Prediction of Postoperative Acute Kidney Injury Risk Factors for Acute Type A Aortic Dissection Patients after Modified Triple-Branched Stent Graft Implantation by a Perioperative Nomogram: A Retrospective Study
Abstract
Objective. Predicting risk factors for acute kidney injury (AKI) after total arch replacement via modified triple-branched stent graft (MTBSG) implantation in patients with acute type A aortic dissection (AAAD) by conducting a nomogram. Methods. We collected the clinical data of 254 patients with AAAD who underwent MTBSG implantation surgery in our center. The independent risk factors of postoperative AKI were screened by univariate and multivariate logistic regression analysis and combined into a nomogram. We use receiver operating characteristic (ROC) curves, decision curve analysis (DCA), clinical impact curve (CIC), and calibration plots to evaluate the accuracy of the nomogram model. Results. Multiple logistic regression analysis showed that the risk factors of AKI after MTBSG implantation were age, malperfusion syndrome, preoperative serum creatinine, cardiopulmonary bypass time, and amount of red blood cell (RBC) transfusion. Based on these five risk factors, we established a nomogram model. The good accuracy and clinical applicability of the model were verified by drawing ROC curve (area under the curve (AUC) = 0.854), DCA curve, CIC curve, and calibration curve. Conclusions. Using perioperative clinical data to establish a nomogram model of AKI in patients with AAAD who received MTBSG implantation can be used as a tool to predict the occurrence of AKI after operation.
1. Introduction
Acute kidney injury (AKI) is a common complication after cardiac surgery [1, 2]. Previous studies have reported that the risk of AKI after aortic arch surgery is significantly higher than that of other types of cardiac surgery [3]. Acute type A aortic dissection (AAAD) is a cardiovascular emergency with acute onset and difficult rescue. Once aortic rupture occurs, the mortality rate is extremely high [4]. At present, the only effective treatment for AAAD is surgery, but because of the complexity and difficulty of surgical operation, there are many complications after operation and the prognosis is poor [5]. It has been reported that the probability of AKI after AAAD surgery is as high as 42% [3], and severe AKI may increase hospital mortality by 3–8 times [6]. Ascending aortic replacement is widely regarded as the first choice for the treatment of AAAD [7]. For the treatment of aortic arch disease, Sun et al. invented a surgical method according to the characteristics of aortic dissection and named it Sun’s procedure, in which the most critical procedure is four-branched artificial vascular total arch replacement and descending aortic frozen trunk stent implantation [8]. At present, there have been many studies on the independent risk factors of AKI after this kind of operation [9]. In order to simplify the steps of aortic arch reconstruction, our center has improved the surgical procedure of aortic arch treatment and invented a modified triple-branched stent graft (MTBSG) to replace four-branch artificial blood vessels. This new stent is implanted during the operation to repair the aortic arch, and it has been proved to be a safe and effective procedure after a long period of clinical practice [10–12]. Unfortunately, there is no research on the risk factors of AKI after MTBSG implantation in the treatment of AAAD, let alone quantitative risk assessment of postoperative AKI, which makes the AKI unpredictable and the intervention lagging behind. Therefore, finding the predictors of AKI after MTBSG implantation is helpful for us to carry out early intervention so as to improve the prognosis of the operation. The purpose of this study is to explore the risk factors of AKI after MTBSG implantation and to establish a competitive risk nomogram model based on the results of the above analysis to predict the degree of risk in order to guide clinical treatment.
2. Materials and Methods
2.1. Patients
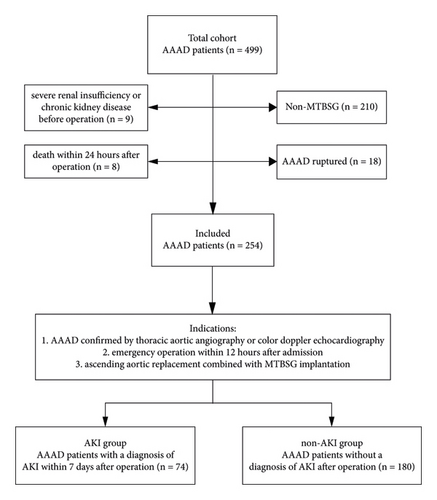
The perioperative clinical data of AAAD patients who underwent emergency surgery in our center from January 2018 to December 2020 were collected retrospectively. In this study, the inclusion criteria were (1) acute type A aortic dissection confirmed by thoracic aortic angiography or color Doppler echocardiography; (2) emergency operation within 12 hours after admission; and (3) ascending aortic replacement combined with modified triple-branched stent graft implantation. The exclusion criteria were (1) severe renal insufficiency or chronic kidney disease before operation and (2) death within 24 hours after operation. This study strictly abides by the Helsinki Declaration (Figure 1).
