Mitigation of Heat Stress in Striped Catfish (Pangasianodon hypophthalmus) by Dietary Allicin: Exploring the Growth Performance, Stress Biomarkers, Antioxidative, and Immune Responses
Abstract
Global warming is a challenge facing the aquaculture industry, and mitigating its effects on aquatic animals’ productivity is mandatory. Hence, the present study investigated the alleviation of heat stress impacts on the performances of striped catfish by dietary allicin. For 60 days, striped catfish were fed dietary allicin at 0, 0.5, 1, 2, and 4%, and then the fish were exposed to 35°C. The results indicated that dietary allicin remarkably enhanced the growth performance (FBW, WG, and SGR) and feed utilization (FCR and PER) in striped catfish in a dose-dependent manner. Further, striped catfish fed allicin at 1 and 2% before heat stress, and those fed on 1% after heat stress had the highest lipase, amylase, and protease activities. The intestinal villi of allicin-treated fish showed increased length and branching. Before heat stress, the AST value was not affected by dietary allicin, but after heat stress, the AST was markedly reduced in fish fed on allicin at 1, 2, and 4%. Significantly, T-CHO and triglycerides were higher after heat stress than before with or without allicin. The blood proteins, albumin, and globulin were markedly lower after heat stress than before heat stress. The glucose and cortisol levels were increased after heat stress, either with or without dietary allicin. Heat stress reduced lysozyme activity, and the best activity was seen in fish fed 2% allicin. In addition, the phagocytic activity before and after heat stress was increased by dietary 1, 2, and 4% allicin. The activities of superoxide dismutase, catalase, and glutathione peroxidase were increased by dietary allicin before and after heat stress. The regression analysis illustrated that the best performances of striped catfish could be achieved by using 1.89–2.28% dietary allicin, leading to high resistance to heat stress.
1. Introduction
The expansion of aquaculture activity to overcome the demand of an overly increasing population is facing several recent manipulations [1]. Striped catfish is one of the potential candidates for warm water aquaculture and showed a high ability for farming under diverse environmental conditions [2, 3]. However, abiotic and biotic stressors cause a severe reduction in biomass productivity and hence enormous economic loss [4, 5]. Global warming has recently negatively affected water quality and led to unstable water temperatures throughout the year [6, 7]. The increased water temperature leads to high thermal stress, activates ammonia toxicity, and results in low feed intake and health status [8, 9]. Undoubtedly, the high temperature can interrupt cellular function by damaging nucleic acids and membranes, leading to gene expression and protein structure alterations [10]. In addition, heat stress generates reactive oxygen species (ROS), which is the primary precursor for oxidative stress [11]. Consequently, fish’s physiological, blood biochemical, and immune responses impair, leading to high susceptibility to infection with disease invaders [4, 12]. In catfish, exposure to high temperatures deteriorates the growth performance and physiological, antioxidative, and immune responses [13–15]. Besides, largemouth bass (Micropterus salmoides) [4], koi (Cyprinus carpio) [16], pufferfish (Takifugu obscurus) [17], and blunt snout bream (Megalobrama amblycephala) [18] exposed to high temperatures revealed a pronounced loss in productivity, immunity, and antioxidative responses.
It is crucial to mitigate the impacts of heat stress by adjusting feeding practices. Markedly, the inclusion of feed additives in fish feeds showed potential effects on aquatic animals, such as beneficial microbes, immunostimulants, and medicinal herbs [19, 20]. Functional additives can enhance the growth performance, digestion of feeds, immunity, and tolerance to stressors in aquaculture [21]. Under heat stress conditions, the inclusion of oregano essential oil, Hericium erinaceus powder, and Yucca schidigera enhanced the resistance of Nile tilapia [22–24], probiotic Clostridium butyricum in kuruma shrimp (Marsupenaeus japonicus) [25], and mannan oligosaccharide in gilthead sea bream (Sparus aurata) [26] via the protection of the immunity and antioxidative responses. One of the most active feed additives used in aquaculture is garlic (Allium sativum) and its extracts (e.g., allicin) for their growth-promoting and immunostimulant effects [27]. Garlic contains several active compounds, including thiosulfinates, diallyl thiosulfate (allicin), vitamins, linoleic acid, flavonoids, silicates, and iodine salts that are involved in several pharmacological effects [28, 29]. Allicin has been proven to enhance the activity of digestive enzymes, feed utilization, feed palatability, and diversity of beneficial intestinal microorganisms in aquatic animals [30, 31]. Consequently, fish exhibit activated intestinal and entire-body immunity with marked antioxidative responses [32]. The inclusion of garlic has been tested in African catfish (Clarias gariepinus) [33], Eurasian Perch (Perca fluviatilis) [32], Asian sea bass (Lates calcarifer) [34], brown trout (Salmo caspius) [35], and Caspian roach (Rutilus rutilus) [36] and resulted in high growth performance, digestion capacity, immune, and antioxidative responses. Further, dietary garlic increased the resistance to pathogenic infection in sobaity sea bream (Sparidentex hasta) [37], Asian sea bass [38], and grouper (Epinephelus coioides) [39]. In terms of abiotic and biotic stressors, dietary garlic increased the resistance of common carp (Cyprinus carpio) to ammonia toxicity [40] and Nile tilapia (Oreochromis niloticus) to carbofuran toxicity [41].
Recently, striped catfish fed dietary garlic showed enhanced growth performance and feed utilization [42]. However, the effects of allicin extract on the blood biochemistry, immune, and antioxidative responses of striped catfish need more investigation. Further, the tolerance against heat stress of striped catfish when feeding with allicin should be reported. Hence, this study tested the mitigation role of dietary allicin extract on heat stress-induced blood biochemical deterioration, immunosuppression, and oxidative stress in striped catfish.
2. Materials and Methods
2.1. Ethical Approval
Experimental procedures followed the local Animal Care and Ethics Committee of the KafrelsheikhUniversity, Egypt (approval no. KFS-IACUC/105/2023).
