Changing Surgical Aortic Valve Size and Choices in the Transcatheter Aortic Valve Replacement Era
Abstract
Objective. The adoption of transcatheter aortic valve replacement (TAVR) has changed the profile of patients referred for surgical aortic valve replacement (SAVR) and drawn more attention to valve sizing and durability. We examined the influence of TAVR on SAVR practice. Methods. Using a statewide database, we evaluated all isolated SAVRs, categorized into three eras: pre-TAVR (2008 to 2011), early TAVR (2012 to 2015), and current-TAVR (2016 to 2022). The primary outcomes of interest were changes in prosthetic valve size and the percentage of mechanical valves used between time periods. Results. There were 6,445 patients included. SAVR volume declined in the current era. Valve size increased over time. In the pre-TAVR era, 41% of patients received a valve smaller than 23 mm, which declined to 33% in the early TAVR era, then to 22% in the current era (p < 0.001 for all). The year of surgery was significantly associated with larger valve selection even after controlling for patient characteristics. Annular enlargement rose in the current-TAVR era (p < 0.001). The use of mechanical valves rose in the current era (p < 0.001 compared to early TAVR). Regression analysis showed that the year of surgery was not predictive of mechanical valve use, suggesting that changes in practice were driven by patient characteristics. Conclusion. Surgical valve choice since the adoption of TAVR has changed, with less frequent use of smaller valves. Increases in mechanical valve usage are likely a reflection of changing patient population.
1. Introduction
The rapid adoption of transcatheter aortic valve replacement (TAVR) has led to a shift in the patient population treated with surgical aortic valve replacement (SAVR), as older and sicker patients are treated with the less invasive option [1]. One area of heightened interest in the TAVR era has been valve sizing. Smaller valves can lead to patient-prosthesis mismatch, which is associated with higher postprocedure gradients and ultimately leads to worse outcomes. This phenomenon is more prevalent in SAVR, but occurs with transcatheter valves as well [2, 3]. A better understanding of valve durability, with the development of definitions for valve deterioration and dysfunction [4], may also have impacted patient management during this time period.
While the changing patient population treated with SAVR has been well studied, the treatment choices operators make once surgery has been chosen are less well understood. Whether the increased focus on valve durability has led to more widespread mechanical valve use and whether a better understanding of patient-prosthesis mismatch has led surgeons to select larger valves are both unanswered questions. We hypothesized that greater awareness of the limitations of smaller valve sizes might impact surgical valve selection or push surgeons to choose mechanical valves more often. The purpose of our study was to determine whether the changes ushered in by the TAVR era have impacted surgical decision-making during SAVR.
2. Patients and Methods
The Virginia Cardiac Services Quality Initiative (VCSQI) is a statewide consortium representing 18 hospitals and capturing 99% of adult cardiac surgery procedures performed in Virginia. Standard Society of Thoracic Surgeons (STS) data is prospectively collected at each institution. In addition to the clinical data, cost data for each of the surgical procedure is captured based on revenue codes and Uniform Billing-04/92 files which are matched to the STS data. Since the consortium data are deidentified with removal of all Health Insurance Portability and Accountability Act patient identifiers, the current study is exempt from the Institutional Review Board review.
2.1. Patient Selection and Endpoints
All isolated SAVR surgeries from January 2008 through June 2022 were included in the study. Patient demographics, type of valve (biological vs. mechanical), size of valve, and other standard preoperative, operative, and postoperative STS data were collected. Patients were excluded if valve type or valve size was missing. There were no other exclusions. Patients were divided into three cohorts, pre-TAVR era (July 2008 to December 2011), early TAVR era (January 2012 to December 2015), and current-TAVR era (January 2016 to June 2022). The cutoffs for the eras were selected based on the publication of the initial TAVR versus SAVR trials in high-risk [5] and intermediate-risk [6] patients.
The primary outcomes of interest were the prosthetic valve size and percentage of mechanical valves used over the three time periods. In addition to treating valve size as a continuous variable, we divided valves into smaller (<23 mm) and larger (≥23 mm) sizes. Prespecified secondary outcomes of interest included analyses limited to bioprosthetic valves and multivariable modeling to determine independent associations with the primary outcomes.
