Compound Heterozygous Mutations in FSIP2 Cause Morphological Abnormalities in Sperm Flagella Leading to Male Infertility
Abstract
Multiple morphological abnormalities of sperm flagella (MMAF) indicate severe teratozoospermia. The fibrous sheath interacting protein 2 (FSIP2) plays an important role in the normal construction of the flagella. In this study, a novel compound heterozygous mutation site of FSIP2, involving c.272_275delinsAGGTTTTTATA (p.L92Vfster74) and c.16788_16791del (p.E5596fs), was identified using whole-exome sequencing in a 32-year-old male. Electron microscope images revealed thick sperm neck, scattered sperm mitochondria, and short sperm tail. In addition, FSIP2 could not be visualized in sperm cells via immunofluorescence staining. Moreover, we used a protein domain prediction tool to identify a potential FSIP2 functional domain (5901-6774), the corresponding deletion of which was responsible for the MMAF phenotype in the infertile man. Finally, we reviewed the literature on FSIP2 and found that FSIP2 mutations are relatively concentrated, with high-frequency mutation regions in exon 16 and exon 17 accounting for 50% (10/20) and 35% (7/20) of cases, respectively. In conclusion, FISP2 is a common pathogenic gene of MMAF, which may provide a rationale for genetic counseling in the next generation of patients with male infertility.
1. Introduction
The World Health Organization (WHO) reported that the incidence of infertility is approximately 8%–15% worldwide, and male factor accounts for approximately 50% of infertility cases, in which genetic factors are often involved [1, 2].
Whole-exome sequencing (WES) refers to high-throughput sequencing after the enrichment of the exon DNA sequence. It is an efficient technique for exploring genetic polymorphisms and mutations [3]. Several genes, including AKAP3 (MIM604689) and AKAP4 (MIM300185), are related to sperm structure and function, particularly the normal composition of the sperm fibrous sheath. In addition, mutations in several genes, such as DNAH1 (MIM603332), CFAP43 (MIM617558), or CFAP44 (MIM617559), can cause severe asthenozoospermia due to multiple morphological abnormalities of the sperm flagella (MMAF) [4–6].
Most pathological mutations in these genes can lead to severe axoneme structural disorganized and the loss of the sperm center doublet. Multiple morphological abnormalities of flagella (MMAF) is a sperm flagellar malformation, and the flagellar morphology is mostly presented as absent, coiled, bent, angulated, irregular, and short, which are some of the most severe sperm flagellar defects that leads to male infertility [7, 8]. Patients with mutations in these genes do not develop symptoms of primary ciliary dyskinesia (PCD), such as recurrent respiratory infections, bronchiectasis, and asthma [9, 10]. However, because MMAF is genetically heterogeneous, the known genetic defects only explain approximately 50%–60% of human MMAF cases. Thus, the relationship between genes and MMAF should be further explored [11, 12].
FSIP2 is the only gene that is focally amplified in 10% of testicular germ cell tumors, which are abnormalities with a strong biological basis [13, 14]. FSIP2 was first reported in a male patient in 2018 [15]. FSIP2 (OMIM 618153) is located on chromosome 2q32.1 and encodes a 6907-amino acid protein. Furthermore, FSIP2 attaches to the sperm tail by binding to AKAP4 and participates in fiber sheath assembly, and FSIP2 mutation potentially leads to MMAF-associated asthenoteratospermia and male infertility [16].
We used WES in a sterile male patient with a high MMAF tendency toward sperm morphology. We identified novel compound heterozygous FSIP2 variants with mutations c.272_275delinsAGGTTTTTATA (p.L92Vfster74) and c.16788_16791del (p.E5596fs). The present study provides useful evidence for the pathogenicity of these two mutations via electron microscopy and protein structure analysis. Thus, this study expands the significance of FSIP2 genotyping in Chinese male patients with infertility.
2. Materials and Methods
2.1. Patient
The patient was a 32-year-old man who presented to the outpatient clinic of our hospital for semen examination due to fertility issues. Physical examination of the testis, epididymis, and vas deferens revealed normal findings; moreover, his blood hormone levels were within the normal range: follicle-stimulating hormone, 4.39 IU/L; luteinizing hormone, 1.5 IU/L; testosterone, 1.57 ng/mL; estradiol, 13.78 pg/mL; and prolactin, 17.7 ng/mL. Neither the patient nor his family had any PCD-related symptoms. The patient was diagnosed with asthenozoospermia and teratozoospermia based on three semen analyses. The control sample was obtained from a fertile man who was planning to have a second child. This study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital, China (No. 2022091), and written informed consent was obtained from the patient (see Supplemental Material (available here)).
