Determinants of Quality of Life in Breast Cancer: Meta-Analytic Structural Equation Modeling of Studies
Abstract
Objective. Quality of life (QoL) is a major concern in breast cancer (BC) patients. Despite efforts, no study has comprehensively addressed determinants of QoL in patients with BC. This study aimed to synthesize evidence on QoL correlations using the meta-analytic structural equation modeling (MASEM) approach. Methods. Our search in PubMed, Web of Science, Scopus, and Cochrane databases resulted in 5,238 initial relevant papers, 73 of which were eligible for final analysis with a total of 44,121 patients. We used a two-stage procedure of correlation-based MASEM to examine the relationship between QoL and body mass index (BMI), physical activity (PA), sleep, depression, fatigue, and stress. Results. Final MASEM model suggested that PA (path coefficient = 0.33, 95% CI = −0.0444; 0.6334), fatigue (path coefficient = −0.23, 95% CI = −0.6825; 0.0361), and stress (path coefficient = −0.22, 95% CI = −0.5143; 0.6875) were the most important factors related to QoL in patients with breast cancer. Final model identified variables responsible for 68% of the variation in QoL in BC. Conclusion. QoL is an important outcome in the treatment course of BC. Large-scale and meta-analysis studies could help patients to have a life with improved quality.
1. Introduction
Breast cancer (BC) has the highest incidence and mortality among females, with an estimated incidence of 24.2% of all cancer types in 2018 worldwide [1]. The estimated 5-year survival rate for women with breast cancer is 80–90%, with poor rates in advanced stages [2]. Hence, enhancing the quality of life (QoL) in these patients is of high importance.
Despite remarkable achievements in control of the disease, nausea, vomiting, pain, insomnia, anorexia, and fatigue are common treatment side effects in patients with BC that result in psychosocial problems and lower activity and worsened QoL [3, 4]. It is claimed that poor QoL is associated with shorter survival, lower treatment adherence, increased cancer mortality, longer hospital stays, and reduced self-care [5, 6]. Some research introduces QoL as a prognostic factor with impacts comparable to pharmacological treatments [7]. However, there is no consensus on the definition of QoL as a multidimensional subjective phenomenon that includes all physical and emotional aspects [8]. The World Health Organization (WHO) defines QoL as “The situation of life resulting from the mixture of the impact of a large number of factors such as those influenced on happiness including being in comfort physical environment, satisfying occupation, intellectual and social attainments, justice, freedom of actions, expression, and also the health aspects” [9].
Irrespective of disease stage and type of treatment, QoL of patients with BC is affected through changes in fatigue, physical inactivity, sleep disorder, and psychological distress immediately after diagnosis [10, 11]. Several studies have assessed determinants of QoL in BC. However, the number of included factors in each study is limited, and the results are sometimes contradictory. No comprehensive study has been conducted to consider a large set of factors in a coherent causal network. The current study aimed to evaluate the impact of the most critical factors in QoL of patients with BC by using a meta-analytic structural equation modeling approach. From the factors assessed in the literature, we selected variables with non-ignorable evidence that includes body mass index (BMI), physical activity (PA), sleep, depression (Dep), stress, and fatigue.
2. Materials and Methods
2.1. Literature Review and Data Extraction
We searched PubMed, Scopus, Cochrane, and Web of Science databases for relevant published papers with the combination of keywords and specific terms as follows: (BMI OR depressive OR Physical activity OR Sleep OR Fatigue OR Mood OR stress) AND (Quality of Life) AND (Breast Cancer Survivors OR Breast Cancer OR Neoplasm). The details of the different search strategies are provided in the online resource materials (search queries). We also reviewed the reference lists of the original articles and reviews to identify other potentially eligible papers. Studies meeting the following criteria were included in the study: (1) being conducted on BC survivors, (2) written in English, and (3) reporting correlation coefficient between variables such as BMI, depression, physical activity, sleep, stress, fatigue, and the quality of life, directly or indirectly. The quality of life, stress, depression, sleep quality, and physical activity were measured by different related questionnaires. Details of the primary data are provided in the online resource material (Table 1: effect size). The full text of potentially relevant articles was obtained. Two authors extracted data independently by using a form based on the Cochrane Collaboration’s data extraction rules, and a third author resolved any discrepancies in the evaluation of the studies. Preferred Reporting Items for Systematic Reviews and Meta-Analysis for Protocols 2015 (PRISMA-IP 2015) were used for preparation and reporting [12].