2.2. Fish and Test Diets
Juveniles of striped catfish were obtained from a private farm in Kilo-21, Alexandria, Egypt, and stocked in the laboratories and greenhouses for the Faculties of Agriculture and Veterinary Medicine, Kafrelsheikh University, Egypt. Fish were acclimated to the laboratory conditions for two weeks and offered basal diets twice daily at 3% of their body weight. After that, similar fish sizes with an initial weight of 5.65 ± 0.22 g were distributed for fifteen glass aquaria (80 L; 70 × 30 × 40 cm) representing five groups with triplicates. Each aquarium was provided with continuous aeration and stocked with 20 fish each. Half of the water was exchanged every two days with fresh chlorine-free water.
Five test diets were formulated by mixing the ingredients presented in Table 1. All ingredients were mixed in the presence of water (35–40%) and fish oil and supplemented with powdered allicin (3% purity, Free Trade Company, Al-Beheira, Egypt) at 0, 0.5, 1, 2, and 4%. Then, diets were pelleted with a laboratory pelletizing machine to produce dough (2-3 mm). Afterward, pellets were dried at room temperature, stocked in plastic bags, and stored in the refrigerator until use. The test diets’ chemical composition (moisture, ash, lipids, and crude protein) was confirmed using the standard method [44]. Fish were offered the test diets twice daily up to the satiation level for 60 days. When the fish rejected the feed, it was assumed that the fish was satiated and the feed intake was recorded for each aquarium. Water quality indices (temperature, dissolved oxygen, and pH) were checked using the Orion Star™ A329 Portable Multiparameter Meter (Thermo Scientific™, Waltham, Massachusetts, U.S), while total ammonia levels were measured calorimetrically using the standard method [45].
| Ingredients | % | Chemical composition | |
|---|---|---|---|
| Fish meal (65% cp) | 10 | Crude protein (%) | 30.56 |
| Soybean meal (44% cp) | 38 | Crude lipids (%) | 6.77 |
| Yellow corn | 15 | Ash (%) | 3.55 |
| Corn gluten | 6 | Fibers (%) | 5.12 |
| Wheat bran | 12 | Gross energy (MJ/kg)2 | 19.17 |
| Wheat middling | 11.92 | Protein/energy (P/E) ratio (mg protein/kJ) | 15.94 |
| Fish oil | 4 | ||
| Vitamin and mineral mix1 | 2 | ||
| Dicalcium phosphate | 1 | ||
| Vitamin C | 0.08 | ||
| Total % | 100 |
- 1The mixture of vitamins and minerals is detailed by Abdel-Latif et al. [43].2Gross energy was calculated based on the values of protein, lipid, and carbohydrates as 23.6, 39.5, and 17.2 kJ/g, respectively.
2.3. Weight Gain and Blood Collection
-
Weight gain (WG, %) = 100 × (final weight (FW, g) − initial weight (IW, g))/IW (g); specific growth rate (SGR, %/day) = 100 × (ln FW (g) − ln IW (g))/days; feed conversion ratio (FCR) = feed intake (g)/WG (g); protein efficiency ratio (PER) = (FW (g) − IW (g))/dry protein intake (g); survival (%) = 100 × final fish number/initial fish number.
Three fish per aquarium were collected and then anesthetized; then, the blood was collected from the caudal vein using 2.5 mL syringes. Serum was collected after centrifugation (3000 rpm for 15 min at 4°C) and kept at −20°C until use. Then, the fish were dissected, and the intestines were collected and used to analyze digestive enzyme activity. Another three fish per aquarium were dissected, and their intestines and livers were removed and kept in formalin (10%) for the histological study.
2.4. Digestive Enzyme Activity
The homogenate was prepared by rinsing the intestines in ice-cold phosphate-buffered saline (PBS) (pH 7.5; 1 g per 10 mL). It was then homogenized and centrifuged at 8000 rpm for 5 minutes, and the supernatant was collected and stored at 4°C for further analysis. The method of Lowry et al. [46] was followed to detect the total protein content. The amylase activity was estimated by following Jiang [47] and Worthington [48]. The lipase activity was analyzed following Borlongan [49] and Jin [50].
2.5. Blood Analysis
According to Doumas et al. [51] and Dumas and Biggs [52], total proteins and albumins were determined, while globulin content was calculated mathematically. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol, and triglycerides were detected by the RA-50 chemistry analyzer (Bayer) using readymade chemicals (kits) supplied by SPINREACT Co. Spain, following the manufacturer’s instructions. Blood glucose and cortisol levels (MG/100 ml) were determined using glucose enzymatic PAP kits obtained from Bio-Merieux (France) [53].
Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were measured using diagnostic reagent kits following the manufacturer’s (Cusabio Biotech Co., Ltd.; China) instructions. The concentration of malondialdehyde (MDA) was detected by adhering to the advice of Uchiyama and Mihara [54] and expressed as nmol MDA/mL.
Serum lysozyme activity was determined using a turbidimetric assay, according to the method described by Ellis [55] based on the lysis of Micrococcus lysodeikticus (Sigma, USA). Briefly, a standard suspension of 0.15 mg/mL of M. lysodeikticus was prepared in 66 mM phosphate buffer (pH 6.0). Serum (50 μL) was added to 1 mL of the bacterial suspension, and the absorbance reduction was recorded at 30-s and 4.5-min intervals at 450 nm using a spectrophotometer (SHIMADZU UV-1600PC). One unit of lysozyme was defined as a reduction in absorbance of 0.001/min.
2.6. Histomorphology
The histological examination was adopted according to Gewaily and Abu Mandour [56]. The dissected intestine and liver samples were cut into approximately 0.5 cm³ and fixed in a neutral buffered10% formaldehyde solution for 24 h. The samples were then dehydrated in ascending grades of alcohol, cleared with xylene, and embedded in paraffin wax. Then, five μm thick sections were cut using a Leica rotatory microtome (RM 20352035; Leica Microsystems, Wetzlar, Germany) and stained with hematoxylin and eosin. Finally, the tissue sections were examined using a BX50/BXFLA microscope (Olympus, Tokyo, Japan).