2.2. Definition and Grouping of AKI
According to KDIGO’s definition of AKI in 2012, AKI was defined as an increase in serum creatinine ≥26.5 μmol/L or 0.3 mg/dl within 48 h after operation, or an increase in creatinine ≥1.5 times of the baseline, or urine output <0.5 ml/kg/h for 6 hours [13, 14]. In this study, the preoperative serum creatinine level was used as the baseline, and postoperative AKI was defined as AKI within 7 days after operation of the aorta. According to the occurrence of postoperative AKI, the patients were divided into AKI group and non-AKI group.
2.3. Operative Procedure
All patients were operated under general anesthesia. This procedure has been described in detail in our previous studies [15, 16]. In short, first of all, according to the pathological changes of the aortic root, choose aortic sinus plasty, Bentall procedure, or Wheat procedure. After the operation of the aortic root was completed, the circulation was stopped when the nasopharyngeal temperature dropped to 25°C, and unilateral cerebral perfusion was established by intubation through the right axillary artery. After the aortic incision is extended upward and the true lumen of the aortic arch and branch artery is determined, the main part of the modified triple-branched stent graft is inserted into the true cavity of the arch and the descending aorta, and then the lateral wall graft is inserted into the aortic branch to open and release the stent. Finally, the end of the stent was anastomosed with the polyester tube graft of the ascending aorta, and the operation was completed.
2.4. Data Collection
Patients’ clinical data were collected from the hospital’s electronic medical record system and entered by two cardiac surgeons. Demographic and baseline risks included gender, age, height, weight, body mass index (BMI), and left ventricular ejection fraction (LVEF). Previous medical history included smoking history, drinking history, hypertension, diabetes, chronic obstructive pulmonary disease, Marfan syndrome, and previous aortic operation. Preoperative complications included shock, moderate or severe pericardial effusion, stroke, preoperative hepatic dysfunction, and malperfusion syndrome. Preoperative indexes included WBC, RBC, HB, PLT, D-dimer, PT, ALB, ALT, AST, γ-GT, CR, BUN, LDH, CRP, and cTnI. Intraoperative indicators included total operative time, cardiopulmonary bypass time, aortic cross-clamp time, cerebral perfusion time, deep hypothermic circulatory arrest time, amount of red blood cell transfusion, and aortic root procedure. Postoperative conditions included thoracic drainage 48 hours after surgery, ICU stay time, mechanical ventilation time, total hospital stay time, and 30-day mortality.
2.5. Statistical Analysis
We used IBM SPSS Statistics (version 22.0 Inc., Chicago, IL, USA) and R programming language (version 3.4.1, Vienna, Austria) for all statistical analyses. Continuous variables with a normal distribution are expressed as mean ± standard deviation (SD) and compared using Student’s t-test; otherwise, they are expressed as median (Q25, Q75) and compared with the Mann–Whitney U test. Categorical data were shown as number (%) and analyzed using the chi-square test or Fisher’s exact test as appropriate. P value <0.05 was considered statistically significant. A multivariate logistic regression model incorporating all factors showing a univariate P value <0.05 was performed to identify risk factors associated with AKI. According to the results of multivariate analysis, a nomogram was established to predict the risk of AKI in patients after MTBSG implantation. We used DCA curve and CIC curve to evaluate its clinical practicability, and the decision curve analysis shows the threshold probability and the net benefit of standardization. The area under ROC curve was used to the model discrimination evaluation.