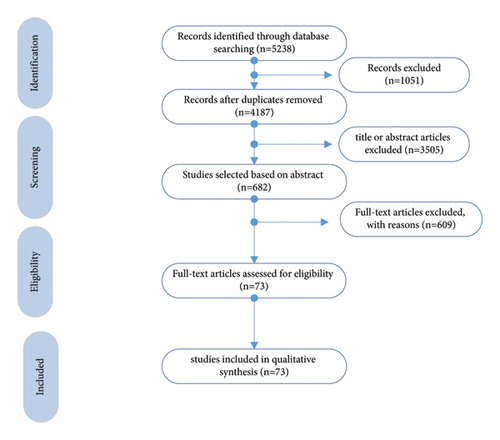
A two-stage meta-analytical structural equation model (MASEM) was applied to test the relationship between QoL and other components between breast cancer survivors. In this approach, we first pooled the multiple correlation matrices available in the studies by meta-analysis, and then the relations were analyzed using structural equation modeling. Various methods have been proposed in the literature to pool the correlation coefficients. In this paper, a two-stage approach synthesizes covariance matrices in meta-analytic structural equation modeling to test the power of correlations between components [13]. The method used in this paper was based on two stages, MASEM. In the first stage, the correlation matrices were tested for heterogeneity assumption. If they were homogeneous, they were combined to a pooled estimate. If there was no homogeneity, the random effect meta-analysis approach was used [13]. In the second stage, we run the SEM with combined effect sizes [13]. Considering moderator, we should correlate the direct and indirect effects in searched paper to combine the effects by meta-analysis. Unfortunately, there were not enough in the papers for considering moderators. When the criteria presented in different articles to evaluate the treatment under study are different (regression beta coefficients, odds ratio, chi-square statistic, F statistic, and Z statistic), they should be transferred to correlation coefficient as the same effect size. If the beta regression coefficient was reported in a study, it transferred to correlation coefficients under the condition that the beta coefficient measure was a number between ±0.5 [14]. If the effect size is reported as OR, it could be converted to the correlation coefficient according to the formula r = (OR3/4 − 1)/(OR3/4 + 1) [15]. For Z statistic computed from testing of equality of two population means, correlation is calculated as [16]. N is the total study sample size. Also, F statistic is computed from ANOVA test, and correlation is calculated as [16]. The following items were extracted for each study: first author name, year of publication, sample size, primary goal of the study, the correlation between variables, mean age, and the nationality or the race of the participants in the study. Studies with missing or unrelated information were deleted. The initial literature search produced 5238 potentially relevant studies, from which 1051 and 3505 studies were removed for being, respectively, duplicate or irrelevant. This led to 73 studies relevant for inclusion in final analysis. The process of article selection is shown in Figure 1. The number of reported correlation was 3–18. None of included articles provided all correlations between variables. Egger’s test was used to evaluate publication bias [17].
2.2. Critical Appraisal: Assessment of Study Bias
The quality of relevant articles was evaluated using the Newcastle-Ottawa Scale (NOS) for cohort studies [18]. Studies were evaluated based on exposure, comparability, selection, and outcome. The maximum possible score (least risk of bias) was nine stars. Moderate to good quality was determined by scores of five stars or more [18].
The Jadad scale was used for quality assessments of the randomized clinical trials (RCTs) [19, 20]. This scale comprises five questions related to the validity of RCTs. The total scores range from 0 to 5 points, where trials with 0–2 points are considered poor quality, where a score of 3–5 denotes a high-quality RCT [20].
The assessment and scoring system is provided in the online resource (assessing the quality). Two review researchers independently evaluated the findings of each study to confirm an unbiased evaluation.
