Anti-dsDNA Is Associated with Favorable Prognosis in Myasthenia Gravis: A Retrospective Study
Abstract
Objectives. To investigate the presence of serum antinuclear antibody (ANA) and anti-double-stranded DNA antibody (anti-dsDNA) in patients with myasthenia gravis (MG) and analyze the clinical characteristics and prognostic factors associated with MG. Methods. We retrospectively enrolled 363 patients with MG and analyzed the clinical characteristics and follow-up data between patients positive and negative for ANA and anti-dsDNA. We defined a Myasthenia Gravis Activities of Daily Living (MG-ADL) reduction as a main prognosis predictor and used logistic regression to determine independent factors associated with prognosis. We built a nomogram to predict prognosis and evaluate the internal validity of the model. Results. Ninety-eight (27.0%) patients were positive for ANA, and 51 (14.0%) were positive for anti-dsDNA. Patients positive for ANA and anti-dsDNA antibodies tended to be female and positive for acetylcholine receptor antibody (AChR-Ab). The rate of thymoma was higher in anti-dsDNA-positive patients with MG (p-dsDNA-MG) than in patients negative for anti-dsDNA (49.0% vs. 26.0%, p = 0.001), and p-dsDNA-MG was associated with reduced MG-ADL score. Regression analysis showed that except for age of onset (OR = 0.986, 95%CI = 0.973–0.999, p = 0.037), anti-dsDNA (OR = 2.800, 95%CI = 1.381–5.679, p = 0.004), ptosis (OR = 2.930, 95%CI = 1.827–4.699, p < 0.001), and eye movement disorder (OR = 2.815, 95%CI = 1.672–4.741, p < 0.001) were independent predictive factors of a favorable prognosis of MG. These predictors were used to generate a nomogram with an excellent predictive value. Conclusions. Being female and the presence of AChR-Ab were features of ANA- or anti-dsDNA-positive MG. The presence of anti-dsDNA was associated with a favorable prognosis of MG.
1. Introduction
Myasthenia gravis (MG) is an antibody-mediated and organ-specific autoimmune disease that often cooccurs with other autoimmune diseases (AD) [1, 2]. A study of MG showed that AD-related autoantibody positivity in serum was 52%, primarily thyroid antibodies and antinuclear antibodies (ANAs), but in 53.85% cases, it was difficult to diagnose AD, which included systemic lupus erythematosus (SLE) [3]. Patients with MG are likely to test positive for AD-related autoantibodies but do not show clinical symptoms. Therefore, a systematic follow-up of autoantibody-positive patients with MG is needed to predict disease progression [3].
Autoantibodies are important components of the classification criteria for many ADs [4]. Among these, detection of ANAs is particularly important because they may appear many years before a patient develops obvious symptoms or signs. Therefore, detection of ANAs is critical for the diagnosis and determination of the prognosis and activity of ADs [5–8]. Antinuclear antibody and anti-double-stranded DNA antibodies (anti-dsDNA) are important antibodies for the diagnosis of SLE [4]. These antibodies may cause organ or tissue damage and induce the inflammatory response [9]. Detection of ANA has high sensitivity, but low specificity, for the diagnosis of SLE [10]. Furthermore, ANA can be found in healthy individuals, or in individuals with various pathologies, resulting in a poor diagnostic value [11]. However, anti-dsDNA is present in >70% of patients with SLE and is particularly prevalent in children and patients with early-onset SLE [12–14]. Anti-dsDNA can appear more than 2 years before a clinical diagnosis of SLE, and a high titer has been shown to predict severe symptoms in the following 6 months [15, 16]. Therefore, anti-dsDNA is a more effective and specific diagnostic tool for SLE than ANA.
To our knowledge, there have been few reports of patients with MG and SLE. However, the probability of developing MG is higher in individuals with SLE than in those without. Similarly, the prevalence of SLE in patients with MG is also higher than that in individuals without MG [17–20]. Previous studies showed that the ANA-positive rate and anti-dsDNA-positive rate in SLE were 90–98% and >70%, respectively; moreover, 85% of patients who were positive for anti-dsDNA but not diagnosed with SLE developed SLE in subsequent years [12, 13, 21]. Therefore, patients with MG having these antibodies are at greater risk for developing SLE. In addition, another study showed that the ANA-positive rate in MG was 38.5% and the anti-dsDNA-positive rate was 19.2% [20]. Therefore, the potential clinical significance of these observations is unclear, and additional studies may be needed to provide valuable information to help neurologists assess and treat ADs.
In this study, we retrospectively analyzed the clinical characteristics and prognosis of patients with MG who were positive for ANA and anti-dsDNA to provide a basis for future research on the pathogenesis and treatment of MG.