2.7. Heat Stress
The heat stress challenge was performed by following Dawood et al. [15]. After the feeding trial, fish were kept in the same aquaria, and each aquarium was fortified with an electrical heater. The temperature increased by 2°C per hour to reach 35°C [57]. Then, the fish were kept under these conditions, and the survivability of the fish was recorded until it reached 50%. During the stress test, water quality indices were checked using the same procedure as before heat stress. Then, the test was terminated, and blood samples were collected, as mentioned previously.
2.8. Statistical Analysis
The Shapiro−Wilk and Levene tests confirmed normal distribution and homogeneity of variance. The obtained data were subjected to a one-way ANOVA. Differences between means were tested at the P < 0.05 level using the Duncan test as a post hoc test. A two-way ANOVA (P value) was also applied to check the effects of allicin, heat stress, and their interactions. All the statistical analyses were conducted via SPSS version 22 (SPSS Inc., IL, USA); t-test was applied to check the significance before and after heat stress. The optimum allicin level was determined using polynomial regression analysis [58].
3. Results
3.1. Water Quality
The water quality indices are presented in Table 2. No interaction effects were seen on the water temperature, dissolved oxygen (DO), pH, and total ammonia. However, heat stress significantly affected the water temperature, DO, and total ammonia. Before heat stress, the temperature was 35.03–35.29°C, DO was 5.69–5.93 mg/L, pH was 7.61–7.83, and total ammonia was 0.02 mg/L. After heat stress, the temperature became 26.14–26.72°C, DO (6.17–6.32 mg/L), pH (7.65–7.85), and total ammonia (0.03–0.05 mg/L).
| Heat stress (±) | Item | 0% | 0.5% | 1% | 2% | 4% | Two-way ANOVA (P value) | ||
|---|---|---|---|---|---|---|---|---|---|
| Allicin | Heat stress | Interaction | |||||||
| − | Dissolved oxygen (mg/L) | 6.32 ± 0.09 | 6.22 ± 0.14 | 6.18 ± 0.07 | 6.29 ± 0.18 | 6.17 ± 0.25 | |||
| + | 5.88 ± 0.21 | 5.92 ± 0.31 | 5.72 ± 0.23 | 5.69 ± 0.22 | 5.93 ± 0.32 | 0.622 | 0.001 | 0.326 | |
| − | Temperature (°C) | 26.14 ± 0.42 | 26.18 ± 0.23 | 26.83 ± 0.24 | 26.72 ± 0.72 | 26.23 ± 0.22 | |||
| + | 35.03 ± 0.06 ∗ | 35.12 ± 0.11 ∗ | 35.19 ± 0.12 ∗ | 35.14 ± 0.14 ∗ | 35.29 ± 0.41 ∗ | 0.528 | 0.001 | 0.421 | |
| − | pH | 7.76 ± 0.21 | 7.65 ± 0.27 | 7.85 ± 0.32 | 7.78 ± 0.44 | 7.67 ± 0.52 | |||
| + | 7.61 ± 0.09 | 7.71 ± 0.11 | 7.76 ± 0.29 | 7.62 ± 0.44 | 7.83 ± 0.6 | 0.328 | 0.716 | 0.286 | |
| − | Ammonia (mg/L) | 0.02 ± 0.005 | 0.02 ± 0.002 | 0.02 ± 0.003 | 0.02 ± 0.004 | 0.02 ± 0.001 | |||
| + | 0.05 ± 0.001 | 0.03 ± 0.002 | 0.04 ± 0.003 | 0.03 ± 0.004 | 0.05 ± 0.005 | 0.364 | 0.001 | 0.267 | |
- Means ± SE (n = 3) with different letters in the same row show significant differences (P < 0.05). The asterisk symbol refers to significant differences before and after heat stress.
3.2. Growth Performance
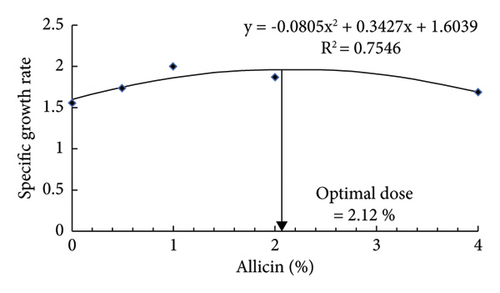
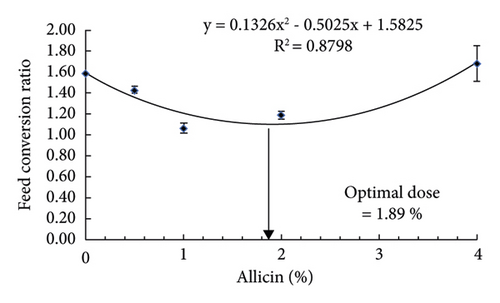
The results indicated that dietary allicin remarkably enhanced the growth performance (FBW, WG, and SGR) and feed utilization (FCR and PER) in striped catfish in a dose-dependent manner (Table 3). Markedly, striped catfish fed allicin at 1 and 2% had the highest FBW, while those fed 1% had the best WG and SGR compared to the remaining groups. The FCR was significantly reduced by 0.5, 1, and 2% of dietary allicin, and the lowest value was observed in striped catfish treated with 1%. While the PER was significantly increased by 0.5, 1, and 2% of dietary allicin, the highest value was observed in striped catfish treated with 1%. The survival rate ranged between 95% and 100% without marked differences among the groups. The regression analysis illustrated that the best SGR could be achieved using 2.12% of dietary allicin (Figure 1(a)), while the best FCR requires 1.89% of allicin (Figure 1(b)).