3. Results
3.1. Clinical Characteristics of the Patients in Two Groups
A total of 254 patients with AAAD who underwent MTBSG implantation were included in this study. According to the diagnostic criteria of AKI, 74 patients developed AKI after operation, accounting for 29.13% of the total. Comparing the demographic and baseline risks data of the two groups before operation, there was a significant difference in age between the two groups (AKI group: 56.95 ± 8.41 years vs. non-AKI group: 52.36 ± 10.80 years, P = 0.000), so age was included in the univariate analysis. There was no significant difference in sex, height, weight, BMI, and preoperative LVEF between the two groups (Table 1).
| Item | AKI group | Non-AKI group | P value |
|---|---|---|---|
| Demographic and baseline risks | |||
| Male gender (n, %) | 50 (67.6%) | 126 (70.0%) | 0.703 |
| Age (years) | 56.95 ± 8.41 | 52.36 ± 10.80 | 0.000 |
| Height (cm) | 169.07 ± 7.25 | 167.37 ± 7.86 | 0.110 |
| Weight (kg) | 72.61 ± 14.56 | 69.78 ± 13.32 | 0.135 |
| Body mass index (kg/m2) | 25.22 ± 3.76 | 24.82 ± 3.90 | 0.456 |
| Preoperative LVEF (%) | 63.55 (60.10, 66.80) | 64.55 (60.43, 67.06) | 0.474 |
| Medical history (%) | |||
| Smoking history | 83 (46.1%) | 38 (51.4%) | 0.447 |
| Drinking history | 22 (29.7%) | 68 (37.8%) | 0.223 |
| Hypertension | 158 (87.8%) | 67 (90.5%) | 0.529 |
| Diabetes | 7 (9.5%) | 15 (8.3%) | 0.772 |
| Chronic obstructive pulmonary disease | 5 (6.8%) | 5 (2.8%) | 0.260 |
| Marfan syndrome | 4 (5.4%) | 9 (5.0%) | 1.000 |
| Previous aortic operation | 7 (9.5%) | 11 (6.1%) | 0.345 |
| Preoperative complications (%) | |||
| Shock | 13 (17.6%) | 13 (7.2%) | 0.013 |
| Moderate/severe pericardial effusion | 18 (24.3%) | 34 (18.9%) | 0.329 |
| Stroke | 3 (4.1%) | 2 (1.1%) | 0.150 |
| Preoperative hepatic dysfunction | 1 (1.4%) | 0 | 0.291 |
| Malperfusion syndrome | 16 (21.7%) | 3 (1.7%) | 0.000 |
| Preoperative indexes | |||
| WBC (×109/L) | 11.63 (10.19, 14.1) | 11.25 (8.97, 13.53) | 0.114 |
| RBC (×1012/L) | 4.26 (3.79, 4.71) | 4.36 (3.98, 4.80) | 0.228 |
| HB (g/L) | 127.14 ± 27.31 | 126.61 ± 28.81 | 0.892 |
| PLT (×109/L) | 105.00 (9.95, 192.50) | 121.00 (10.30, 203.50) | 0.586 |
| D-dimer (ug/mL) | 16.80 (5.76, 20.00) | 9.25 (2.75, 20.00) | 0.010 |
| PT (s) | 15.89 ± 5.04 | 15.03 ± 10.77 | 0.912 |
| ALB (g/L) | 37.34 ± 7.33 | 38.13 ± 5.92 | 0.371 |
| ALT (IU/L) | 57.22 ± 110.11 | 46.77 ± 119.14 | 0.497 |
| AST (IU/L) | 58.47 ± 100.12 | 51.99 ± 163.18 | 0.751 |
| γ-GT (IU/L) | 46.15 ± 41.46 | 42.39 ± 47.76 | 0.555 |
| CR (μmol/L) | 111.97 ± 32.18 | 99.92 ± 29.98 | 0.005 |
| BUN (mmol/L) | 8.20 ± 5.99 | 6.80 ± 3.88 | 0.067 |
| LDH (IU/L) | 433.11 ± 656.92 | 294.40 ± 552.45 | 0.087 |
| CRP (mg/L) | 25.10 (5.31, 49.50) | 18.40 (8.77, 76.30) | 0.596 |
| cTnI (ug/L) | 0.03 (0.01, 0.49) | 0.02 (0.004, 0.13) | 0.192 |
- Continuous variables were presented as mean ± standard deviation (SD). Categorical variables were shown as number (%). Student’s t-test or Mann–Whitney U test was used for continuous variables, and chi-square test was used for categorical variables. LVEF, left ventricular ejection fraction; WBC, white blood cell; RBC, red blood cell; HB, hemoglobin; PLT, platelet; PT, prothrombin time; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transpeptidase; CR, creatinine; BUN, blood urea nitrogen; LDH, lactate dehydrogenase; CRP, C-reactive protein; cTnI, troponin I. Bold values indicate p values less than 0.05.