2. Materials and Methods
2.1. Study Design and Patients
We collected data from patients with MG diagnosed at the First Affiliated Hospital of Sun Yat-sen University between June 1, 2016, and June 30, 2019. The patient’s clinical characteristics and follow-up data with analysis of ANA or anti-dsDNA were retrospectively analyzed.
Patients who met the following criteria were diagnosed with MG: (1) typical pathological fatigue of skeletal muscles and (2) one of the following: (a) positive neostigmine test; (b) positive neurophysiologic testing, with repetitive nerve stimulation (RNS) showing a decrement of >10% in the compound muscle action potential (CMAP) amplitude at a frequency of 3 Hz or abnormal voluntary single-fiber electromyography (SFEMG) showing a mean jitter value exceeding the upper limit of the normal value, or >10% of pairs showing increased jitter (>55 μs) or occurrence of a block; or (c) positive serum antibody including antibodies against acetylcholine receptor (AChR-Ab) or muscle-specific kinase (MuSK-Ab) or lipoprotein receptor-related protein 4 (LRP4-Ab) [1]. Patients with MG who were followed-up for >2 years and had been tested for ANAs were included. The exclusion criteria were as follows: (1) patients with incomplete clinical data or follow-up data; (2) patients with severe cardiac, pulmonary, liver, or renal disorders; and (3) patients with other tumors except thymoma. The study was approved by the Ethical Committee of the First Affiliated Hospital of Sun Yat-sen University.
2.2. Data
Demographic data included sex and age of onset. Clinical data included serum MG antibodies, Myasthenia Gravis Foundation of America Classification (MGFA classification), history of myasthenia crisis, neostigmine test, RNS or SFEMG, computed tomography (CT) of the thymus, thymectomy, and involvement of muscles including extraocular muscles, levator palpebrae superioris, facial, neck, limb, and truncal (involving chewing, breathing, and bulbar involvement) muscles. The total follow-up time was 2 years, with follow-up once every 3 months in the first year and once every 6 months in the second year. Follow-up data included drug exposure, Myasthenia Gravis Activities of Daily Living (MG-ADL) score, and MGFA postintervention status. We set the treatment response as a prognosis. In patients with high score of initial MG-ADL (≥3), we defined an MG-ADL score reduction of no less than 3 points as a clinical improvement [22] and an indicator of a good prognosis, while in patients with low initial score (<3), we defined drug reduction without MG-ADL increase or MG-ADL reduction without drug adding as good prognosis. Thymectomy was performed in patients with MG complicated with thymoma or in patients with generalized AChR-MG.
Antibodies related to MG were detected using enzyme-linked immunosorbent assay (ELISA) kits (RSR, Cardiff, UK). The following levels were considered positive for each antibody: AChR − Ab > 0.45 nmol/L, MuSK − Ab > 0.4 U/mL, and LRP4 − Ab > 0 U/mL. Serum ANA and anti-dsDNA were detected using an ELISA kit (AESKU, Wendelsheim, Germany) and a chemiluminescence immunoassay kit (YHLO, Shenzhen, China), respectively. The following levels were considered positive: ANA > 12 U/mL and anti − dsDNA > 12 IU/mL.
2.3. Statistical Analysis
Continuous variables were tested for data normality using the Shapiro–Wilk (W method) test. Normally distributed data were analyzed using independent sample t-tests and reported as the mean (standard deviation). Nonnormally distributed data were analyzed using the Wilcoxon rank-sum test and reported as the median (interquartile range, IQR). Categorical variables were reported as frequencies (percentages) and analyzed using the chi-square test or Fisher’s exact test (when theoretical number T < 1 or n < 40). p < 0.05 was considered to indicate statistically significant differences. Univariate analysis and clinical knowledge were used to screen for possible predictors of outcomes, and variables with p < 0.1 were further subjected to multivariate logistic regression to estimate odds ratios (OR) and 95% confidence intervals (CI). All data were analyzed using IBM SPSS version 25.0 (IBM, Armonk, NY, USA) software. The results of the multivariate logistic regression analysis were used to generate a nomogram, which was evaluated for predictive values using a receiver operating characteristic (ROC) curve and for accuracy by plotting the calibration curve. Internal validation was performed using the 1000 bootstrapping sample method, and the C-index and 95% confidence interval (CI) from the bootstrapping sample datasets were used to assess the stability of the model. These procedures were performed using R statistical software package (version 4.1.3; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
We collected data from 453 patients diagnosed with MG but excluded 50 cases without data for ANA or anti-dsDNA, 28 cases with incomplete clinical data, two patients who died during follow-up, and 10 cases with connective tissue disorder (CTD). Finally, 363 patients were enrolled in the study. A flow chart of the study is shown in Figure 1. The median age of the patients was 43 (range: 13–88) years. In addition, 169 (46.6%) were male and 194 (53.4%) were female. Furthermore, 146 (40.2%) patients had ocular MG (OMG) and 217 (59.8%) had generalized MG (GMG). The AChR-Ab positivity rate was 75.2% (273 patients). The rate of thymoma was 29.2% (106 patients), and 135 patients underwent thymectomy. Facial involvement was not included in the analysis because only two patients showed relevant symptoms.