| Item | 0% | 0.5% | 1% | 2% | 4% |
|---|---|---|---|---|---|
| IBW (g) | 5.60 ± 0.06 | 5.58 ± 0.04 | 5.60 ± 0.06 | 5.62 ± 0.07 | 5.63 ± 0.07 |
| FBW (g) | 14.34 ± 0.52c | 15.87 ± 0.10b | 18.55 ± 0.44a | 17.32 ± 0.26a | 15.64 ± 0.34b |
| WG (%) | 156.26 ± 11.81 d | 184.21 ± 1.87c | 231.47 ± 11.08a | 208.29 ± 0.67b | 177.59 ± 4.68c |
| SGR (%/day) | 1.56 ± 0.08 d | 1.74 ± 0.01c | 2.00 ± 0.06a | 1.88 ± 0.00b | 1.70 ± 0.03c |
| FCR | 1.59 ± 0.08a | 1.43 ± 0.03b | 1.07 ± 0.05d | 1.19 ± 0.03c | 1.68 ± 0.17a |
| PER | 2.06 ± 0.10 d | 2.29 ± 0.05c | 3.08 ± 0.13a | 2.76 ± 0.08b | 1.99 ± 0.19 d |
| Survival (%) | 95 ± 2.89 | 98.33 ± 1.67 | 100 ± 0 | 100 ± 0 | 98.33 ± 1.67 |
- Means ± SE (n = 3) with different letters in the same row show significant differences (P < 0.05). IBW: initial body weight, FBW: final body weight, WG: weight gain, SGR: specific growth rate, FCR: feed conversion ratio, and PER: protein efficiency ratio.
3.3. Digestive Enzyme Activity
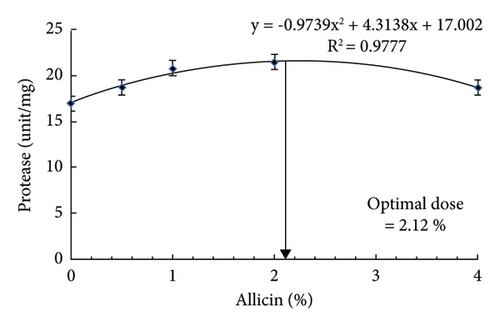
Dietary allicin, heat stress, and their interactions were significant factors in the lipase and amylase activities of striped catfish. The results indicated that dietary allicin remarkably enhanced the lipase, protease, and amylase activities in striped catfish in a dose-dependent manner (Table 4). Markedly, striped catfish fed allicin at 1 and 2% before heat stress had the highest lipase activity. At the same time, fish fed 1% had the best lipase activity after heat stress compared to the remaining groups. Similarly, striped catfish fed allicin at 1 and 2% before heat stress had the highest amylase activity. While fish fed on 1% had the best amylase activity after heat stress compared to the remaining groups. The protease activity showed higher values in striped catfish fed 1 and 2% allicin before and after heat stress. The regression analysis illustrated that the best protease activity could be achieved by using 2.12% of dietary allicin (Figure 1(c)).
| Heat stress (±) | Item | 0% | 0.5% | 1% | 2% | 4% | Two-way ANOVA (P value) | ||
|---|---|---|---|---|---|---|---|---|---|
| Allicin | Heat stress | Interaction | |||||||
| − | Lipase activity (unit/mg) | 16.97 ± 0.42c | 19.86 ± 0.18b | 22.04 ± 0.48a | 22.54 ± 0.61a | 20.69 ± 0.34b | |||
| + | 13.40 ± 0.37d ∗ | 15.86 ± 0.32b ∗ | 18.56 ± 0.63a ∗ | 16.26 ± 0.56b ∗ | 14.17 ± 0.49c ∗ | 0.001 | 0.001 | 0.004 | |
| − | Amylase activity (unit/mg) | 17.64 ± 0.42c | 18.31 ± 0.25b | 20.85 ± 0.25a | 21.41 ± 0.54a | 18.72 ± 0.23b | |||
| + | 13.40 ± 0.44b ∗ | 14.50 ± 0.31b ∗ | 17.40 ± 0.44a ∗ | 14.93 ± 0.41b ∗ | 14.06 ± 0.21b ∗ | 0.001 | 0.001 | 0.005 | |
| − | Protease activity (unit/mg) | 16.97 ± 0.44c | 18.71 ± 0.50b | 20.78 ± 0.54a | 21.49 ± 0.39a | 18.71 ± 0.46b | |||
| + | 13.21 ± 0.42c ∗ | 14.90 ± 0.27b ∗ | 16.64 ± 0.71a ∗ | 15.56 ± 0.68a ∗ | 13.35 ± 0.05b ∗ | 0.001 | 0.001 | 0.117 | |
- Means ± SE (n = 3) with different letters in the same row show significant differences (P < 0.05). The asterisk symbol refers to significant differences before and after heat stress.
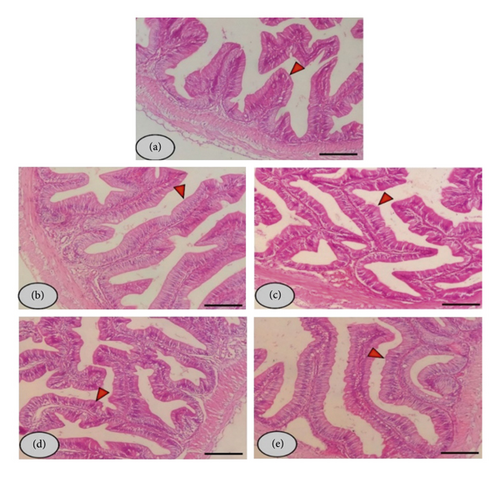
3.4. Histology Features of Intestines and Livers
The histological study investigated the striped catfish intestine in the control and allicin-treated groups and exposed normal structures of the intestinal wall and villi. The intestinal villi of allicin-treated fish showed increased length and branching in accordance with the increased level of allicin in the fish diet (Figure 2).