The comparison of preoperative medical history between the two groups showed that there was no significant difference between the two groups. Among the preoperative complications, the incidence of preoperative shock (17.6% vs. 7.2%, P = 0.013) and malperfusion syndrome (21.7% vs. 1.7%, P = 0.000) was significantly higher in the AKI group. D-dimer (16.80 (5.76, 20.00) vs. 9.25 (2.75, 20.00), P = 0.010) and serum CR (111.97 ± 32.18 vs. 99.92 ± 29.98, P = 0.005) were significantly increased in AKI group, but there was no significant difference in other laboratory indexes. Therefore, preoperative shock, malperfusion syndrome, preoperative D-dimer, and serum CR were included in the univariate study (Table 1).
The results of intraoperative indicators of the two groups showed that compared with the non-AKI group, the CPB time (174.89 ± 64.85 vs. 147.13 ± 41.17, P = 0.001) of the AKI group was significantly longer and the amount of RBC transfusion (5.50 ± 2.08 vs. 3.46 ± 1.76, P = 0.000) was more, and the difference between the two groups was statistically significant. But there was no significant difference in total operation time, ACC time, CP time, and DHCA time. Therefore, we included CPB time and amount of RBC transfusion in the univariate study (Table 2).
| Item | AKI group | Non-AKI group | P value |
|---|---|---|---|
| Intraoperative indicators | |||
| Total operative time (min) | 325.27 ± 73.78 | 309.41 ± 65.48 | 0.092 |
| Cardiopulmonary bypass time (min) | 174.89 ± 64.85 | 147.13 ± 41.17 | 0.001 |
| Aortic cross-clamp time (min) | 54.24 ± 28.96 | 49.27 ± 22.15 | 0.140 |
| Cerebral perfusion time (min) | 9.00 (8.00, 11.00) | 8.00 (8.00, 10.00) | 0.161 |
| DHCA time (min) | 2.50 (1.50, 3.50) | 2.00 (2.00, 3.00) | 0.634 |
| Amount of RBC transfusion (U) | 5.50 ± 2.08 | 3.46 ± 1.76 | 0.000 |
| Aortic root procedure | |||
| No treatment (n, %) | 28 (37.8%) | 55 (30.6%) | 0.099 |
| Sinus plasty (n, %) | 34 (45.9%) | 78 (43.3%) | |
| Bentall procedure (n, %) | 11 (14.9%) | 47 (26.1%) | |
| David procedure (n, %) | 1 (1.4%) | 0 | |
| Postoperative conditions | |||
| Thoracic drainage (ml/48 h) | 450.00 (292.50, 650.00) | 350.00 (250.00, 546.75) | 0.081 |
| ICU stay time (day) | 12.00 (5.00, 20.50) | 4.00 (3.00, 7.00) | 0.000 |
| Mechanical ventilation time (h) | 133.00 (66.50, 287.25) | 43.50 (28.25, 74.00) | 0.000 |
| Total hospital stay time (day) | 23.86 ± 15.00 | 18.53 ± 7.71 | 0.005 |
| 30 d mortality (%) | 7 (3.9%) | 9 (12.2%) | 0.029 |
- Continuous variables were presented as mean ± standard deviation (SD) or median (Q25, Q75). Categorical variables were shown as number (%). Student’s t-test or Mann–Whitney U test was used for continuous variables, and chi-square test was used for categorical variables. DHCA, deep hypothermic circulatory arrest. Bold values indicate p values less than 0.05.
Postoperative conditions showed that postoperative mechanical ventilation time was significantly prolonged (133.00 (66.50, 287.25) vs. 43.50 (28.25, 74.00), P = 0.000), ICU stay time and total hospital stay were significantly increased in AKI group, and 30-day mortality was higher, but there was no significant difference in thoracic drainage volume between the two groups within 48 hours after operation (Table 2).