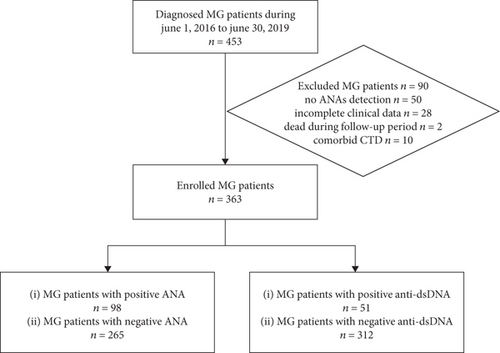
3.1. Clinical Characteristics of Patients with MG Positive for ANA (p-ANA-MG) and Patients with MG Positive for Anti-dsDNA (p-dsDNA-MG)
Of the 363 enrolled patients, 98 (27.0%) were positive for ANA and 51 (14.0%) were positive for anti-dsDNA. The median age of onset was 41.5 years (IQR: 26.8–55.3 years) for p-ANA-MG and 46 years (IQR: 35.0–59.0 years) for p-dsDNA-MG. Compared to patients with MG who were ANA-negative (n-ANA-MG), those with p-ANA-MG tended to be female (68.4% vs. 47.9%), had a higher rate of AChR-Ab (85.7% vs. 71.3%), and had the generalized type (68.4% vs. 56.6%) with limb (42.9% vs. 30.2%) involvement. Compared to anti-dsDNA-negative patients with MG (n-dsDNA-MG), those with p-dsDNA-MG tended to be female (66.7% vs. 51.3%), had a later age of onset (p = 0.008), had a higher rate of AChR-Ab (90.2% vs. 72.8%), had thymoma (49.0% vs. 26.0%), and had generalized MG with bulbar (52.9% vs. 33.3%) and neck involvement (19.6% vs. 7.4%). Furthermore, the MG-ADL scores showed that p-dsDNA-MG at 2 years was more likely to show good treatment response (p = 0.022) (Table 1).
| p-ANA-MG | n-ANA-MG | p value | p-dsDNA-MG | n-dsDNA-MG | p value | |
|---|---|---|---|---|---|---|
| n = 98 (%) | n = 265 (%) | n = 51 (%) | n = 312 (%) | |||
| Gender | 0.001 ∗ | 0.041 ∗ | ||||
| Male | 31 (31.6) | 138 (52.1) | 17 (33.3) | 152 (48.7) | ||
| Female | 67 (68.4) | 127 (47.9) | 34 (66.7) | 160 (51.3) | ||
| Age of onset (years, median, quartile) | 41.5 (26.8, 55.3) | 38.0 (21.5, 51.0) | 0.059 | 46.0 (35.0, 59.0) | 38.0 (22.0, 51.0) | 0.008 ∗ |
| Age of onset (years) | 0.096 | 0.221 | ||||
| <18 | 7 (7.1) | 42 (15.8) | 3 (5.9) | 46 (14.7) | ||
| 18-49 | 61 (62.2) | 147 (55.5) | 31 (60.8) | 177 (56.7) | ||
| >49 | 30 (30.6) | 76 (28.7) | 17 (33.3) | 89 (28.5) | ||
| Presence of AChR-Ab | 84 (85.7) | 189 (71.3) | 0.005 ∗ | 46 (90.2) | 227 (72.8) | 0.007 ∗ |
| MGFA classification | 0.042 ∗ | 0.090 | ||||
| Type I | 31 (31.6) | 115 (43.4) | 15 (29.4) | 131 (42.0) | ||
| Types II-V | 67 (68.4) | 150 (56.6) | 36 (70.6) | 181 (58.0) | ||
| Symptom | ||||||
| Limb involvement | 42 (42.9) | 80 (30.2) | 0.023 ∗ | 23 (45.1) | 99 (31.7) | 0.061 |
| Bulbar involvement | 43 (43.9) | 88 (33.2) | 0.060 | 27 (52.9) | 104 (33.3) | 0.007 ∗ |
| Neck involvement | 13 (13.3) | 20 (7.5) | 0.092 | 10 (19.6) | 23 (7.4) | 0.014a |
| Breathing involvement | 19 (19.4) | 48 (18.1) | 0.781 | 6 (11.8) | 61 (19.6) | 0.184 |
| Chewing involvement | 14 (14.3) | 28 (10.6) | 0.325 | 10 (19.6) | 32 (10.3) | 0.053 |
| Neostigmine test | 0.294 | 0.460a | ||||
| Negative | 5 (5.1) | 12 (4.5) | 2 (3.9) | 15 (4.8) | ||
| Positive | 90 (91.8) | 233 (87.9) | 48 (94.1) | 275 (88.1) | ||
| Not performing | 3 (3.1) | 20 (7.5) | 1 (2.0) | 22 (7.1) | ||
| RNS or SFEMG | 0.748 | 0.346 | ||||
| Normal | 11 (11.2) | 23 (8.7) | 2 (3.9) | 32 (10.3) | ||
| Abnormal | 67 (68.4) | 184 (69.4) | 38 (74.5) | 213 (68.3) | ||
| Not performing | 20 (20.4) | 58 (21.9) | 11 (21.6) | 67 (21.5) | ||