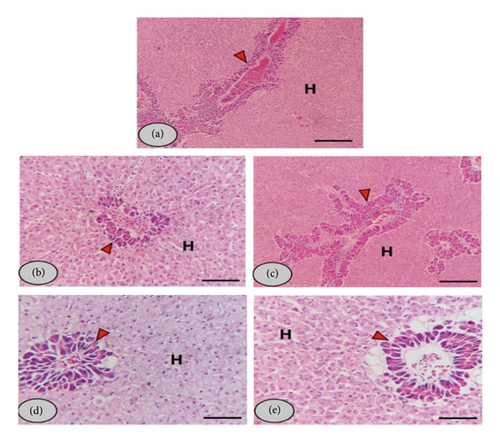
The liver revealed normal structure, including hepatic and pancreatic tissue, without any inflammatory or degenerative changes in all groups, in addition to solid glycogen deposits at low and medium doses (0.5 and 1%) of allicin (Figure 3). However, there was perifatty acinar degeneration and vacuolation at the higher doses (2 and 4%).
3.5. Blood Biochemical Indices
The blood biochemical profile of striped catfish treated with allicin is shown in Table 5. Dietary allicin, heat stress, and their interaction were significant factors in the blood biochemical profile of striped catfish except for the total protein, globulin, globulin, and glucose contents. Striped catfish fed allicin at 0.5, 1, and 2% before heat stress had lower ALT than those fed 0 and 4%. After heat stress, striped catfish fed dietary allicin had lower ALT than the control, while fish fed 2% had the lowest ALT value. Before heat stress, the AST value was not affected by dietary allicin, but after heat stress, the AST was markedly lower in striped catfish fed allicin at 1, 2, and 4% than in fish fed 0 and 0.5%. Before heat stress, striped catfish fed allicin at 1 and 2% had lower total cholesterol (T-CHO) than those fed at 0, 0.5, and 4%. However, after heat stress, fish fed allicin at 1% had lower T-CHO than those fed 0, 0.5, 2, and 4%. The triglycerides were reduced in striped catfish fed allicin, with the lowest value in the case of 1 and 2% before heat stress. After heat stress, fish fed allicin at 1, 2, and 4% had lower triglycerides than those fed at 0 and 0.5%. Significantly, ALT, AST, T-CHO, and triglycerides were higher after heat stress than before heat stress in striped catfish fed with or without allicin.
| Heat stress (±) | Item | 0% | 0.5% | 1% | 2% | 4% | Two-way ANOVA (P value) | ||
|---|---|---|---|---|---|---|---|---|---|
| Allicin | Heat stress | Interaction | |||||||
| − | ALT (U/I) | 3.24 ± 0.04a | 3.15 ± 0.03b | 3.17 ± 0.06b | 3.14 ± 0.03b | 3.24 ± 0.04a | |||
| + | 3.71 ± 0.08a ∗ | 3.62 ± 0.03b ∗ | 3.40 ± 0.01c ∗ | 3.29 ± 0.04d ∗ | 3.46 ± 0.04c ∗ | 0.001 | 0.001 | 0.002 | |
| − | AST (U/I) | 72.26 ± 0.58 | 72.83 ± 0.82 | 72.91 ± 0.33 | 74.04 ± 1.27 | 72.87 ± 0.36 | |||
| + | 81.49 ± 0.44a ∗ | 80.50 ± 0.50a ∗ | 78.36 ± 0.48b ∗ | 76.55 ± 0.35b ∗ | 77.86 ± 0.62b ∗ | 0.001 | 0.001 | 0.001 | |
| − | T-CHO (mg/dl) | 76.56 ± 0.38a | 75.51 ± 0.56a | 71.76 ± 0.58b | 69.41 ± 0.69b | 75.51 ± 0.42a | |||
| + | 79.95 ± 0.56a ∗ | 79.17 ± 0.44a ∗ | 79.07 ± 1.46a ∗ | 72.62 ± 0.54b ∗ | 77.69 ± 0.45a ∗ | 0.001 | 0.001 | 0.013 | |
| − | Triglycerides (mg/dl) | 129.69 ± 0.88a | 124.01 ± 0.67b | 122.41 ± 0.90c | 118.22 ± 1.44c | 124.04 ± 0.73b | |||
| + | 139.33 ± 1.85a ∗ | 137.34 ± 0.84a ∗ | 136.88 ± 0.41b ∗ | 127.91 ± 0.35c ∗ | 131.40 ± 0.52c ∗ | 0.001 | 0.001 | 0.009 | |
| − | Total protein (g/dl) | 4.15 ± 0.05b ∗ | 4.37 ± 0.03a ∗ | 4.43 ± 0.04a ∗ | 4.56 ± 0.06a ∗ | 4.36 ± 0.06a ∗ | |||
| + | 3.88 ± 0.02b | 3.72 ± 0.31b | 4.12 ± 0.02a | 4.28 ± 0.01a | 4.08 ± 0.02a | 0.001 | 0.005 | 0.326 | |
| − | Albumin (g/dl) | 2.32 ± 0.03b ∗ | 2.50 ± 0.07a ∗ | 2.69 ± 0.07a ∗ | 2.74 ± 0.08a ∗ | 2.57 ± 0.06a ∗ | |||
| + | 2.10 ± 0.03d | 2.25 ± 0.05c | 2.33 ± 0.05b | 2.56 ± 0.05a | 2.31 ± 0.03b | 0.001 | 0.001 | 0.573 | |
| − | Globulin (g/dl) | 1.83 ± 0.08 ∗ | 1.87 ± 0.10 ∗ | 1.75 ± 0.09 | 1.82 ± 0.04 ∗ | 1.79 ± 0.11 | |||
| + | 1.78 ± 0.04 | 1.47 ± 0.26 | 1.79 ± 0.07 | 1.72 ± 0.04 | 1.77 ± 0.05 | 0.759 | 0.141 | 0.305 | |
| − | Glucose (mg/dl) | 13.16 ± 0.44a | 12.92 ± 0.20ab | 11.88 ± 0.11c | 11.73 ± 0.78c | 12.27 ± 0.24b | |||
| + | 17.58 ± 0.57a ∗ | 15.83 ± 0.27b ∗ | 15.05 ± 0.12b ∗ | 14.01 ± 0.11b ∗ | 15.11 ± 0.10b ∗ | 0.001 | 0.001 | 0.088 | |
| − | Cortisol (ng/dl) | 25.32 ± 0.44 | 25.54 ± 0.49 | 25.21 ± 0.36 | 24.72 ± 0.21 | 25.17 ± 0.33 | |||
| + | 32.83 ± 0.22a ∗ | 31.22 ± 0.38b ∗ | 28.71 ± 0.28c ∗ | 27.33 ± 0.51c ∗ | 30.59 ± 0.65b ∗ | 0.001 | 0.001 | 0.001 | |
- Means ± SE (n = 3) with different letters in the same row show significant differences (P < 0.05). Asterisk symbols refer to significant differences before and after heat stress. ALT: alanine aminotransferase, AST: aspartate aminotransferase, and T-CHO: total cholesterol.