2.2. Statistical Plan
Continuous data are expressed as the mean ± standard deviation (SD) and were compared with t-tests for normally distributed data or expressed as median (interquartile range) and compared using the Wilcoxon rank sum test for non-normally distributed data. Categorical variables are summarized as percentages and were compared using the χ2 test. An analysis of variance was used for comparisons of continuous data across time periods. In order to determine whether changes over time were independently associated with the time period versus changes in the patient population, we performed multivariable regression. To assess valve size over time, we performed linear regression, treating valve size as a continuous variable, and binary logistic regression, using smaller and larger thresholds as above. For mechanical valve use, we performed binary logistic regression. Nonlinear regression was performed to determine the trend of the increase in valve size over time. All statistical analyses were performed with SPSS version 28 (SPSS, Chicago, IL, USA). All significance tests were 2-sided, and p < 0.05 was considered significant.
3. Results
3.1. Patient Characteristics
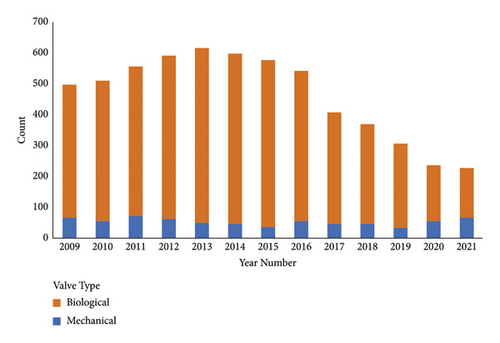
There were 6,484 patients treated with SAVR during the study period, of whom 39 were excluded for missing valve type or size, leaving 6,445 in the study cohort. There was an increase in SAVR volume from the pre-TAVR to the early TAVR eras and then a decline in cases during the current-TAVR era (Figure 1).
Patient characteristics are shown in Table 1. There were significant differences in almost every characteristic in overall comparisons, driven primarily by differences between the pre- and early TAVR eras compared to the current-TAVR era. Patient age was not different between the pre-TAVR and early TAVR eras (p = 0.5) but declined significantly in the current-TAVR era (p < 0.001). Similarly, the percentage of patients treated for aortic stenosis was similar in the pre-TAVR and early TAVR eras (p = 0.2) but decreased in the current-TAVR era (p < 0.001).
| Pre-TAVR era (n = 1,792) | Early TAVR era (n = 2,422) | Current-TAVR era (n = 2,231) | p value | |
|---|---|---|---|---|
| Age | 67.4 (±13.6) | 67.12 (±12.3) | 62.4 (±12.5) | <0.001 |
| Female gender | 41% (n = 738) | 39% (n = 945) | 33% (n = 746) | <0.001 |
| White race | 87% (n = 1,564) | 88% (n = 2121) | 86% (n = 1888) | 0.14 |
| Hypertension | 77% (n = 1385) | 78% (n = 1899) | 75% (n = 1670) | 0.01 |
| Diabetes | 29% (n = 518) | 33% (n = 802) | 27% (n = 603) | <0.001 |
| Lung disease (moderate or severe) | 11% (n = 194) | 12% (n = 285) | 10% (n = 214) | <0.001 |
| End-stage renal disease | 3% (n = 52) | 2% (n = 49) | 2% (n = 42) | 0.07 |
| Endocarditis | 5% (n = 91) | 6% (n = 143) | 12% (n = 264) | <0.001 |
| Coronary artery disease | 23% (n = 411) | 20% (n = 489) | 16% (n = 357) | <0.001 |
| Heart failure | 41% (n = 727) | 45% (n = 1097) | 46% (n = 1025) | <0.001 |
| Aortic stenosis | 89% (n = 1550) | 88% (n = 2109) | 78% (n = 1710) | <0.001 |
| Height (cm) | 170 (±11) | 171 (±10) | 172 (±)10 | <0.001 |
| Weight (kg) | 87 (±22) | 88 (±21) | 90 (±21) | <0.001 |
| STS risk of mortality (%) | 2.0 (1.0–3.6) | 1.8 (1.1–3.3) | 1.3 (0.7–2.1) | <0.001 |
- STS = society of thoracic surgeons; TAVR = transcatheter aortic valve replacement.