| A history of myasthenia crisis | 16 (16.3) | 31 (11.7) | 0.244 | 6 (11.8) | 41 (13.1) | 0.786 |
| Thymoma | 35 (35.7) | 71 (26.8) | 0.097 | 25 (49.0) | 81 (26.0) | 0.001 ∗ |
| Thymectomy | 37 (37.8) | 98 (37.0) | 0.892 | 23 (45.1) | 112 (35.9) | 0.208 |
| Thymoma and thymectomy | 0.095 | 0.003 ∗ | ||||
| NN | 51 (52.0) | 157 (59.2) | 21 (41.2) | 187 (59.9) | ||
| TN | 10 (10.2) | 10 (3.8) | 7 (13.7) | 13 (4.2) | ||
| NT | 12 (12.2) | 37 (14.0) | 5 (9.8) | 44 (14.1) | ||
| TT | 25 (25.5) | 61 (23.0) | 18 (35.3) | 68 (21.8) | ||
| Treatment | 0.685 | 0.190 | ||||
| PYR | 5 (5.1) | 19 (7.2) | 3 (5.9) | 21 (6.7) | ||
| PYR+PRE | 44 (44.9) | 124 (46.8) | 18 (35.3) | 150 (48.1) | ||
| PYR+PRE+IMM | 49 (50.0) | 122 (46.0) | 30 (58.8) | 141 (45.2) | ||
| Treatment response at two years | 0.755 | 0.022 ∗ | ||||
| Bad | 40 (40.8) | 113 (42.6) | 14 (27.5) | 139 (44.6) | ||
| Good | 58 (59.2) | 152 (57.4) | 37 (72.5) | 173 (55.4) | ||
| MGFA post intervention status | 0.261 | 0.444 | ||||
| Worse+unchanged+improved | 46 (46.9) | 107 (40.4) | 24 (47.1) | 129 (41.3) | ||
| MM+PR+CSR | 52 (53.1) | 158 (59.6) | 27 (52.9) | 183 (58.7) |
- Abbreviations: AChR-Ab: acetylcholine receptor antibodies; CSR: complete stable remission; MGFA: Myasthenia Gravis Foundation of America; RNS: repetitive nerve stimulation; SFEMG: single-fiber electromyography; NN: not performing thymectomy in nonthymomatous MG; TN: not performing thymectomy in thymomatous MG; NT: performing thymectomy in nonthymomatous MG; TT: performing thymectomy in thymomatous MG; PYR: pyridostigmin; PRE: prednisone; IMM: immunosuppressant; MM: minimal manifestation; PR: pharmacologic remission. ∗p value < 0.05: statistically significant. aFisher exact test.
3.2. Prognostic Factors for MG
Good treatment response versus bad treatment response was associated with younger age of onset (p = 0.010); higher prevalence of dsDNA-Ab (17.6% vs. 9.2%); higher rate of ocular MG (46.2% vs. 32.0%); reduced rate of a history of myasthenia crisis (9.0% vs. 18.3%); reduced rate of thymectomy (32.9% vs. 43.1%); significantly increased rates of ptosis (69.5% vs. 37.9%), diplopia (48.6% vs. 21.6%), and eye movement disorder (49.5% vs. 20.3%); and lower rate of breathing involvement (14.3% vs. 24.2%) (Table 2). Univariate analysis showed that young age of onset, ocular type, ptosis, diplopia, eye movement disorder, and presence of anti-dsDNA were statistically significant parameters (p < 0.1) and indicators of good prognosis. In contrast, a history of myasthenia crisis, breathing involvement, and thymectomy were factors for poor prognosis. Multivariate logistic regression showed that except for age of onset (OR = 0.986, 95%CI = 0.973–0.999, p = 0.037), anti-dsDNA (OR = 2.800, 95%CI = 1.381–5.679, p = 0.004), ptosis (OR = 2.930, 95%CI = 1.827–4.699, p < 0.001), and eye movement disorder (OR = 2.815, 95%CI = 1.672–4.741, p < 0.001) were independent predictors of a good prognosis in MG (Table 3).