3.2. The Selection of Risk Factors and Model Performance Test of Nomograms
Univariate analysis showed that there were significant differences in age, preoperative shock, malperfusion syndrome, D-dimer, CR, CPB time, and amount of RBC transfusion (Table 3). These variables were included in multivariate logistic analysis, and the results showed that age (OR: 1.053; 95% CI: 1.015,1.092; P = 0.006), malperfusion syndrome (OR: 11.224, 95% CI: 2.474, 50.920; P = 0.002), CR (OR: 1.016, 95% CI: 1.005, 1.027; P = 0.004), CPB time (OR: 1.009, 95% CI: 1.001, 1.016; P = 0.022), and amount of RBC transfusion (OR: 1.676, 95% CI: 1.403, 2.000; P = 0.000) were independent risk factors for AKI after MTBSG implantation (Table 4).
| Index | OR | 95% CI | P value |
|---|---|---|---|
| Age (years) | 1.047 | 1.018, 1.078 | 0.002 |
| Shock (n, %) | 2.738 | 1.202, 6.233 | 0.016 |
| Malperfusion syndrome (n, %) | 16.276 | 4.579, 57.856 | 0.000 |
| D-dimer (μg/mL) | 1.048 | 1.011, 1.087 | 0.010 |
| CR (μmol/L) | 1.013 | 1.004, 1.022 | 0.006 |
| Cardiopulmonary bypass time (min) | 1.010 | 1.005, 1.016 | 0.000 |
| Amount of RBC transfusion (U) | 1.654 | 1.410, 1.940 | 0.000 |
| Item | OR | 95% CI | P value |
|---|---|---|---|
| Age (years) | 1.053 | 1.015, 1.092 | 0.006 |
| Malperfusion syndrome (n, %) | 11.224 | 2.474, 50.920 | 0.002 |
| CR (μmol/L) | 1.016 | 1.005, 1.027 | 0.004 |
| Cardiopulmonary bypass time (min) | 1.009 | 1.001, 1.016 | 0.022 |
| Amount of RBC transfusion (U) | 1.676 | 1.403, 2.000 | 0.000 |
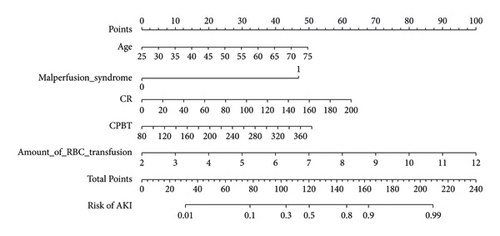
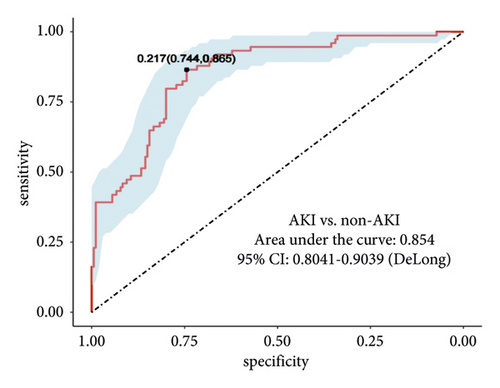
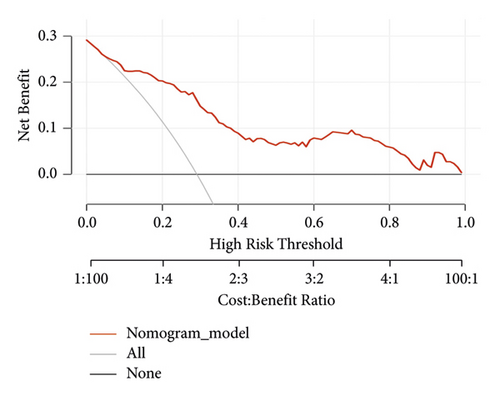
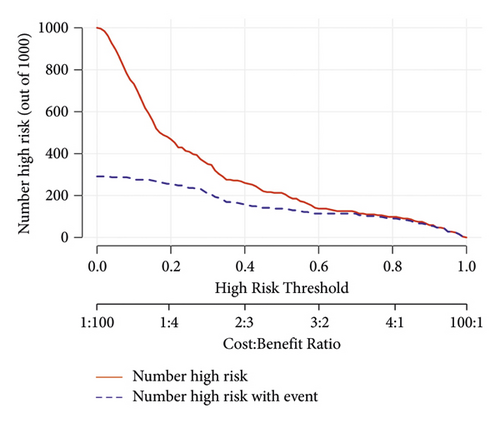
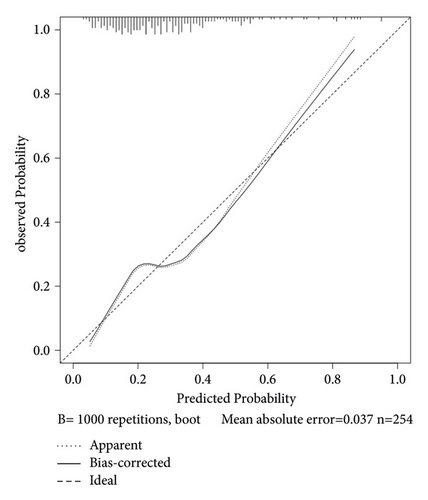
We set up a nomogram prediction model based on the factors of AKI obtained by multivariate logistic regression analysis and the contribution weight of each variable. The risk of AKI after MTBSG implantation is determined by the total score of the risk factors at the top of the nomogram (Figure 2). The ROC curve is drawn according to the risk score and clinical characteristics. The results show that the AUC is 0.854 (95% CI = 0.8041∼0.9039) (Figure 3). We also used the DCA curve and CIC curve to evaluate the net benefit of intervention based on the nomogram model on patients with AKI. The results show