic small pulmonary embolism according to the left atrial enhanced computed tomography before discharge. After 3 months of oral anticoagulation, repeated enhanced computed tomography did not display any thrombosis in the pulmonary artery in these patients. One patient in CA group experienced transient ischemic attack after ablation and another patient implanted permanent pacemaker.
| SA group (n = 51) | CA group (n = 102) | P value | |
|---|---|---|---|
| Procedural complications, n (%) | 4 (7.8) | 3 (2.9) | 0.223 |
| Death | 0 | 0 | |
| Cerebrovascular accident | 0 | 1 | |
| Pericardial effusion | 0 | 0 | |
| Pleural effusion | 0 | 0 | |
| Groin hematoma | 0 | 1 | |
| Permanent pacemaker implantation | 0 | 1 | |
| Pulmonary embolism | 2 | 0 | |
| Iatrogenic conversion to thoracotomy | 2 | 0 | |
| Pulmonary vein bleeding | 2 | 0 |
- CA, catheter ablation; SA, surgical ablation.
3.4. Follow-Up Results
3.4.1. Heart Rhythm
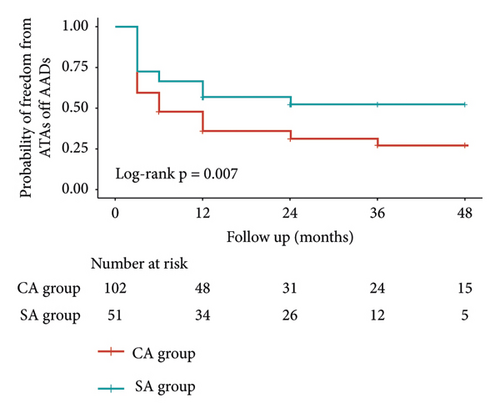
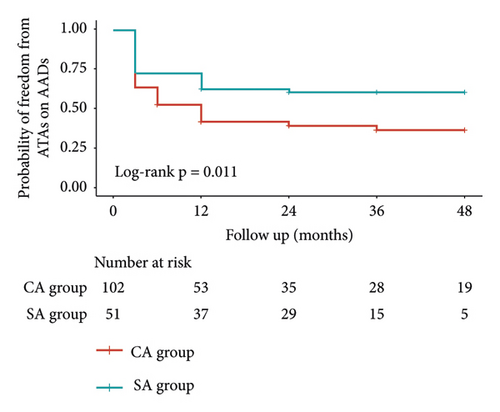
All patients were followed up for at least 6 months (mean: 36.2 ± 15.7 months). Freedom from atrial tachyarrhythmias off AADs was 56.9%, 52.5%, and 52.5% in SA group and 36.0%, 31.4%, and 27.5% in CA group at 12, 24, and 36 months, respectively (p = 0.007; Figure 4(a)). Freedom from atrial tachyarrhythmias on AADs was 62.7%, 60.6%, and 60.6% in SA group and 42.0%, 39.6%, and 36.7% in CA group at 12, 24, and 36 months, respectively (p = 0.011; Figure 4(b)). During follow-up, 11 patients in SA group and 23 patients in CA group underwent redo CA. Freedom from atrial tachyarrhythmias after redo CA off AADs was 68.6%, 66.3%, and 66.3% in SA group and 44.9%, 42.5%, and 39.7% in CA group at 12, 24, and 36 months, respectively (p = 0.004).
3.4.2. Stroke, Hospitalization for Heart Failure, and Death
Three patients in each group experienced ischemic stroke/transient ischemic attack confirmed by cranial computed tomography or magnetic resonance image (p = 0.401). Five patients were hospitalized for heart failure in CA group and one patient in SA group (p = 0.664). One patient in CA group underwent permanent pacemaker implantation during follow-up. One patient died in SA group at 12 months after operation due to a car accident.
3.4.3. Predictors of Atrial Tachyarrhythmia Recurrence
The univariable Cox regression analysis demonstrated that SA was significantly associated with a lower risk of atrial tachyarrhythmia recurrence off AADs after blanking period (HR: 0.586, 95% CI 0.369–0.931, p = 0.024). After adjustment of age, sex, LAD, and AF duration history, multivariable Cox regression analysis suggested that SA procedure was an independent factor to reduce risk of atrial tachyarrhythmia recurrence (HR: 0.589, 95% CI 0.370–0.937, p = 0.025) (Supplementary Table 2).