| Good response | Bad response | p value | |
|---|---|---|---|
| n = 210 (%) | n = 153 (%) | ||
| Gender | 0.265 | ||
| Male | 103 (49.0) | 66 (43.1) | |
| Female | 107 (51.0) | 87 (56.9) | |
| Age of onset (years, median, quartile) | 35.5 (20.8, 51.3) | 44.0 (28.0, 52.0) | 0.010 ∗ |
| Age of onset (years) | 0.026 ∗ | ||
| <18 | 37 (17.6) | 12 (7.8) | |
| 18-49 | 114 (54.3) | 94 (61.4) | |
| >49 | 59 (28.1) | 47 (30.7) | |
| Presence of AChR-Ab | 156 (74.3) | 117 (76.5) | 0.634 |
| Presence of ANA | 58 (27.6) | 40 (26.1) | 0.755 |
| Presence of anti-dsDNA | 37 (17.6) | 14 (9.2) | 0.022 ∗ |
| MGFA classification | 0.007 ∗ | ||
| Type I | 97 (46.2) | 49 (32.0) | |
| Types II-V | 113 (53.8) | 104 (68.0) | |
| A history of myasthenia crisis | 19 (9.0) | 28 (18.3) | 0.010 ∗ |
| Thymoma | 58 (27.6) | 48 (31.4) | 0.437 |
| Thymectomy | 69 (32.9) | 66 (43.1) | 0.045 ∗ |
| Thymoma and thymectomy | 0.128 | ||
| NN | 126 (60.0) | 82 (53.6) | |
| TN | 15 (7.1) | 5 (3.3) | |
| NT | 26 (12.4) | 23 (15.0) | |
| TT | 43 (20.5) | 43 (28.1) | |
| Neostigmine test | 0.106 | ||
| Negative | 12 (5.7) | 5 (3.3) | |
| Positive | 189 (90.0) | 134 (87.6) | |
| Not performing | 9 (4.3) | 14 (9.2) | |
| RNS or SFEMG | 0.565 | ||
| Normal | 20 (9.5) | 14 (9.2) | |
| Abnormal | 149 (71.0) | 102 (66.7) | |
| Not performing | 41 (19.5) | 37 (24.1) | |
| Symptom | |||
| Ptosis | 146 (69.5) | 58 (37.9) | <0.001 ∗ |
| Diplopia | 102 (48.6) | 33 (21.6) | <0.001 ∗ |
| Eye movement disorder | 104 (49.5) | 31 (20.3) | <0.001 ∗ |
| Limb involvement | 66 (31.4) | 56 (36.6) | 0.303 |
| Bulbar involvement | 73 (34.8) | 58 (37.9) | 0.538 |
| Neck involvement | 21 (10.0) | 12 (7.8) | 0.480 |
| Breathing involvement | 30 (14.3) | 37 (24.2) | 0.016 ∗ |
| Chewing involvement | 25 (11.9) | 17 (11.1) | 0.815 |
| Treatment | 0.230 | ||
| PYR | 12 (5.7) | 12 (7.8) | |
| PYR+PRE | 105 (50.0) | 63 (41.2) | |
| PYR+PRE+IMM | 93 (44.3) | 78 (51.0) |
- Abbreviations: AChR-Ab: acetylcholine receptor antibodies; ANA: antinuclear antibodies; anti-dsDNA: anti-double-stranded deoxyribonucleic acid antibodies; MGFA: Myasthenia Gravis Foundation of America; RNS: repetitive nerve stimulation; SFEMG: single-fiber electromyography; NN: not performing thymectomy in nonthymomatous MG; TN: not performing thymectomy in thymomatous MG; NT: performing thymectomy in thymomatous MG; TT: performing thymectomy in thymomatous MG; PYR: pyridostigmin; PRE: prednisone; IMM: immunosuppressant. ∗p value < 0.05: statistically significant.
| Variables | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| β | OR (95% CI) | p value | β | OR (95% CI) | p value | |
| Age of onset | -0.015 | 0.985 (0.973-0.996) | 0.010# | -0.014 | 0.986 (0.973-0.999) | 0.037 ∗ |
| History of myasthenia crisis | -0.812 | 0.444 (0.238-0.829) | 0.011# | |||
| MGFA classification | -0.600 | 0.549 (0.355-0.848) | 0.007# | |||
| Anti-dsDNA | 0.753 | 2.123 (1.104-4.085) | 0.024# | 1.030 | 2.800 (1.381-5.679) | 0.004 ∗ |
| Ptosis | 1.318 | 3.737 (2.408-5.798) | <0.001# | 1.075 | 2.930 (1.827-4.699) | <0.001 ∗ |
| Diplopia | 1.234 | 3.434 (2.145-5.499) | <0.001# | |||
| Eye movement disorder | 1.351 | 3.861 (2.394-6.228) | <0.001# | 1.035 | 2.815 (1.672-4.741) | <0.001 ∗ |
| Breathing involvement | -0.649 | 0.523 (0.306-0.892) | 0.017# | |||
| Thymectomy | -0.438 | 0.645 (0.419-0.992) | 0.046# | |||
| Thymoma and thymectomy | ||||||
| NN | 1 | |||||
| TN | 0.669 | 1.952 (0.683, 5.577) | 0.212 | |||
| NT | -0.307 | 0.736 (0.393, 1.376) | 0.337 | |||
| TT | -0.430 | 0.651 (0.392, 1.079) | 0.096# | |||
- Abbreviations: anti-dsDNA: anti-double-stranded DNA antibodies; NN: not performing thymectomy in nonthymomatous MG; TN: not performing thymectomy in thymomatous MG; NT: performing thymectomy in nonthymomatous MG; TT: performing thymectomy in thymomatous MG; #p value < 0.1: statistically significant. ∗p value < 0.05: statistically significant.