that the nomogram model in this study has a good benefit in predicting the occurrence of AKI after MTBSG implantation (Figures 4 and 5). We also use the calibration curve to evaluate the difference between the predicted results of the nomogram model and the actual results. The results show that the calibration curve of the model is close to the diagonal, so it is considered that the predicted results of the nomogram model in this study are in satisfactory consistency with the actual results (Figure 6). The nomogram model of AKI after MTBSG implantation was verified by bootstrap resampling which indicated that the model had good discrimination.
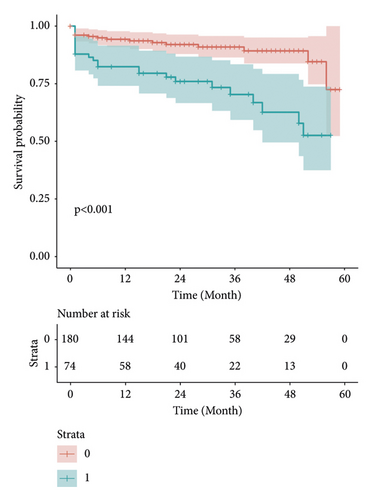
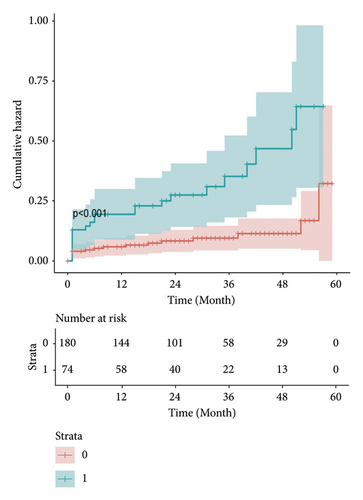
3.3. Kaplan–Meier Survival Curves and Cumulative Hazard Curves
Kaplan–Meier estimates of the impact of postoperative AKI on patients survival. Our results show that the long-term survival rate of patients in AKI group is significantly different from that in non-AKI group. With the increase of time, the survival rate and survival time of patients in AKI group decreased significantly, and the survival risk increased significantly (Figures 7 and 8).
4. Discussion
With the progress of medical treatment, the surgical treatment of AAAD is also more diversified. It has been proved to be a safe and effective surgical method to repair the aortic arch by MTBSG implantation [10–12, 15, 16]. However, there is no research on the risk factors of AKI after MTBSG implantation. AKI after AAAD is a common and fatal complication [1, 2], which significantly increases the risk of death in hospital and the prognosis is poor [5]. Therefore, we believe that early identification of risk factors for AKI after MTBSG implantation is of great significance to reduce the incidence of AKI and improve the prognosis of patients.
In this study, the incidence of AKI after MTBSG implantation was 29.13%, which was roughly the same as previous studies [3]. Through multivariate analysis, we determined the independent risk factors of AKI after MTBSG implantation and, combined with clinical practice, developed a practical and novel nomogram model to predict the risk of AKI after MTBSG implantation. The nomogram model was verified by ROC curve, DCA curve, CIC curve calibration curve, and bootstrap verification.