3.2. Valve Choice
Operative and postprocedure characteristics are shown in Table 2. Valve selection changed significantly between eras. Utilization of mechanical valves declined from the pre-TAVR to the early TAVR era (p < 0.001) and then rose again in the current-TAVR era (p < 0.001 compared to early TAVR). Annular enlargement did not change significantly from the pre-TAVR to early TAVR eras (p = 0.2), but then rose in the current-TAVR era (p < 0.001), doubling compared to the pre-TAVR time period.
| Pre-TAVR era (n = 1,792) | Early TAVR era (n = 2,422) | Current-TAVR era (n = 2,231) | p value | |
|---|---|---|---|---|
| Operative | ||||
| Mechanical valve | 13% (n = 230) | 8% (n = 200) | 16% (n = 348) | <0.001 |
| Valve size (mm) | 23 (21–25) | 23 (21–25) | 23 (23–25) | <0.001 |
| Annular enlargement | 4% (n = 75) | 5% (n = 123) | 8% (n = 168) | <0.001 |
| Cross-clamp time (min) | 74 (59–92) | 74 (60–94) | 75 (60–95) | 0.4 |
| Bypass time (min) | 102 (85–125) | 104 (85–129) | 104 (84–130) | 0.7 |
| Postoperative | ||||
| Ventilator time (hours) | 7.2 (4.8–14.2) | 5.6 (4.0–10.4) | 4.6 (3.4–6.9) | <0.001 |
| ICU time (hours) | 44 (24–72) | 47 (26–76) | 48 (26–77) | 0.8 |
| In-hospital mortality | 2.5% (n = 45) | 1.2% (n = 30) | 0.9% (n = 20) | <0.001 |
| Total cost ($) | 33,391 (27,766–43,257) | 34,377 (27,352–44,766) | 37,538 (30,537–50,366) | <0.001 |
- TAVR = transcatheter aortic valve replacement.
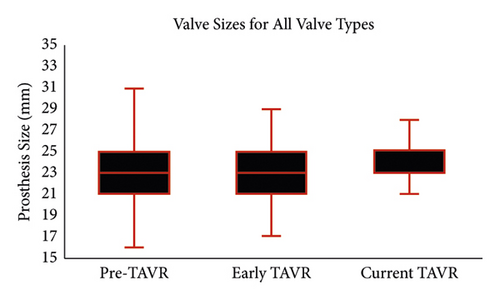
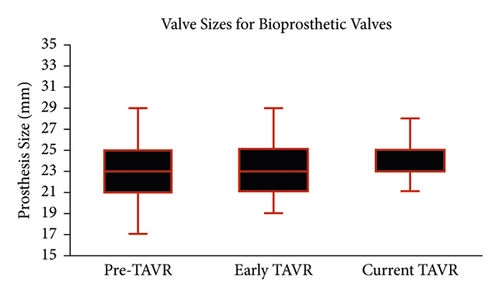
Valve size rose significantly during the study period (Figure 2). Although the median valve size remained 23 mm, valve size rose continuously throughout the study period (p < 0.001 for all between-group comparisons), driven by a reduction in the use of smaller valves. In the pre-TAVR era, 41% of patients received a valve smaller than 23 mm, which declined to 33% in the early TAVR era (p < 0.001) and then declined ever further in the current-TAVR era to 22% (p < 0.001 compared to both). When limited to biological valves, the increase in valve size during the three time periods was similarly pronounced. Median biological valve sizes in the pre-TAVR era and early TAVR era were both 23 mm (21–25 mm), which increased to 25 mm (23–25 mm) in the current era (p < 0.001 for all between-group comparisons). The use of smaller valves (<23 mm) among bioprosthetic SAVRs declined from 40% in the pre-TAVR era to 33% in the early TAVR era to 18% in the current era (p < 0.001 for all comparison).
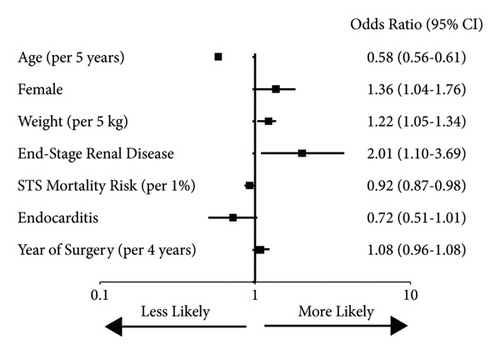
Because the patient population treated with SAVR changed significantly during the time periods studied, we performed multivariable regression to determine whether the time period itself was independently associated with valve size. In a linear regression model including all the patient characteristics, as well as the valve type, the year of surgery was significantly associated with larger valve selection (p < 0.001). Binary logistic regression showed several clinical variables independently associated with larger valve selection (Figure 3). The year of surgery had a strong correlation with larger valve selection, with about a 60% increase in larger valve use per 4-year increment. A similar analysis of variables associated with mechanical valve selection showed several with independent associations, but the year of surgery did not (Figure 4). This suggests that the increased use of mechanical valves over time was driven primarily by patient characteristics.