2.3. Statistical Analysis
Atwo-stagemeta-analytical structural equation model (MASEM) was applied to test the relationship between QoL and other components between breast cancer survivors. In this approach, we first pooled the multiple correlation matrices available in the studies by meta-analysis, and then the relations were analyzed using structural equation modeling. We tested the homogeneity of the correlation matrices from individual studies using I2 and Q statistic. I2 values above 75% indicate serious heterogeneity where values lower than 25% showed minor heterogeneity. p value <0.05 indicates heterogeneity among studies and the need to use a random effect model [18]. The null hypothesis for the Q test also declares homogeneity [18]. Then, a weighted pooled correlation matrix was calculated. To build a pooled matrix, the patterns of correlations between independent and response variables need to be fairly similar in different studies. A key issue is choosing a fixed or random effect model based on the study target [21]. In fixed effect models, the size of the actual effect is shared in all studies. In contrast, in random effect models, effect sizes are assumed to differ among studies and are usually assumed to follow a normal distribution [22].
MASEM provides standardized path coefficients and tests the correlations between components based on the following goodness-of-fit criteria with desired ranges in parentheses: root mean square error of approximation (RMSEA < 0.06), comparative fit index (CFI > 0.95), standardized root mean square residual (SRMR < 0.08), and TLI index [23].
MASEM package is a package for conducting meta-analysis using structural equation modeling [18]. Analysis has been performed using R package (v.3.3.2; R Development Core Team, 2014) [24] and the metafor package [20].
3. Results
Most of the retrieved eligible studies (55 of 73) were dated to 2010 and were conducted in the United States of America. Among the searched databases, PubMed had the most relevant articles. The I2 values for the assessed correlations ranged from 14.31 to 98.96%, and the Q test had a value of less than 0.001 in most cases, both indicating high heterogeneity among studies. Hence, we adopted the random effect model in this study.
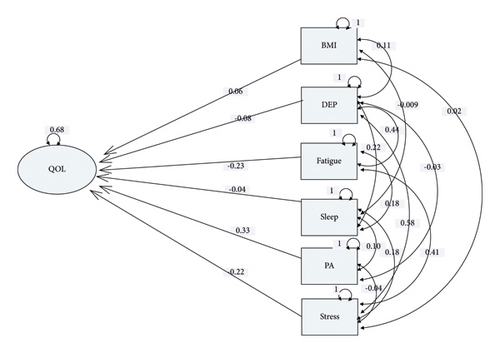
Our model assumed the following correlation between variables: sleep with BMI and PA; Dep with BMI, PA, and sleep; fatigue with sleep and Dep.; and stress with PA, Dep, BMI, fatigue, and sleep (Figure 2). The χ2 value for this model was 4.24, with a p value of 0.23, indicating a good fit. RMSEA and SRMR values were 0.003 and 0.0312, respectively, which confirm the suitability of the model. The TLI value of 0.97 and the CFI value of 0.99 indicate an acceptable fit of the final model (Table 1). In structural equation modeling, the degree of freedom is calculated from the df = 0.5 × (p) × (p + 1) − k formula. P is the number of observable variables, and k is the number of parameters that the software will calculate in the model. This model has