The blood total protein and albumin were increased in striped catfish fed allicin at 0.5, 1, 2, and 4% before heat stress, while total protein increased by 1, 2, and 4% and albumin by 2% allicin after heat stress. Dietary allicin did not affect the globulin content either before or after heat stress. Generally, the blood proteins, albumin, and globulin were markedly lower in striped catfish after heat stress than before heat stress. Before and after heat stress, the glucose content was lower in striped catfish fed allicin than in those fish in control. Striped catfish fed allicin at 1, 2, and 4% before heat stress had lower glucose than those fed 0 and 0.5%, while after heat stress, fish fed dietary allicin had lower glucose than the control regardless of the inclusion levels. Before heat stress, the cortisol value was not affected by dietary allicin. However, after heat stress, the cortisol was markedly lower in striped catfish fed allicin at 0.5, 1, 2, and 4% than in fish fed the control. The glucose and cortisol levels were higher in striped catfish before and after heat stress, either with or without dietary allicin.
3.6. Immune and Antioxidative Responses
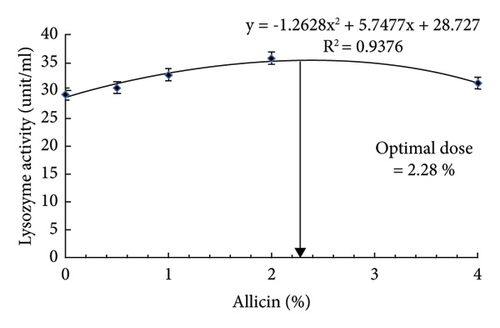
Blood immunity and antioxidative responses of striped catfish fed dietary allicin before and after heat stress are presented in Table 6. Dietary allicin and heat stress were significant factors in the blood immunity and antioxidative responses of striped catfish. However, no significant interaction effects were observed on the measured blood immunity and antioxidative responses (P > 0.05). Before heat stress, the lysozyme activity was increased by dietary 1, 2, and 4% allicin. While after heat stress, dietary allicin enhanced the lysozyme activity regardless of the dose. Lysozyme activity after heat stress was lower than before, and the best activity was seen in fish fed 2% allicin either before or after heat stress. In addition, the phagocytic activity before and after heat stress was increased by dietary 1, 2, and 4% allicin, and the highest activity was seen by dietary 2%. Before heat stress, the phagocytic index was higher in striped catfish fed allicin regardless of the dose. After heat stress, the phagocytic index increased with allicin and the highest was observed in fish fed 2%. The phagocytic activity and index were lower after heat stress than before. The regression analysis illustrated that the best lysozyme activity could be achieved by using 2.28% of dietary allicin (Figure 1(d)).
| Heat stress (±) | Item | 0% | 0.5% | 1% | 2% | 4% | Two-way ANOVA (P value) | ||
|---|---|---|---|---|---|---|---|---|---|
| Allicin | Heat stress | Interaction | |||||||
| − | Lysozyme activity (unit/mL) | 33.35 ± 0.46d ∗ | 34.58 ± 0.65d ∗ | 37.36 ± 0.49b ∗ | 40.10 ± 0.80a ∗ | 36.54 ± 0.47c ∗ | |||
| + | 29.37 ± 0.68c | 30.53 ± 0.47b | 32.82 ± 0.16b | 35.79 ± 0.55a | 31.39 ± 0.47b | 0.001 | 0.001 | 0.826 | |
| − | Phagocytic activity (%) | 31.02 ± 0.46c ∗ | 32.25 ± 0.65c ∗ | 35.03 ± 0.49b ∗ | 37.77 ± 0.80a ∗ | 34.21 ± 0.47b ∗ | |||
| + | 26.31 ± 0.38d | 28.44 ± 0.35c | 31.20 ± 0.24b | 33.74 ± 0.62a | 30.26 ± 0.39b | 0.001 | 0.001 | 0.894 | |
| − | Phagocytic index | 2.33 ± 0.06b ∗ | 2.55 ± 0.04a ∗ | 2.46 ± 0.04a ∗ | 2.60 ± 0.01a ∗ | 2.44 ± 0.07a ∗ | |||
| + | 2.16 ± 0.03c | 2.37 ± 0.02b | 2.34 ± 0.02b | 2.50 ± 0.01a | 2.32 ± 0.05b | 0.001 | 0.001 | 0.791 | |
| − | Superoxide dismutase (IU/L) | 29.99 ± 0.41c ∗ | 33.14 ± 0.86b ∗ | 35.31 ± 0.45b ∗ | 38.38 ± 0.46a ∗ | 31.18 ± 0.94c ∗ | |||
| + | 25.90 ± 0.40d | 29.19 ± 0.61c | 32.36 ± 0.55b | 34.81 ± 0.34a | 29.23 ± 0.57c | 0.001 | 0.001 | 0.386 | |
| − | Catalase (IU/L) | 31.27 ± 0.48c ∗ | 33.33 ± 0.69b ∗ | 38.62 ± 0.46a ∗ | 39.58 ± 0.42a ∗ | 33.10 ± 0.56b ∗ | |||
| + | 27.58 ± 0.38d | 30.53 ± 0.36c | 33.29 ± 0.53b | 35.65 ± 0.71a | 30.36 ± 0.46c | 0.001 | 0.001 | 0.121 | |
| − | Glutathione peroxidase (IU/L) | 29.24 ± 0.61c ∗ | 32.62 ± 0.36b ∗ | 33.70 ± 1.23b ∗ | 35.28 ± 0.53a ∗ | 31.40 ± 0.33b ∗ | |||
| + | 26.03 ± 0.16c | 28.13 ± 0.52b | 31.47 ± 0.62b | 32.57 ± 0.34a | 28.70 ± 0.25b | 0.001 | 0.001 | 0.361 | |
| − | Malondialdehyde (nmol/mL) | 27.14 ± 0.16a | 25.32 ± 0.69b | 24.61 ± 0.32b | 23.37 ± 0.68c | 25.38 ± 0.68b | |||
| + | 31.91 ± 0.23a ∗ | 30.14 ± 0.18a ∗ | 28.42 ± 0.77b ∗ | 26.90 ± 0.31c ∗ | 27.81 ± 0.35b ∗ | 0.001 | 0.001 | 0.133 | |
- Means ± SE (n = 3) with different letters in the same row show significant differences (P < 0.05). The asterisk symbol refers to significant differences before and after heat stress.