4. Discussion
In the present study, surgical biatrial ablation was superior to CA in terms of long-term SR maintenance in patients with PerAF. These results suggested that surgical biatrial ablation was a promising treatment strategy for PerAF, which could serve as a reference for clinicians to make decisions.
Our study was unique by means of comparing surgical biatrial ablation versus CA in patients with PerAF. Prior studies have noticed the intricate mechanism of PerAF, including less PV sources involved and more atrial electrical and structural remodeling progresses, which poses a particular challenge for the ablation treatment [17]. With the development of AF from paroxysmal to persistent, the sustain of AF is driven by the macro-reentrant circuits in both atria without triggers from PVs, and simple PVI or box lesion is not enough to achieve SR maintenance [18]. Based on the electrophysiological mapping findings, the right atrium might play an important role in triggering and maintaining PerAF, especially with an enlarged right atrium [9, 10]. Miller et al. revealed sources in the right atrium in 85% of patients with PerAF, underscoring the importance of right atrial ablation for treating PerAF [10].
Considering the left and right atrium as an electrical continuum, Cox maze procedure achieved excellent long-term SR maintenance rate for treating stand-alone AF [19]. Nonetheless, the invasiveness and morbidity of Cox maze hampered its popularity [20]. Thoracoscopic epicardial ablation, with less trauma and postoperative severe complications compared with conventional Cox maze, spread around the world for treating stand-alone AF after being introduced in 2005 [21]. However, the lesion sets of thoracoscopic ablation, including PV isolation, Dallas line, and box lesions, were not equivalent to that of Cox maze and right atrial lesion set was lacking [22, 23]. Subsequent studies have shown that thoracoscopic ablation is not as effective as conventional Cox maze for long-term SR maintenance [24]. Our previous study demonstrated that adding right atrial lesion could improve the SR maintenance rate in thoracoscopic SA [11].
Previous studies did not show significant difference on the efficacy of CA versus SA in treating PerAF [6–8]. In FAST trial, SA was shown to be superior to CA in achieving freedom of atrial arrhythmias after 12 months of follow-up, whereas there were only 26% of patients with PerAF in SA group [6]. The 7-year follow-up results of FAST trial demonstrated that there was no significant difference between CA and SA for the treatment of PerAF [8]. The lesion set of patients in SA group in FAST trial only included PVI, ganglionic plexi ablation, or box ablation, and no right atrial lesion set was mentioned. In CASA-AF trial, SA was not superior to CA in treating long-standing PerAF [7]. However, only the left atrial lesion set, including left atrial box lesion, left atrial appendage occlusion, and ganglionic plexi ablation, was performed in SA group in CASA-AF trial. Lesion set with only left atrium involved might diminish the efficacy of SA. In our study, we performed biatrial lesion set in SA group consistent with the principle of Cox maze, and surgical biatrial ablation was shown to be more effective than CA for treating PerAF. During SA procedure, four patients were converted to SR at the ablation of right atrium, which supported the important role of right atrium in triggering and maintaining AF.
A recent meta-analysis described the overall results of minimally invasive SA compared to CA. Freedom from AF in the whole cohort at 1 year was 64.8% ± 1.5% vs 73.1% ± 1.5% between CA and SA, respectively (log-rank p < 0.001) [25]. Another recent meta-analysis reported that the 5-year success rate after single CA procedure in PerAF was 33.3% [26]. The success rates of CA procedure in both of the meta-analyses were higher than the results reported in our study. This might be related to several reasons: first, the patients enrolled in our study were PerAF and did not include paroxysmal AF; second, after propensity score matching, the mean LAD of patients in CA group reached 45.8 mm in our study, which was higher than that in both of the meta-analyses.