six obvious variables, so six factors and six measurement errors are calculated for the model. Also, the six coefficient paths must be calculated. So, 6 + 6 + 6 is equal to 18. Then, the was 3 (df = 0.5(6) (6 + 1) − 18 = 3). Table 2 in online resource materials shows the summery of data from studies included in the final analysis. For each effect, the value and 95% confidence interval for the merged correlations are provided. The largest correlation was between Dep-Stress (0.62, 95% CI = 0.4748; 0.7468), Fatigue-Dep (0.47, 95% CI = 0.3453; 0.5922), Stress-Fatigue (0.45, 95% CI = 0.1463; 0.6875), PA-QoL (0.44, 95% CI = 0.2295; 0.6143), and QoL-Fatigue (−0.43, 95% CI = −0.7087; 0.0361) (Table 2). The quality of included studies was also evaluated and presented in the online resource materials (Tables 3 and 4)
| χ 2 (3) = 4.25 | RMSEA | SRMR | TLI | CFI |
| p value = 0.24 | 0.003 | 0.031 | 0.97 | 0.99 |
| Construct association | k | N | Weighted r(95% CI) | Q(p value) | I 2(95% CI) |
|---|---|---|---|---|---|
| BMI-PA | 11 | 6676 | −0.1764 (−0.2725, −0.0768) | 119.01 (<0.001) | 92.52 (82.87–97.88) |
| BMI-Sleep | 4 | 4504 | 0.0519 (−0.1342, 0.2345) | 34.10 (<0.001) | 95.29 (83.10–99.69) |
| BMI-Depression | 8 | 1538 | 0.1177 (−0.0384, 0.2681) | 47.08 (<0.001) | 88.05 (70.87–97.48) |
| BMI-Stress | 4 | 11057 | −0.025 (−0.2666, 0.2196) | 36.93 (<0.001) | 95.18 (82.80–99.69) |
| BMI-Fatigue | 7 | 2731 | 0.0464 (−0.0756, 0.1669) | 22.39 (<0.001) | 86.26 (60.18–98.02) |
| BMI-QoL | 5 | 3218 | 0.0528 (−0.1886, 0.2882) | 79.10 (<0.001) | 96.38 (89.26–99.58) |
| PA-Sleep | 4 | 6158 | 0.1384 (0.0971, 0.1791) | 7.00 (0.0718) | 40.29 (0.00–99.77) |
| PA-Depression | 12 | 12220 | −0.1278 (−0.331, 0.0867) | 1725.68 (<0.001) | 98.96 (97.86–99.64) |
| PA-Stress | 9 | 11704 | −0.0801 (−0.2932, 0.1406) | 110.58 (<0.001) | 96.73 (92.54–99.18) |
| PA-Fatigue | 17 | 6140 | −0.0206 (−0.2084, 0.1580) | 311.90 (<0.001) | 97.68 (95.74–99.05) |
| PA-QoL | 5 | 1192 | 0.442 (0.2295, 0.6143) | 78.23 (<0.001) | 92.96 (79.77–99.11) |
| Sleep-Depression | 14 | 12067 | 0.2213 (0.0768, 0.3567) | 396.47 (<0.001) | 97.45 (94.64–99.05) |
| Sleep-Stress | 5 | 457 | 0.2363 (−0.1824, 0.5824) | 48.23 (<0.001) | 94.73 (83.68–99.48) |
| Sleep-Fatigue | 12 | 1725 | 0.1889 (−0.0265, 0.3875) | 206.05 (<0.001) | 94.63 (88.76–98.21) |
| Sleep-QoL | 3 | 2639 | −0.0626 (−0.4279, 0.3203) | 49.21 (<0.001) | 96.57 86.50–99.92) |
| Depression-Stress | 8 | 1215 | 0.6297 (0.4748, 0.7468) | 67.54 (<0.001) | 93.25 (84.03–98.54) |
| Depression-Fatigue | 19 | 6595 | 0.4782 (0.3453, 0.5922) | 586.48(<0.001) | 97.34 (95.23–98.83) |
| Depression-QoL | 8 | 4015 | −0.3968 (−0.5671, −0.1939) | 428.91 (<0.001) | 97.18 (93.38–99.30) |
| Stress-Fatigue | 6 | 1901 | 0.4584 (0.1463, 0.6875) | 71.33 (<0.001) | 96.16 (89.62–99.39) |
| Stress-QoL | 6 | 792 | −0.4009 (−0.4658, −0.3318) | 5.0340 (0.4117) | 14.31 (0.00–87.02) |
| Fatigue-QoL | 7 | 3778 | −0.4304 (−0.7087, −0.0361) | 665.40 (<0.001) | 99.13 (97.87–99.82) |
- QoL, quality of life; BMI, body mass index; PA, physical activity.