Before heat stress, allicin increased superoxide dismutase (SOD) and the highest levels were observed in fish fed 2%. After heat stress, SOD was increased by dietary 0.5, 1, 2, and 4% allicin and the highest activity was seen at dietary 2%. The catalase (CAT) and glutathione peroxidase (GPx) activities were higher in striped catfish fed dietary allicin before and after heat stress. The CAT showed the highest activity by dietary 1 and 2% before heat stress and by 2% after heat stress. The GPx was markedly increased by dietary allicin at 2% before and after heat stress. The malondialdehyde level (MDA) was reduced by dietary allicin, and fish fed 2% had the lowest MDA level before heat stress. After heat stress, fish fed 1, 2, and 4% of allicin had lower MDA than fish fed 0 and 0.5%, with the lowest being in striped catfish fed 2%. Striped catfish had lower SOD, CAT, and GPx and higher MDA levels after heat stress than before heat stress.
4. Discussion
Medicinal plants, including garlic, exhibited multiple functional roles in aquaculture, and their beneficial properties increased the tolerance to stressful farming conditions [30, 31]. Under optimal farming conditions, including suitable water temperature, dietary garlic enhanced growth performance, health status, immunity, and antioxidative status of aquatic animals. Concurrently, this study revealed enhanced growth performance of striped catfish-fed dietary allicin extract. The results are in line with Patel et al. [42], Adineh et al. [59], Ghehdarijani et al. [36], Zaefarian et al. [35], Zare et al. [32], and Karimi Pashaki et al. [60], who stated that dietary garlic enhanced the growth performance of striped catfish, rainbow trout, Caspian roach, brown trout, Eurasian perch, and common carp. The improvement in growth performance is usually related to an increased feed intake and utilization, which is also proved in the current study. Considerably, striped catfish fed dietary allicin had increased feed intake and PER while decreasing the FCR. The enhancement in feed utilization probably results from the enhancement in the digestive enzyme activity by dietary allicin [61]. With the appetizer function of garlic extracts, the improved feed intake can also be related to enhanced palatability [30, 31]. Further, improved intestinal histomorphological features in the present study would lead to high feed intake, absorption of nutrients, and high metabolic rates. In this regard, the results showed activated amylase, lipase, and protease in striped catfish fed dietary allicin before and after heat stress, which is in line with Jahanbakhshi et al. [62], who indicated that dietary garlic enhanced the digestive enzyme activity in giant freshwater prawn (Macrobrachium rosenbergii). Besides, allicin can show vital antibacterial roles involved in activating beneficial microorganisms and suppressing harmful intestinal microorganisms [63]. As a result, fish show high digestion capacity and local intestinal immunity, thereby improving digestion capacity.
The evaluation of the serum bioindicators is a precise tool for evaluating the health status, metabolic function, immunity, and tolerance to stressors [64]. Among them, the liver function-related enzymes (ALT and AST) should show optimal levels as their rises refer to liver damage [65]. Before heat stress, the results showed that striped catfish fed dietary allicin showed no marked effects on the ALT and AST levels in the serum. The results are in line with Zare et al. [32], who reported no marked effects of dietary garlic on the ALT and AST in Eurasian perch. Further, Abdelwahab et al. [34] stated that Asian sea bass treated with garlic also showed no alteration in ALT and AST. The absence of liver function alterations refers to the safe use of allicin in striped catfish feeding and the lack of stress resulting from supplementing allicin in striped catfish diets. However, after heat stress, the levels of ALT and AST are increased in all fish compared to before heat stress. Interestingly, striped catfish fed dietary allicin after heat stress had lower ALT and AST than the control. Generally, higher levels of ALT and AST after heat stress than before heat stress are probably related to the stress-induced failure of liver function [15]. Indeed, heat stress is known for its damaging effect on the general health status and for causing oxidation, disrupting several physiological functions in fish tissues, including the liver organ [12]. Nevertheless, the reduction of ALT and AST in striped catfish treated with allicin is associated with the role of allicin as a natural antioxidant supplement involved in mediating antioxidative response, physiological status, and liver function [27]. Similarly, including garlic lowered ALT and AST levels in Nile tilapia [66] and common carp [40]. The high content of flavonoids rutin, tangeretin, S-allyl cysteine, diallyl-di-sulfide, and nobiletin [28, 29] in garlic is involved in the inhibition of the antioxidation of lipids in liver cell membranes, thereby regulating the secretion of ALT and AST [37].