Age, malperfusion syndrome, serum CR, CPB time, and the amount of RBC transfusion were identified as independent risk factors for AKI after MTBSG implantation. Previous studies have shown that elderly patients have a higher risk of developing AKI after surgery, which is consistent with our findings [17]. With the increase of age, the renal blood perfusion of elderly patients decreased, and the regulatory response of renal vessels to vasodilation factor also decreased [18], so the ability of kidney to adapt to hemodynamic changes became worse and the tolerance to ischemic injury decreased [19]. At the same time, due to the deterioration of renal function, elderly patients are more likely to be exposed to renal damage drugs such as diuretics or contrast media due to certain complications or examinations. Therefore, in some elderly patients with AAAD, we should pay more attention to the protection of renal function before and during operation and try to avoid the use of drugs that may cause renal function damage.
Previous studies have found that the increase of preoperative CR level is an independent risk factor for postoperative AKI. The increase of preoperative CR level is closely related to preoperative kidney injury [20], and any preoperative kidney injury will affect the recovery of postoperative renal function and increase the risk of AKI. In our study, the baseline level of CR in patients with AKI increased significantly before operation, which made the kidney less tolerant to surgery. In addition, the hemodynamic instability, hypoxic injury, and inflammation of the kidney after operation would further promote the occurrence of AKI [21]. Most preoperative kidney injuries are caused by malperfusion syndrome [22], which is a serious complication of AAAD. Poor renal perfusion is considered to be the most important risk factor for AKI [21], which is confirmed by our study. Therefore, we should be more alert to the patient with preoperative imaging suggested the dissection involving the renal artery, and early reperfusion treatment is helpful to improve the prognosis.
Cardiopulmonary bypass support is a necessary condition for cardiovascular surgery, but its nonpulsatile advective blood flow is a nonphysiological state. Long-term cardiopulmonary bypass will aggravate the destruction of blood components and promote the release of inflammatory factors into the blood [23]. Roh et al. reported that the risk of postoperative AKI in patients with CPB time >180 min was 4 times higher than that in patients with CPB time <120 min [24]. Compared with conventional cardiac surgery, AAAD surgery needs to undergo DHCA, and a large change in temperature will further damage renal function. CPB time in our nomogram is a risk factor for postoperative AKI, which is consistent with previous literature reports.
In recent years, the risk of blood transfusion and the incidence of AKI have been the focus of research, but the relationship between them has not been clarified. Some studies have shown that the volume of blood transfusion is related to the high incidence of AKI [25–27], which is similar to our research results. The results of a nonrandomized trial by Koch et al. showed that the increase of RBC transfusion significantly increased the incidence of complications and mortality in patients undergoing simple coronary artery bypass grafting surgery, which may be related to T cell-related immune function damage [28]. However, some studies have also reported that there is no direct relationship between AKI and blood transfusion, which may be due to the heterogeneity and insufficient number of samples in the study. In cardiovascular surgery, acute bleeding is a common intraoperative emergency, so transfusion of RBC to supplement hemoglobin is a common procedure in surgery. With the loss of blood, the content of hemoglobin will be significantly reduced, which will affect the oxygen carrying capacity of blood and then affect the oxygen supply of kidney tissue, resulting in hypoxic injury. At the same time, the stored red blood cells may be deformed and hemolyzed during storage, and the infusion of RBC may increase the accumulation of free hemoglobin and iron, which have nephrotoxicity and will further aggravate kidney dysfunction [29–31]. Therefore, by reducing the amount of RBC transfusion, the incidence of AKI and related transfusion complications may decrease.
Hypothermic circulatory arrest (HCA) is a necessary step in repairing the aortic arch, which is often considered as one of the factors that can affect renal function after surgery [32]. There is still controversy regarding the direct relationship between intraoperative HCA and postoperative AKI. In a study by Vekstein et al. [33], it was found that the degree of systemic hypothermia was not associated with the incidence or severity of AKI in patients undergoing proximal aortic surgery, including arch replacement requiring cardiopulmonary bypass. However, further prospective research is needed to validate this conclusion. In the study conducted by Arnaoutakis et al. [34], the results also indicated that, compared to DHCA, moderate hypothermic circulatory arrest (MHCA) does not result in worse renal function. Therefore, most studies currently suggest that the potential risk factor affecting postoperative AKI is the duration of HCA, rather than the degree of hypothermia. In our center, the application of modified triple-branched stent grafts has greatly simplified the complex arch reconstruction procedure, which has significantly reduced the duration of DHCA during surgery for patients undergoing total arch repair for AAAD. This is beneficial in minimizing the impact of HCA on overall organ function.