According to the results of MASEM (Figure 2), the highest positive effect on QoL was for PA (path coefficient = 0.33, 95% CI = −0.0444; 0.6334), where fatigue (path coefficient = −0.23, 95% CI = −0.6825; 0.0361) and stress (path coefficient = −0.22, 95% CI = −0.5143; 0.6875) had the most detrimental effect on QoL in patients with BC. In this model, approximately 68% of QoL variance is determined with the variables included in the model. There was no publication bias according to Egger’s test (P = 0.78) (Table 3).
| BMI | PA | Sleep | Depression | Stress | Fatique | QoL | |
|---|---|---|---|---|---|---|---|
| BMI | 1 | ||||||
| PA | 0.9252 | 1 | |||||
| Sleep | 0.9529 | 0.4029 | 1 | ||||
| Depression | 0.8805 | 0.9896 | 0.9745 | 1 | |||
| Stress | 0.9518 | 0.9673 | 0.9473 | 0.9325 | 1 | ||
| Fatique | 0.8626 | 0.9769 | 0.9463 | 0.9734 | 0.9616 | 1 | |
| QoL | 0.9638 | 0.9296 | 0.9657 | 0.9718 | 0.1431 | 0.9913 | 1 |
4. Discussion
- (1)
There were a sufficient number of articles on that variable.
- (2)
The current approach (MASEM) presented by extracting the correlation between different variables from study units then pooling the multiple correlation matrices available in the studies by meta-analysis, secondly the measure of relations were analyzed using structural equation modeling. According to these descriptions, having the correlation coefficient information on that variable with QoL and each other predictor was the second criterion for considering that variable in modeling.
We had no chance of considering more predictors or presenting different moderators in our model for these two reasons.
In this paper, we used a correlation-based MASEM model. According to these issues, the mean age was insufficient to calculate the correlation effect size as the model input. On the other hand, the latent effect of age or the cancer stage can be seen in physical activity, fatigue, or other predictors.
Our findings highlight the significance of the physical activity, stress, and fatigue in this regard and the results indicate that the null hypothesis (equality of regression coefficients across predictors) was rejected and the effect of variables was not the same (x2 = 32.7564, p value < 0.001, R = 0.45). It is noteworthy that most studies have been conducted over the past ten years, which underscores the increasing concerns about the QoL in patients with breast cancer in recent years. QoL is also recognized as a significant predictor of prognosis in cancer patients [25]. However, to our knowledge, no research has assessed determinants of QoL in this population from accumulated data thus far, and all original research studies have focused on a very few factors with contradictory results in some cases. Despite the improvements in the treatment of BC and the increasing number of survivors, there is currently no study that comprehensively addresses the factors affecting the quality of life of these people, and in all studies, only one or two aspects of these factors have been mentioned. Also, the results obtained in these studies are sometimes contradictory or different. Therefore, future research should examine the quality of life in all aspects of interaction with other variables. The strengths of this study are the use of both meta-analysis and structural equation modeling and cumulating the results of other studies. According to the fitting values of the model, it is evident that these models are largely satisfactory and represent the factors affecting the quality of life.
4.1. Study Limitation
Only papers published in English were included in this study.
4.2. Clinical Implication
QoL is the crucial factor in breast cancer survivors. Enhancing physical activity and reducing fatigue and stress could improve QoL in patients with breast cancer.
5. Conclusion
Findings of the current meta-analytic study indicate that physical activity is critical in enhancing the quality of life in patients with breast cancer. Controlling fatigue and stress is of high importance and maintains a high quality of life in these patients. Further large-scale studies are essential to fortify these findings, find other vital factors, and assess their interrelations.
Abbreviations
-
- BC:
-
- Breast cancer
-
- BMI:
-
- Body mass index
-
- QoL:
-
- Quality of life
-
- WHO:
-
- World Health Organization
-
- PA:
-
- Physical activity
-
- Dep:
-
- Depression
-
- MASEM:
-
- Meta-analytic structural equation modeling
-
- N.R.:
-
- Not reported.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Marjan Mansourian was responsible for data curation, formal analysis, investigation, methodology, resources, and software. Razieh Bazrafshan was responsible for data curation, investigation, and original draft preparation. Zahra Malakoutikhah was responsible for investigation, original draft preparation, and review and editing. Tohid Jafari was responsible for formal analysis, software, and methodology. Golnaz Vaseghi was responsible for conceptualization, funding acquisition, project administration, and supervision.
Acknowledgments
The authors would like to thank Dr. Mahboobeh Akhlaghi for her kindness. Isfahan University of Medical Sciences granted permission for this research (grant ID: 396548).
Open Research
Data Availability
The data used to support the findings of this study are included within the supplementary information file.