The special structure of the right atrium deserves some consideration during mapping and CA. The pectinate muscles at the lateral RAA may be separated by a thin wall of myocardium, which potentially increases the risk of perforation [27]. In addition, complete RAA isolation may be difficult owing to the large size of the RAA orifice and catheter stability [28]. Therefore, CA is mainly focused on left atrial ablation at present. Surgical right atrial ablation can be easily performed through right-side transthoracic approach under direct vision. However, the biatrial lesion set performed in our study could not be equivalent to the Cox maze lesion set. The ablation lines to the mitral isthmus and tricuspid annulus are difficultly created by stand-alone epicardial ablation, and it is very easily performed in CA. Furthermore, transmural lesions cannot be reliably created by transpolar radiofrequency pens from the epicardial surface due to the heat sink of the beating heart [29]. When intramural/transmural reentry coexists with nontransmural tissue lesions, the risk of AF recurrence would increase [30]. Hybrid ablation technique combining epicardial SA and endocardial CA on beating heart might have the potential to create transmural tissue lesions and improve SR maintenance rate [13].
Previous studies demonstrated that the perioperative complication rate was higher in SA group than that in CA group [6, 7, 25]. In our study, there was no significant difference in the perioperative complication rate between SA group and CA group (p = 0.223), which might be associated with the small sample size. In addition, procedures in both groups were performed by experienced surgeons/electrophysiologists in our institution.
5. Limitations
First, the retrospective single-center study design with small sample size and the relatively short follow-up time limited the drawing of broad conclusions. Second, selection bias still existed in our study, although we used propensity score matching to minimize the bias. The fact that the one patient that died in a car accident was not considered a competing event but rather censored. Third, Holter testing was performed at every follow-up time point and symptomatic moment, which possibly resulted in missed asymptomatic atrial tachyarrhythmia episodes. Finally, in previous cases of hybrid ablation in our institution, endocardial mapping showed almost all PVs were completely isolated by bipolar clamp; however, transmural lesions cannot be reliably created by transpolar radiofrequency pens from the epicardial surface due to the heat sink of the beating heart [13]. Therefore, epicardial mapping was not routinely performed during SA procedure. Endocardial mapping and CA were recommended for recurrent patients who did not respond to medical therapy. Furthermore, we did not perform right atrial mapping to evaluate the right atrial trigger before SA, but we applied the concept of maze procedure and performed surgical biatrial ablation to all patients. Further multicenter randomized trials are expected to confirm our results.
6. Conclusions
In patients with PerAF, thoracoscopic surgical biatrial ablation might provide better freedom from atrial tachyarrhythmia recurrence than CA procedure. Randomized control trials are warranted to validate our results.
Abbreviations
-
- AADs:
-
- Antiarrhythmic drugs
-
- AF:
-
- Atrial fibrillation
-
- CA:
-
- Catheter ablation
-
- CI:
-
- Confidence interval
-
- CTI:
-
- Cavotricuspid isthmus
-
- CS:
-
- Coronary sinus
-
- HR:
-
- Hazard ratio
-
- IVC:
-
- Inferior vena cava
-
- LAD:
-
- Left atrial diameter
-
- LAA:
-
- Left atrial appendage
-
- LIPV:
-
- Left inferior pulmonary vein
-
- PerAF:
-
- Persistent atrial fibrillation
-
- PV:
-
- Pulmonary vein
-
- PVI:
-
- Pulmonary vein isolation
-
- RAA:
-
- Right atrial appendage
-
- RIPV:
-
- Right inferior pulmonary vein
-
- SA:
-
- Surgical ablation
-
- SD:
-
- Standard deviation
-
- SR:
-
- Sinus rhythm
-
- SVC:
-
- Superior vena cava.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors’ Contributions
Chunyu Yu, Haojie Li, and Shuo Yuan contributed equally to this article and they are co-first authors of this article. Chunyu Yu, Haojie Li, and Shuo Yuan wrote the original draft, curated the data, did formal analysis, and investigated the study; Lihui Zheng, Lingmin Wu, and Ligang Ding validated the study and provided resources; Yan Yao and Zhe Zheng conceptualized the study, developed methodology, did project administration, supervised the study, and reviewed and edited the manuscript.
Acknowledgments
This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant number: 2021-I2M-1-063).
Open Research
Data Availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study.