Our results also found that the occurrence of AKI after operation will not only prolong the length of stay and increase the cost of hospitalization but also affect the prognosis of patients and increase the risk of hospital death. Therefore, it is very important to establish a visual risk prediction model. The nomogram established in this study can be used to individually predict the risk of AKI after MTBSG implantation and quantify the risk factors of AKI. The higher the score of each item in the nomogram model, the greater the risk of AKI. The ability to predict the risk of AKI can help identify high-risk patients and make appropriate clinical decisions, effectively grasp the timing of continuous renal replacement therapy, avoid invasive treatment operations as far as possible, and maximize the benefits of treatment.
There are several limitations to our study. Firstly, as a single-center retrospective study, there may be inherent selection bias associated with retrospective research. Secondly, due to the excellent efficacy and favorable clinical outcomes of the modified triple-branched stent graft, the majority of AAAD patients in our center have undergone the placement of a modified triple-branched stent graft implantation during total arch replacement. The sample size of cases using other arch surgical methods was too small and therefore not included in this study; this may have an impact on the applicability of the model. Finally, because of the limited sample size from a single center, lack of multicenter data hinders further external validation of the model and its clinical applicability needs further exploration. Therefore, before final application in clinical practice, large-scale multicenter prospective randomized controlled trials should be conducted, and reliable external verification of the model should be carried out to evaluate its usefulness.
Abbreviations
-
- AAAD:
-
- Acute type A aortic dissection
-
- AKI:
-
- Acute kidney injury
-
- MTBSG:
-
- Modified triple-branched stent graft
-
- ROC:
-
- Receiver operating characteristic
-
- DCA:
-
- Decision curve analysis
-
- CIC:
-
- Clinical impact curve
-
- AUC:
-
- Area under the curve
-
- BMI:
-
- Body mass index
-
- LVEF:
-
- Left ventricular ejection fraction
-
- CPB:
-
- Cardiopulmonary bypass
-
- ACC:
-
- Aortic cross-clamp
-
- CP:
-
- Cerebral perfusion
-
- DHCA:
-
- Deep hypothermic circulatory arrest
-
- WBC:
-
- White blood cell
-
- RBC:
-
- Red blood cell
-
- HB:
-
- Hemoglobin
-
- PLT:
-
- Platelet
-
- PT:
-
- Prothrombin time
-
- ALB:
-
- Albumin
-
- ALT:
-
- Alanine aminotransferase
-
- AST:
-
- Aspartate aminotransferase
-
- γ-GT:
-
- γ-Glutamyl transpeptidase
-
- CR:
-
- Creatinine
-
- BUN:
-
- Blood urea nitrogen
-
- LDH:
-
- Lactate dehydrogenase
-
- CRP:
-
- C-reactive protein
-
- cTnI:
-
- Troponin I.
Ethical Approval
This study was approved by the Ethics Committee of the affiliated Union Hospital of Fujian Medical University.
Consent
Since this is a retrospective analysis of medical records, the need for informed consent was abandoned.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
The design concept was put forward by FX, LX, JH, QW, YH, and LC. FX and LX were responsible for data collection and input. YH and LC were responsible for data processing and icon drawing. JH, QW, and XL were responsible for typesetting and language censorship. All the co-authors were involved in the preparation of the manuscript. All co-authors reviewed and approved the final version of the manuscript. LC and YH contributed equally to this work.
Acknowledgments
This work was supported by the Natural Science Foundation of Fujian Province (2020J01998), Joint Funds for the Innovation of Science and Technology, Fujian province, China (2018Y9050), and Startup Fund for Scientific Research, Fujian Medical University (2017XQ1032), and Joint Funds for the innovation of Science and Technology, Fujian province, China (2018Y9047); Natural Science Foundation of Fujian Province, China (2020J011032); Fujian Provincial Health Technology Project, China (2019-1-29).
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.