2.2. Semen Parameters and Sperm Morphological Analyses
The patient and healthy control were asked to abstain from sex for 2–7 days before collecting their masturbation semen samples in the hospital semen retrieval room. The semen samples were completely liquefied after being placed in a 37°C incubator for 30 min. Semen parameters, including semen volume, sperm progressive motility, and sperm concentration, were evaluated using computer-assisted sperm analysis (SAS medical, SAS-II, Beijing, China). Simultaneously, sperm morphology was assessed using Papanicolaou stain, and the samples were observed under bright field at 1,000x magnification. All semen parameters were evaluated according to the 5th edition of the WHO laboratory manual for the examination and processing of human semen [17].
2.3. Scanning Electron Microscopy (SEM)
Prepared spermatozoa samples were immobilized overnight with 2.5% phosphate-buffered glutaraldehyde at 4°C. Immobilized spermatozoa samples were dehydrated using a series of 50%, 70%, and 90% graded ethanol, wherein the concentration was changed after every 15 min. Finally, the samples were incubated in 100% ethanol twice for 20 min each. After drying, the sperm samples were observed via SEM at 10 kV (Nova nano450, Thermo Fisher FEI).
2.4. Transmission Electron Microscopy (TEM)
The prepared sperm samples were fixed overnight with 2.5% phosphate-buffered glutaraldehyde. These samples were washed thrice with 0.1 M phosphate buffer (PB; pH 7.2) and fixed with 1% osmium tetroxide in 0.1 M PB for 1 h at 4°C. Dehydration was performed sequentially using 50%, 70%, and 90% ethanol as well as 100% acetone. The samples were then allowed to polymerize for 48 h at 60°C. Subsequently, ultrathin sections were stained with 3% uranyl acetate and lead citrate. The ultrastructure of these samples was observed and photographed via TEM at 100 kV (Tecnai, Thermo Fisher FEI).
2.5. Immunostaining
The sperm samples were fixed on slides with 4% paraformaldehyde for 30 min. These samples were then permeabilized with 0.2% Triton-X for 15 min and blocked with 1% BSA for 60 min, followed by overnight incubation at 4°C with FSIP2 (rabbit monoclonal, Invitrogen, 1 : 100) and acetylated α-tubulin (mouse monoclonal, Santa Cruz, 1 : 200) antibodies. Next, the primary antibody was washed with PBS, and anti-rabbit (Abcam6802, 1 : 500, green) and anti-mouse (Abcam6789, 1 : 500, red) secondary antibodies were added to the slides and incubated with sperm for 1 h. Finally, the sperm samples were stained with DAPI for 3–5 min and observed under a scanning confocal immunofluorescence microscope (Leica sp8, Germany). Among the FSIP2 key domains, the protein domains were predicted using CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Moreover, we visualized the 3D structural model of FSIP2 key domains using I-TASSER (http://zhang.bioinformatics.ku.edu/I-TASSER/) (see Supplemental Material) and PyMOL2.5.2 (https://pymol.org) to identify the impact of FSIP2 mutation sites on protein structure and function.