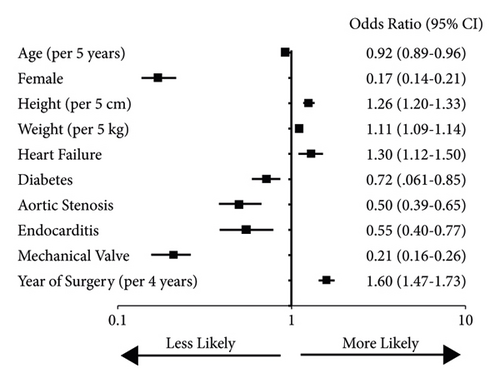
To better understand the timing of increasing use of larger valves, we fitted linear and nonlinear (quadratic, S-shaped) models to valve size by year. The best fit was a linear model (p < 0.001), suggesting that there were no abrupt increases in valve size but rather a continuous increase over time.
4. Discussion
We evaluated a statewide cardiac surgery database to determine whether the increased utilization of TAVR had impacted valve selection in patients treated with SAVR. Our study has two primary findings: (1) Surgical aortic valve size has increased over the past decade, a finding that persists even after controlling for patient characteristics. (2) Mechanical valve use has increased in the current era, but this finding appears to be explained by patient characteristics. Taken together, our findings suggest that TAVR and a better understanding of how valve characteristics impact patient outcomes have impacted SAVR decision-making, with the avoidance of smaller biological surgical valves.
We noted several patient characteristics associated with surgical valve size. Taller and heavier patients were more likely to get larger valves, as were men. This is unsurprising, as these groups would be expected to have larger annuli. Aortic stenosis as an indication for surgery was also associated with smaller valve sizes. This is likely an inverse reflection of patients who are treated for aortic regurgitation, which is commonly caused by a dilated aortic root, likely corresponding to a larger annulus [7]. As the percentage of patients treated for aortic regurgitation increases (and as patients with aortic stenosis are more likely to be treated with TAVR), the valve size would be expected to increase. Still other patient characteristics with strong associations with valve size cannot easily be explained by variations in anatomy. Younger patients were treated with larger valves, which may reflect the surgeons’ assessment of the necessity of better durability and an understanding that larger valves may deteriorate less quickly. Surgeons may also be more aware of the potential need for a valve-in-valve procedure in a patient’s future, whereby TAVR is performed on a dysfunctional SAVR valve. In one large registry, TAVR-in-SAVR survival was much higher in patients whose original bioprosthesis was >20 mm, compared to those with a prosthesis ≤20 mm (41% vs. 33% at 8 years, p = 0.01), a relationship that remained significant after multivariable regression [8].
Around the same time as the initial TAVR trials were being published, the Valve Academic Research Consortium (VARC) was established to standardize definitions for TAVR trials [9]. In its most recent iteration VARC-3 proposes definitions for bioprosthetic valve dysfunction, comprised of structural and nonstructural valve deterioration, and ultimately bioprosthetic valve failure [4]. Among the nonstructural deterioration variables that contribute to valve dysfunction and worse patient outcomes is patient-prosthesis mismatch, typically calculated using the valve manufacturer’s orifice area and the patient’s body surface area. Patient-prosthesis mismatch is usually considered significant when the indexed orifice area is <0.85 cm2/m2. Because of the direct correlation between valve size and valve area, patient-prosthesis mismatch is more common when smaller valves are used.
In a recent large SAVR series, moderate patient-prosthesis mismatch occurred in 32% of patients, severe in almost 3% [10]. In that same study, patients with valves sized ≤21 mm were almost twice as likely to have an elevated postoperative gradient. Both smaller valves and patient-prosthesis mismatch were linked to valve deterioration, which portended a doubling in the risk of death during follow-up (hazard ratio 2.18, p < 0.001). In a study focusing on the interaction between paradoxical low-flow and patient-prosthesis mismatch in patients treated with SAVR, 55% had patient-prosthesis mismatch, a finding that was linked to lower survival over 10 years of follow-up [11]. Valve size is also an important consideration for long-term valve function in patients treated with TAVR, although with less clear clinical implications. Smaller valve size and postprocedure patient-prosthesis mismatch are both associated with higher gradients over time in TAVR-treated patients [2]. Similar findings were reported in a recent analysis of the Transcatheter Valve Therapies Registry. In that study, smaller annuli were linked to more patient-prosthesis mismatches and higher postprocedure gradients [12]. Interestingly, there was no association in that analysis between patient-prosthesis mismatch and one-year outcomes, prompting some to suggest that true severe patient-prosthesis mismatch is “quasi-obsolete” in the current-TAVR era [13]. While this may one day prove to be the case, limited long-term data hinder our ability to say so with certainty, and a recent meta-analysis suggests that at least a severe patient-prosthesis mismatch portends worse survival [14].