The results also revealed reduced total cholesterol and triglyceride levels in striped catfish fed dietary allicin before and after heating stress. Although total cholesterol and triglyceride levels were higher after heat stress than before heat stress, dietary allicin reduced these levels. Concurrently, Zare et al. [32], Talpur and Ikhwanuddin [38], Mohebbi et al. [67], and Shalaby et al. [66] illustrated that dietary garlic reduced total cholesterol and triglyceride levels in Eurasian perch, Asian sea bass, rainbow trout, and Nile tilapia, respectively. The increased total cholesterol and triglycerides after heat stress are also detected in Wuchang bream [68] and channel catfish [69]. Heat stress induces failure in the metabolic function of lipids and results in a high accumulation of lipids in the blood, thereby resulting in high cholesterol and triglyceride levels [70]. However, garlic derivatives such as allicin are proven to regulate the formation of cholesterol due to their hypolipidemia and hypocholesterolemia roles [71, 72].
Under stressful conditions, the values of the blood protein profile (total proteins, albumin, and globulin) can be affected, resulting in immune suppression [73]. Blood proteins are involved in fortifying nutrients and energy required for cellular structures, hormone production, and immune response [74]. Albumin is also a proteinous content that inhibits the blood drain from the liver tissue [75]. In this context, the study investigated the effects of dietary allicin on the blood protein profile and showed improved values in striped catfish before and after heat stress. However, the levels of the blood protein profile are lower in striped catfish after heat stress than before heat stress, either with or without allicin supplementation. The increased protein profile in this study by dietary allicin is similar to that reported by Jahanjoo et al. [37], Jha et al. [76], Chesti et al. [77], and Nya and Austin [78], who indicated elevated blood proteins in sobaity sea bream, Catla catla, amur carp, and rainbow trout, respectively. The enhancement of blood proteins may result from the abundant content of amino acids in allicin and the positive role of sulfur compounds, including S-allyl cysteine sulfoxide, involved in forming proteins in the liver tissue [28, 29].
Exposure to stress requires enough energy to alleviate stress’ impacts on fish and regulate the suitable response [79]. In this regard, cortisol and glucose levels are customarily analyzed to check the effects of using feed additives or when fish are exposed to stressful conditions such as heat stress. The results showed no alterations in the cortisol and glucose contents in the blood of striped catfish fed dietary allicin before heat stress. While heat stress increased cortisol and glucose levels, fish-fed allicin showed lowered levels. These results are in line with Hamed et al. [41], Adineh et al. [80], and Zare et al. [32], who reported reduced cortisol and glucose levels in Nile tilapia, grass carp, and Eurasian perch fed dietary garlic additives. The rise of cortisol and glucose levels was also seen in Nile tilapia [81] and channel catfish [69], which were stressed by high temperatures. During stress, cortisol affects the hepatocytes to cause glycolysis and gluconeogenesis and, thereby, glucose production [82]. Hence, high glucose levels refer to stressful conditions and the necessity for energy expenditure [83, 84]. The reduction of cortisol and glucose levels in striped catfish fed dietary allicin can be attributed to the high bioactive compound content in regulating glucocorticoids [85].
Fish immunity can be evaluated by detecting phagocytic and lysozyme activities, which protect against infection with pathogenic bacteria [86]. The results showed activated phagocytic and lysozyme elements in striped catfish fed dietary allicin before or after heat stress. Nevertheless, heat-stressed fish revealed lower phagocytic and lysozyme activities than before heat stress. Similarly, Nile tilapia [87] exposed to heat stress had impaired phagocytic and lysozyme activities. On the other hand, supplementation with dietary garlic enhanced the lysozyme activity in Nile tilapia [41], rainbow trout [88], guppy (Poecilia reticulata) [89], brown trout (Salmo caspius) [35], and Asian sea bass [38]. Further, Talpur and Ikhwanuddin [38] and Nya and Austin [78] reported that dietary garlic activated the phagocytic activity in Asian sea bass and rainbow trout. Increased phagocytic and lysozyme activities are related to the immunostimulant role of allicin [90]. Besides, allicin has a decisive antioxidative role which can limit the lipid peroxidation induced by stressors and protect the immune cells [27].
The study revealed enhanced antioxidative responses (SOD, CAT, and GPx) in striped catfish fed dietary allicin before and after heat stress. The results are in line with Mahmoud et al. [91], Yousefi et al. [40], Mosbah et al. [92], and Adineh et al. [59], who noted enhanced antioxidative capacity in Nile tilapia, common carp, European sea bass, and rainbow trout fed garlic. The study also indicated low MDA levels in the blood of striped catfish fed dietary allicin. The MDA levels are the product of lipid peroxidation induced by excessive reactive oxygen metabolites (ROS) [93]. Stressful conditions are responsible for the release of ROS involved in the oxidation of lipids in membranes and thereby damage cellular function [94]. The increased MDA levels in striped catfish after heat stress may explain the deteriorated immunity and antioxidative capacity of striped catfish. However, striped catfish fed dietary allicin showed lowered MDA levels after heat stress which confirms the antioxidative role of allicin. As a garlic component, allicin has a noticeable antioxidative effect that inhibits oxidative stress. It contains diallyl disulphide, s-allyl cysteine, and flavonoids known to activate the antioxidative response [28, 29].
5. Conclusion
The present study investigated the alleviation of heat stress impacts on the performances of striped catfish by dietary allicin. The dietary allicin supplementation markedly improved growth performance, digestive enzyme activity, feed utilization, and intestinal health. Consequently, striped catfish revealed improved biochemical blood metabolites and immune and antioxidative responses after heat stress. The regression analysis illustrated that the best performances of striped catfish could be achieved by using 1.89–2.28% of dietary allicin, leading to high resistance to heat stress.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
All authors have contributed equally to this work.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