2.6. WES and Sanger Sequencing
Peripheral blood samples (2 mL) were collected from the patient and his parents, and DNA was extracted from whole blood using a blood genomic DNA extraction kit (QIAamp DNA Blood Mini Kit, Qiagen). WES was performed using Novaseq 6000 system based on Illumina sequencing technology. Patient gene sequences were aligned with normal human genome sequences, and loci with pathogenic potential were screened via Mutation Taster, SIFT, Polyphen2, and GERP++ software analysis and then matched according to genotype-phenotype. The frequency of genomic variants in the general population was assessed using 1,000 Genomes Project, ESP6500 and ExAC, and gnomADMAX (see Supplemental Material). FSIP2 mutations in the proband and patient’s parents were verified using PCR and Sanger methods. The PCR cycle consists of three steps: (1) high-temperature denaturation, in which the DNA is converted to single strands at 95°; (2) low-temperature annealing (approximately 55°C), wherein the primer and single chain bind according to base complementary pairing; and (3) mid-temperature extension, in which the temperature is adjusted to the optimal reaction temperature of the DNA polymerase (approximately 72°C) to facilitate the synthesis of the complementary strand in the direction of the phosphate to five-carbon sugar (5′–3′). The following two primers were used to amplify exon 3 and exon 17 of FSIP2 (5′→3′). Exon 3 (c.272_275delinsAGGTTTTTATA), forward: GGTGAGGAGAGAGGAAAGGA and reverse: ACCCACTTTATTATTGCTGGTGA. Exon 17 (16788_16791del), forward: TGAAAAGCGGCATGATTAACCT and reverse: TGGACTTCAAGGAGGATAACTGA. PCR products were sequenced using Ex Taq DNA Polymerase Kit (Bio-Rad, Hercules, CA, USA). Finally, sequence analysis was performed using 3730XL system (GSL Biotech).
3. Results
3.1. Routine Semen Analysis
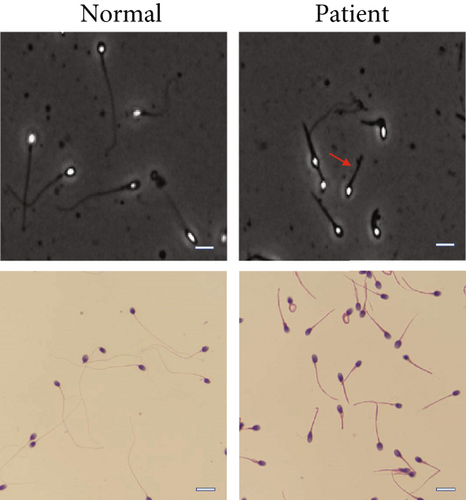
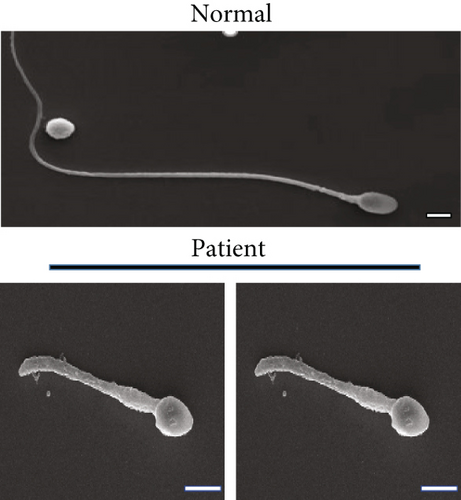
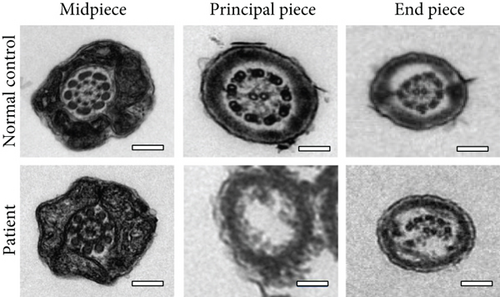
Routine semen analysis revealed a normal sperm concentration and reduced sperm motility according to WHO guidelines (Table 1). Further, light microscopy and SEM analyses confirmed that approximately 90% of the patient’s sperm had an abnormal morphology, including several short tails and a few curled flagella (Figures 1(a) and 1(b)). In both healthy control and patient, TEM analysis revealed that the cross-sectional ultrastructure of the sperm mid-piece exhibited an intact “9+2” axonal architecture. However, a cross-section of the principal piece and end piece of the patient’s sperm revealed the loss of axonal structure and central pair (CP), with a disordered arrangement of the microtubule doublets (Figure 1(c)).
| First ejaculate | Second ejaculate | Third ejaculate | Reference valuesa | |
|---|---|---|---|---|
| Semen parameters | ||||
| Semen volume (mL) | 4 | 3.5 | 4.0 | ≥1.5 |
| Sperm concentration (106/mL) | 111.7 | 107.6 | 111.3 | ≥15 |
| Sperm progressive motility (%) | 6.8 | 9.8 | 5.0 | ≥32 |
| Sperm morphology | ||||
| Normal spermatozoa (%) | 1 | 1 | 1 | ≥4 |
| Anomalies of the head (%) | 93 | 93 | 93 | ─ |
| Short flagella (%) | 71 | 68 | 75 | ─ |
| Absent flagella (%) | 0 | 1 | 1 | ─ |
| Coiled flagella (%) | 7 | 8 | 8 | ─ |
| Flagella of irregular caliber (%) | 2 | 4 | 2 | ─ |
- aAccording to the World Health Organization standards (5th edition).