Surgical valve sizing is done intraoperatively. After resecting the aortic valve leaflets and debriding calcifications or other debris, manufacturer-provided valve sizers are inserted into the annulus, and the largest possible valve size is selected. George and colleagues compared this methodology with multidetector computed tomography, the method commonly used to size TAVR valves [15]. They found that the intraoperative valve sizing method resulted in an undersized valve over 40% of the time compared with computed tomography. Even if manual assessment correlated perfectly with noninvasive sizing, there would be large differences between surgical and transcatheter valve orifices because of the sewing ring, which is an element of surgical prostheses that may take away over 5 mm of inner diameter. TAVR, which uses a stent frame much thinner than the sewing ring, and does not have to be sutured in place, has the ability to oversize valves and stretch the annulus with reasonable risk. While surgeons may not be able to stretch the annulus as safely as can be done during TAVR, they do have the option of performing annular enlargement concomitantly with SAVR, which allows for the delivery of a larger valve. The other option for surgeons facing a small aortic annulus is a stentless valve which can be implanted as a freestyle conduit or as a root replacement. Both methods provide a larger internal orifice while providing a bioprosthesis to the patient without a need for annulus enlargement. We found a doubling in the use of annular enlargement from the pre-TAVR to the current-TAVR era. Previously thought to impart a higher operative risk, it now appears that annular enlargement can be used without increasing that risk [16].
Our second hypothesis was that an increased focus on valve durability would lead to an increase in the percentage of patients treated with a mechanical valve, as these last longer than bioprosthetic ones. We did note an increase in mechanical valves in the current-TAVR era, but with multivariable analysis, it appears that this increase can be explained by patient characteristics, rather than a change in surgical strategy. Younger age, female gender, and lower predicted procedural mortality were all independently associated with mechanical valve use, but the year of the surgery did not maintain a significant relationship. Current guidelines recommend a mechanical valve for patients under 50 years of age, a bioprosthetic valve for those 65 and older, and shared decision-making for those in between [17]. In the absence of randomized trials comparing mechanical and bioprosthetic valves, large registries offer the best insight into treatment decisions. In a Swedish registry including 1,099 propensity score-matched pairs of patients aged 50–69, those treated with mechanical valves had better survival, although this result appeared limited to those aged 50–59 [18]. In a study of almost 10,000 patients aged 45–64 undergoing aortic valve replacement in California, those treated with a mechanical valve had lower mortality in the 45–54 cohort, but no mortality difference in those over age 54 [19]. Both studies were notable for including surgeries from as far back as the 1990s, and neither included long enough follow-up to outlast the typical durability of a tissue valve, so the very long-term benefit of a mechanical valve may not have manifested. Whether improved longevity and lower anticoagulation goals in the modern era have changed the risk-benefit of the treatment options awaits further study. But just as TAVR seems to have encouraged larger valve use, it may also reduce the incentive towards mechanical valve use, as a greater awareness of valve-in-valve procedures as a future treatment option makes the limited durability of a tissue valve less of a concern [8].
4.1. Study Limitations
Our study is retrospective in nature and subject to the usual selection biases inherent to such research. Registry data is limited to the quality of the registry, although in this case the Society of Thoracic Surgeons data is generally considered excellent quality. We hypothesized that the difference in surgical practice over time is attributable to the uptake of TAVR, but it is possible that a clinical variable not studied or some other explanation exists to explain our findings. We also acknowledge that our study is based on a statewide data which may not be applicable to all surgical practices in and out of the USA. We did not have long-term patient outcomes.
5. Conclusion
We evaluated a statewide surgical database and found that in surgical aortic valve replacement, the size of the aortic valve prosthesis has increased over time, independent of changes in the clinical profile of the patient. During the same time period, mechanical valve use also increased, but this change was explained by patient characteristics.
Abbreviations
-
- SAVR:
-
- Surgical aortic valve replacement
-
- STS:
-
- Society of Thoracic Surgeons
-
- TAVR:
-
- Transcatheter aortic valve replacement
-
- VARC:
-
- Valve Academic Research Consortium
-
- VCSQI:
-
- Virginia Cardiac Services Quality Initiative.
Conflicts of Interest
The authors declare that they no conflicts of interest.
Open Research
Data Availability
The data supporting the conclusions of the study are available upon request to the corresponding author.