3.3. Construction of the Nomogram
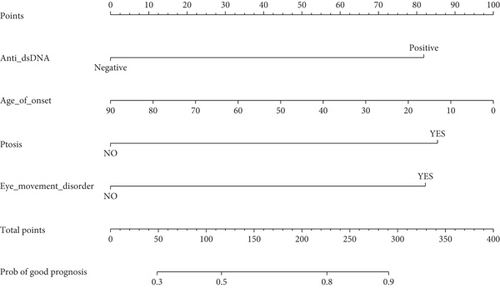
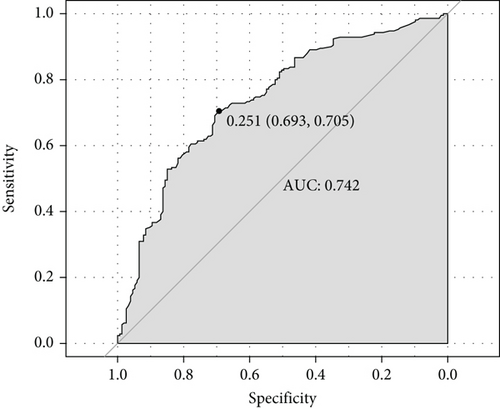
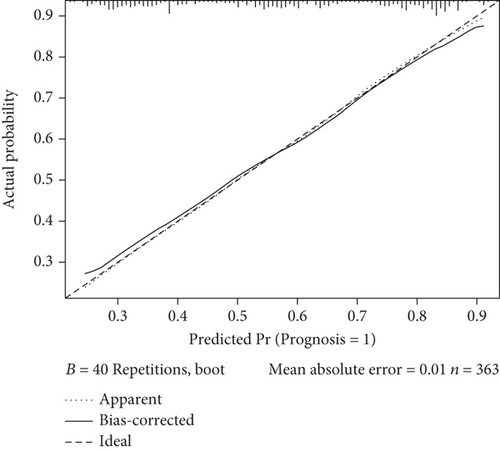
We constructed a nomogram to predict the prognosis of MG based on the results of the multivariate analysis. Prognosis was estimated by calculating the scores corresponding to protective factors. The score of each influencing factor was calculated in predicting the prognosis. For example, a 50-year-old patient with p-dsDNA-MG having only ptosis and eye movement disorder might have an age of onset score of 44, anti-dsDNA score of 82, ptosis score of 85, and eye movement disorder score of 82. In this patient, the total score would be 293. The probability of predicting a good prognosis in patients with MG was 90% based on the total score using this scoring system (Figure 2(a)). For the nomogram, the area under the ROC curve (AUC) was 0.742, which indicated that the model could discriminate between MG patients with favorable or poor prognosis (Figure 2(b)). The calibration curve demonstrated good curve fitting between the predicted outcome and the observed prognosis, whereby the line with a slope of 1 exhibited the best predictive value (Figure 2(c)). Internal validation using bootstrapping showed that the model had high discriminatory ability (C − index = 0.742, 95% CI: 0.687–0.785).
4. Discussion
We analyzed the association of ANA and anti-dsDNA with the clinical characteristics and prognosis of MG and then identified several factors associated with treatment response (mainly MG-ADL reduction), age of onset, ptosis, eye movement disorder, and anti-dsDNA. We constructed a predictive model for MG prognosis and estimated its discriminatory ability by using an ROC curve and evaluated the accuracy of the model using a calibration curve. The results showed that the model, which included anti-dsDNA as a significant factor, had an excellent predictive value.