3.2. Gene Mutation Sites
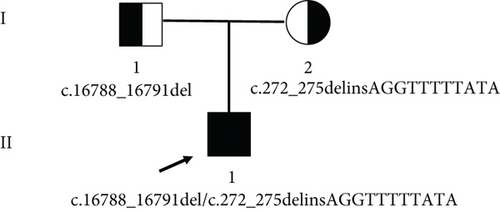
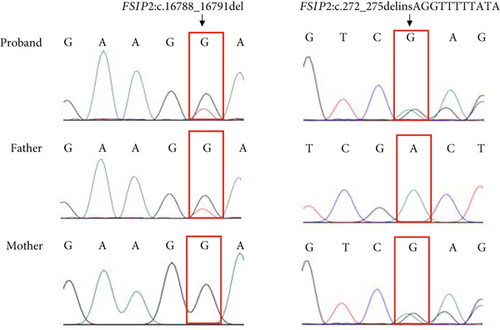
Two novel FSIP2 mutations, exon3: c.272_275delinsAGGTTTTTATA (p.L92Vfster74) and exon 17: c.16788_16791del (p.E5596fs), were identified in the patient using WES (Figure 2). Detailed information on the variants is provided in Table 2. Sanger sequencing revealed that the patient’s father had c.16788_16791del heterozygous mutation and his mother had c.272_275delinsAGGTTTTTATA heterozygous mutation (Figure 2(a)). Functional predictions based on SIFT and Polyphen2 databases suggested that these mutations have never been reported, whereas Mutation Taster classified them as disease-causing (Table 2). Both mutations (p.L92Vfster74 and p.E5596fs) are new mutations whose gene frequencies have not been described in ExACMAX, 1000G_ALL, or GnomAD (Table 2). The two mutation sites are pathogenic according to American College of Medical Genetics and Genomics (ACMG) guidelines (Table 2).
| Gene ∗ | Chromosome location # | Mutation | Amino acid change | Mutation type | Exon | SIFT | Polyphen2 | Mutation taster | ExACMAXa | 1000G_ALLb | GnomADc | ACMGd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FSIP2 | Chr2:186607906-186607909 | c.272_275 delinsAGGTTTTTATA | p.L92Vfster74 | Frameshift deletion/insertion | Exon 3 | NA | NA | Disease-causing | 0 | 0 | 0 | Pathogenic |
| Chr2:186670820-186670824 | c.16788_16791del | p.S5597Gfster6 | Frameshift deletion | Exon 17 | NA | NA | Disease-causing | 0 | 0 | 0 | Pathogenic |
- Note: ∗Gene: FSIP2 (NC_000002.12); #chromosome location: GRCh37/hg19 position; aExAC: http://exac.broadinstitute.org; b1000G_ALL: ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp;cGnomAD: http://gnomad.broadinstitute.org; dACMG: American College of Medical Genetics and Genomics.
3.3. Analysis of the Sequence and Protein Structure of the FSIP2 Mutation Sites
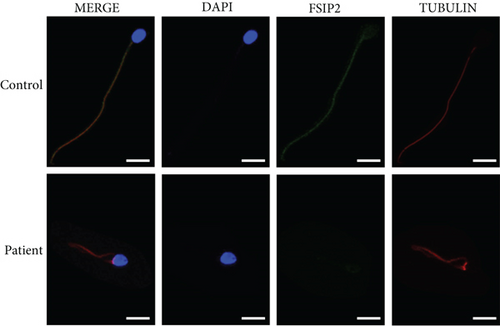
FSIP2 expression in the patient and control samples was determined via immunofluorescence staining. The patient’s sperm showed weak FSIP2 expression, whereas the control sperm showed positive FSIP2 expression (Figure 3).