Antinuclear antibodies and anti-dsDNA are important in SLE diagnosis, and anti-dsDNA is the specific autoantibody associated with SLE. A previous study showed that 16 patients with MG with concurrent SLE were all positive for ANA, and the anti-dsDNA positive rate was 93.8% [23]. Our study also found that 98 (27.0%) patients with MG were positive for ANA and 51 patients (14.0%) were positive for anti-dsDNA. Furthermore, most patients with MG positive for anti-dsDNA were also positive for ANA (94.1%). Negative ANA results in the p-dsDNA-MG group may have been associated with low anti-dsDNA titers, which may have contributed to the inability to detect ANA [24]. However, the levels of anti-dsDNA in our study ranged from 66.59 IU/mL to 101.37 IU/mL. Moreover, a positive test result for ANAs may indicate the presence of a systemic autoimmune disease except SLE, such as Sjögren’s syndrome and rheumatoid arthritis or an organ-specific autoimmune disease such as Graves’ disease and Hashimoto’s thyroiditis [25].
Moreover, 15%-24% of patients with MG have another associated autoimmune disease, particularly with early-onset MG [26]. Thyroid disease is the most common disease associated with MG, but rarely rheumatoid arthritis, SLE, and other autoimmune diseases [27]. Autoimmune diseases seem to occur more often in female and seropositive patients with MG [28]. We found a higher antibody-positive rate in women than in men, which was consistent with previous studies [20]. It is possible that estrogen induces receptor interactions in immune cells during local inflammatory responses, increases the immune response of Th2 lymphocytes, and plays an important role in the development of autoimmune diseases [29]. Generalized MG accounted for 87.5% of patients in a study of MG with high ANA titers [30], which was consistent with the results of our study. Furthermore, patients with MG and SLE were previously shown to predominantly have generalized MG [31]. Most MG patients coexisting with primary Sjögren syndrome were classified as generalized MG, [32] while thyroid diseases showed the highest association rate with ocular early-onset MG [33].
Thymoma, a rare tumor of the thymus, was found to be associated with many ADs including MG, pure red cell aplasia, and SLE [34]. A previous study showed that MG patients with Hashimoto’s thyroiditis also had a higher rate of thymoma, while others showed that MG and autoimmune thyroid diseases (AITD) had a lower frequency of thymic abnormalities [3, 35]. Our results indicate that p-dsDNA-MG with a better prognosis was more likely to have thymoma and this seems to be unreasonable. However, the effect of thymoma on MG is controversial both in the field of neurology and thoracic surgery. While a study suggested that MG with thymoma had a worse prognosis than MG alone [36], another study argued that the prognosis of MG with thymoma was equivalent to that of MG without thymoma [37]. In our previous study, we found an interesting phenomenon, likely due to the sufficiently long follow-up time. Although the thymomatous MG group had a higher rate of unfavorable prognosis (unchanged+worse+death) than the MG group (26.0% vs. 6.3%, respectively), the crude 5-year cumulative complete stable remission and pharmacologic remission rates were also higher in the thymomatous MG group than that in the MG group (24.0% vs. 10.4%, respectively) [38].
Patients with thymomatous MG had a favorable prognosis following treatment, and early surgical intervention improved neurologic outcomes [39]. However, patients with nonresectable or recurrent thymoma tended to present with severe forms of MG and a low survival rate [40]. Postoperative complications make surgery a related factor of a poor prognosis. On the other hand, the pathogenesis of MG with AD antibodies such as anti-DNA antibodies and anti-cardiolipin antibodies may also be related to thymectomy [41]. Thymectomy, which can reduce MG symptoms, may promote the development of new onset ADs at the same time [42–44]. Different ADs may share common mechanisms, and the possible inducing genes, components, and triggering factors to ADs are similar. All these factors may lead to more complicated clinical manifestations and prognoses [45].
Multivariate logistic regression showed that except age of onset, anti-dsDNA, ptosis, and eye movement disorder were independent factors associated with favorable prognosis as defined mainly by an MG-ADL score reduction of ≥3 points. Ocular MG (OMG) is typically associated with milder symptoms and a better prognosis [46, 47]. We further investigated the association of involvement of different muscles with MG prognosis and identified several protective factors including ptosis and eye movement disorder. A study showed that ocular or bulbar weakness had resolved more frequently than “neck/limb/respiratory” (NLR) weakness [48].
Moreover, our study showed that late-onset MG was an independent risk factor associated with favorable prognosis, which is consistent with previous studies. Beghi et al. reported younger age at onset of MG was correlated to the chance of complete remission while Kapinas et al. found that symptom onset at or after the age of 50 predicts unfavorable outcome in myasthenia gravis [49, 50]. Moreover, late-onset MG was associated with a higher risk of exacerbations of MG and the necessity of emergency treatments [51]. In an Iranian study including 146 patients, MG was more severe in older patients [52]. And in a cohort of 168 Caucasian patients, conversion to GMG occurred in patients with later onset of disease. And late onset of disease significantly affected clinical worsening, mainly worsening in total QMG score (T-QMGS) [53]. In addition, adult-onset OMG patients have a higher risk of generalized development, and the second generalization group comprised more late-onset patients [54, 55].
Another unexpected finding was that anti-dsDNA was associated with MG-ADL score reduction. This finding was contrary to findings in previous studies wherein anti-dsDNA was involved in multiple organ injury in SLE, especially in the kidney, skin, and central nervous system [56]. However, the results of these previous studies may have been due to different pathogeneses. For example, MG and SLE may not have a common pathogenesis. B cells are important in the pathogenesis of SLE, while T cells play a significant role in the pathogenesis of MG [31]. The pathogenesis of SLE is characterized by the defective clearance of apoptotic and necrotic cells, resulting in the release of autoantigens. Various immune pathways are dysregulated, resulting in the release of proinflammatory cytokines, production of autoantibodies, and activation of the complement system, thereby resulting in the loss of autoimmune tolerance [57]. Anti-dsDNA can bind to DNA antigens or cross-reactive antigens in kidney cells, resulting in the activation of the complement cascade with immune cell infiltration and cytokine release. This process induces renal inflammation and fibrosis [58]. In the nervous system, anti-dsDNA mainly binds to cross-reactive antigens such as NMDAR, resulting in increased neuronal calcium influx and subsequent excitotoxicity and cell death [59]. The pathogenic antibodies for MG are AChR-Ab, MuSK-Ab, and LRP4-Ab, and the markers for MG disease severity are intracellular antibodies such as anti-titin antibodies [1].
A previous study showed that patients with MG and SLE had milder symptoms and higher remission rates than patients with either MG or SLE alone [60]. The results of our study agreed with the findings of the aforementioned previous studies. In addition, although one study showed an increased incidence of crisis in MG with SLE [31], only five cases were evaluated, and these patients developed SLE before MG. Therefore, the finding of increased crisis may have been due to serious multiorgan damage resulting from SLE. A study of patients indicated that the pathogenesis of MG and SLE differed depending on which occurred first [61]. The relationship between MG and other ADs is also still controversial to date. Similar to the findings observed in patients with SLE, MG combined with AITD or RA tended to be a milder form of the disease, [3, 26] and comorbidity with MG did not seem to adversely affect the course of SS [32]. However, some studies showed that patients with both MG and HT had a more severe MG status and that MG in association with other autoimmune disorders had a less favorable prognosis [35, 62]. Our study showed that the presence of anti-dsDNA in MG was associated with milder symptoms and better prognosis than the absence of anti-dsDNA. Therefore, detection of anti-dsDNA levels in patients with MG is important for clinical characterization and prognosis determination. Finally, we generated a nomogram based on multivariate logistic regression analysis that showed good differentiation and accuracy for prognosis prediction at the 2-year follow-up.
Our study has some limitations. First, this was a retrospective study. In addition, the study likely had a selection bias because it was a single center study. Furthermore, some follow-up indices lacked detail, and drug doses were not reported or were unclear. The sample size was not large enough to clarify the roles of all variables in disease progression and prognosis. Moreover, the study only used internal validation. Finally, the prognostic variable, MG-ADL, has a lower sensitivity for generalized weakness [63]. Multicenter studies are needed to optimize the model and establish a more suitable database with a larger sample size to validate our findings.
5. Conclusion
This study found that being female and having AChR-Ab were characteristic features of ANA- or anti-dsDNA-positive MG. In the analysis of clinical characteristics, the p-ANA-MG group tended to have generalized MG with limb involvement, and the p-dsDNA-MG group was more likely to have thymoma with bulbar and neck involvement. Nevertheless, our regression analysis results showed that patients with anti-dsDNA had a good treatment response (mainly MG-ADL score reduction ≥ 3 points), which indicated a good prognosis. Moreover, the predictive model that included age of onset, anti-dsDNA, ptosis, and eye movement disorder had an excellent prognostic value.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors’ Contributions
Shiyin Li and Jiaxin Chen contributed equally to this work.
Acknowledgments
We express our gratitude to Yin Chen, Yaru Lu, Jieni Zhang, and Qihang Zou (Department of Neurology, The First Affiliated Hospital of Sun Yat-sen University) for their help. We also express our gratitude to the Guangdong Provincial Key Laboratory of Diagnosis and Treatment of Major Neurological Diseases (2020B1212060017), Guangdong Provincial Clinical Research Center for Neurological Diseases (2020B1111170002), Southern China International Joint Research Center for Early Intervention and Functional Rehabilitation of Neurological Diseases (2015B050501003 and 2020A0505020004), Guangdong Provincial Engineering Center for Major Neurological Disease Treatment, Guangdong Provincial Translational Medicine Innovation Platform for Diagnosis and Treatment of Major Neurological Disease, and Guangzhou Clinical Research and Translational Center for Major Neurological Diseases (201604020010). This work was supported by the Guangdong Natural Science Foundation Committee (2022A1515010478) and the Guangdong Basic and Applied Basic Research Foundation (2020A1515110909).
Open Research
Data Availability
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.