To identify the mechanism underlying the loss of FSIP2 expression, we analyzed the sequence and protein structural features of the FSIP2 mutation site. We also compared the sequences of wild-type FSIP2 and the two patient mutation sites, c.272_275delinsAGGTTTTTATA and c.16788_16791del (Figure 4(a)). We found that c.272_275delinsAGGTTTTTATA mutation site ends early, leaving only 165 amino acids at the N-terminus, resulting in protein truncation. Notably, this serious deletion results in a significant loss of protein function. Furthermore, c.16788_16791del mutation site shared its first 5596 amino acids with wild-type FSIP2. However, subsequently, only five amino acids remained at the mutant C-terminus, whereas the wild-type C-terminus had a sequence of approximately 1400 amino acids.
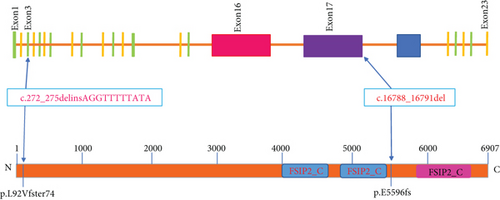
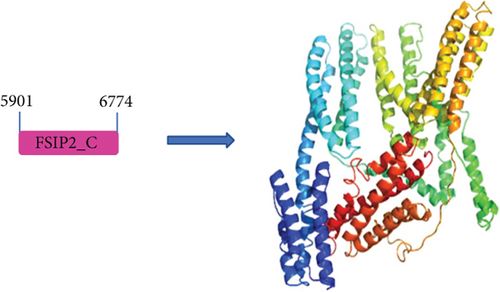
Notably, the loss of informational sequence in c.272_275delinsAGGTTTTTATA resulted in the loss of protein structure and function. In the case of c.16788_16791del mutation, the loss of protein function can lead to the deletion of a key functional domain. To the best of our knowledge, the crystallographic structure of FSIP2 has not yet been reported in the literature. However, using a protein domain prediction tool, we could identify a potential functional domain in FSIP2 (5901-6774). In addition, we compared the sequences of FSIP1 and FSIP2 and found a sequence similarity of 12.5, indicating some structural similarity (see Supplemental Material). A structural model of FSIP2 and FSIP1 domains was constructed using homology modeling, which revealed the possible structure of the FSIP2 domain (5901-6774) (Figure 4(b)). The c.16788_16791del mutation led to the deletion of this critical domain, resulting in the loss of protein function and pathogenicity, as evidenced by the MMAF phenotype observed in this infertile patient.
4. Discussion
In this study, we identified novel FSIP2 compound heterozygous mutation sites associated with short-tailed sperms in a Chinese man; the two new mutations were c.272_275delinsAGGTTTTTATA (p.L92Vfster74) and c.16788_16791del (p.E5596fs).
The sperm is composed of three parts: head, middle part, and tail [22]. The cytoskeleton of normal mobile sperm flagellum follows a “9+2” axonemal configuration and is surrounded by a fibrous sheath, mainly composed of nine outer microtubule doublets, two CPs, and an outer dense fiber [23–25]. Abnormal structure of the sperm fiber sheath and a lack of CP may cause MMAF, resulting in abnormal sperm tail morphology, including short, curly, or absent tails. In this study, a patient with FSIP2 mutation had a normal sperm concentration, but the sperm showed loss of axonal structure and CPs, with disordered arrangement of the microtubule doublets. Moreover, in contrast to the sperm flagella of patients with DNAH1, CFAP43, and CFAP44 mutations, those of patients with MMAF mostly contained short tails [26–28].
A major component of the skeletal structure of the sperm flagella is the fibrous sheath, and fibrous sheath dysplasia leads to male infertility [29]. Notably, FSIP2 is unique to spermatogenic cells and may attach to sperm tails by binding to the fibrous sheath via AKAP4, although AKAP4 was not identified in any sperm samples from patients with FSIP2 mutation [15]. Previous mouse model data suggest that FSIP2 is only expressed in the testis [18, 30], and the FSIP2 protein emerges in the testis of mice 21 days after birth and gradually accumulates until adulthood. Moreover, FSIP2 transcription begins in late spermatocyte developmental stage, and the gene is expressed in sperm cells and flagella at different spermatogenesis stages [18, 20]. Further, immunofluorescence staining was performed using the sperm samples of patients with FSIP2 mutation and controls, which revealed that the FSIP2 antibody could not be observed in the piece of the sperm tail. However, after staining with tubulin, the fluorescence of both antibodies in the control group could be visualized. This suggests that FSIP2 plays a major role in sperm tail formation. A recent study reported that FSIP2 mutations severely affect sperm acrosome development, suggesting that FSIP2 mutations are responsible for the development of globozoospermia [21].
By analyzing the sequence and protein structure of FSIP2 mutation sites, we identified a potential functional domain (5901-6774) that was affected by the mutations identified in the patient. The mutation ultimately leads to changes in sperm protein expression and function [15, 20]. Simultaneously, by combining the previously reported mutation sites in FISP2 and those identified in the present study, we revealed that most of the 15 identified FISP2 mutation sites were in exons 16 (50% [10 of 20]) and 17 (35% [7 of 20]), suggesting that the high-frequency FSIP2 mutation region exists within these two exons with relatively concentrated features (Table 3).
| FSIP2 variants | Exon | Amino acid change | Status | Reference |
|---|---|---|---|---|
| c.910delC | Exon 8 | p.Gln304Lys12ter3 | ho | [15] |
| c.[1606_1607insTGT; 1607_1616delAAAGATTGCA] | Exon 16 | p.Lys536Metfster1 | ho | |
| c.2282dupA | Exon 16 | p.Asn761llefster4 | ho | |
| c.16389_16392delAATA | Exon 17 | p.Glu5463Glufster7 | ho | |
| c.1907C>A | Exon 16 | p.S636ter | ho | [8] |
| c.8030_8031insA | Exon 16 | p.T2680Nfster9 | ho | |
| c.16246_16247insCCCAAATATCACC | Exon 16 | p.T5416fster7 | he | [18] |
| c.17323C>T | Exon 17 | p.Q5774ter | he | |
| c.1750T>A | Exon 16 | p.C584S | he | [19] |
| c.13600A>G | Exon 17 | p.I4534V | he | |
| c.8368_8369insC | Exon 16 | p.2790fs | ho | [20] |
| c.19981 C>T | Exon 18 | p.R6661ter | he | [21] |
| c.18448G>A | Exon 17 | p.V6150I | he | |
| c.5238-5240del | Exon 16 | p.E1746del | he | |
| c.5480A>T | Exon 16 | p.D1827V | he | |
| c.9056T>C | Exon 16 | p.I3019T | he | |
| c.10823T>C | Exon 17 | p.S5933F | he | |
| c.17798C>T | Exon 17 | p.D1827V | he | |
| c.272_275delinsAGGTTTTTATA | Exon 3 | p.L92Vfster74 | he | This paper |
| c.16788_16791del | Exon 17 | p.E5596fs | he |
- Note: ho: homozygote; he: heterozygote.
Most sperms in patients with MMAF were immobile due to the completely defective flagella, and ICSI-assisted technology was used to address fertility problems in these patients. In a patient with FSIP2 mutation reported in 2021, two blastocysts were transferred after ICSI, but the outcome was unsuccessful [18]. Fortunately, in the patient with this newly reported FSIP2 compound heterozygous mutation (c.1750T>A and c.13600A>G), the embryos cultured on day 3 of the transfer resulted in a successful clinical pregnancy [19]. ICSI enables couples with obstructive azoospermia, severe oligospermia, or asthenospermia to have children. However, there are concerns about the health of these children because of the risk of genetic transmission via ICSI to male offspring [31]. As only few cases of FSIP2 mutation have been reported, more cases are needed to clarify the ART outcome of patients with FSIP2 mutation.
In conclusion, we discovered a novel compound heterozygous mutation site in FSIP2 that can cause male reproductive infertility and summarized the high-frequency FSIP2 mutation sites. This finding may help clinicians better understand FSIP2 to have a clear diagnostic basis for assessing asthenozoospermia.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We would like to thank the participants described in this report for their consent and support. We also thank Professor Zhang Feng of Fudan University for his guidance. This study is supported by the Zhejiang Medical and Health Science and Technology Project (2019RC109 and 2020RC104).
Open Research
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.