Perampanel Monotherapy for Focal and Generalized Epilepsy in Clinical Practice
Abstract
Objectives. To investigate the effectiveness, safety, and tolerability of perampanel (PER) when used as monotherapy to treat focal or generalized epilepsy in everyday clinical practice, using data from the PERMIT study. Methods. PERMIT was a pooled analysis of 44 real-world studies from 17 countries, in which people with focal and generalized epilepsy were treated with PER. This post hoc analysis included people with epilepsy (PWE) from PERMIT who were treated with PER monotherapy at baseline. Retention and effectiveness were assessed after 3, 6, and 12 months. Effectiveness assessments included ≥50% responder rate and seizure freedom rate (no seizures since at least the prior visit). Safety and tolerability were assessed by evaluating adverse events (AEs) and discontinuation due to AEs. Results. Overall, 268 PWE were treated with PER monotherapy at baseline. Retention was assessed for 168 PWE, effectiveness for 183 PWE, and safety and tolerability for 197 PWE. Retention rates were 91.1%, 87.3%, and 73.3% at 3, 6, and 12 months, respectively. At 12 months, responder rates were 84.2% overall, 82.9% in PWE with only focal-onset seizures at baseline, and 88.0% in those with only generalized-onset seizures at baseline; corresponding freedom rates were 62.9%, 57.7%, and 80.0%, respectively. AEs were reported for 45.2% of PWE. The most frequently reported AEs (≥5% of PWE) were dizziness/vertigo (16.8%), irritability (11.2%), somnolence (9.1%), and depression (6.6%). Over 12 months, 13.7% discontinued due to AEs. Conclusions. PER was effective when used as monotherapy in clinical practice, particularly in those with generalized-onset seizures, and was generally well tolerated.
1. Introduction
Perampanel (PER) is a first-in-class, potent, selective, orally active, and noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist [1, 2]. The European Medicines Agency licensed PER for adjunctive treatment of focal-onset seizures, with or without focal to bilateral tonic-clonic seizures, in people with epilepsy (PWE) aged ≥4 years, and primary generalized tonic-clonic seizures in those aged ≥7 years with idiopathic generalized epilepsy (IGE) [3]. The US Food and Drug Administration (FDA) approved PER for monotherapy and adjunctive therapy for the treatment of focal-onset seizures, with or without focal to bilateral tonic-clonic seizures, in PWE aged ≥4 years, and as adjunctive therapy for primary generalized tonic-clonic seizures in PWE aged ≥12 years [4].
Clinical trial evidence supporting the use of PER as monotherapy was initially limited to a small number of PWE who withdrew to PER monotherapy during open-label extension studies investigating PER as adjunctive therapy [5]. Of 2245 PWE enrolled in these studies, only seven withdrew to PER monotherapy, all of whom had refractory focal-onset seizures, and seizure data were only available for six PWE [5]. Approval of PER as monotherapy in the USA was therefore primarily based on an FDA strategy allowing the extrapolation of data from adjunctive therapy trials [6, 7]. More recently, a single-arm, open-label, Phase III study, conducted in Japan and South Korea, demonstrated that PER monotherapy was efficacious and generally well tolerated when administered for up to 26 weeks to PWE (aged ≥12 years) with primarily newly diagnosed focal epilepsy [8]. Real-world clinical practice data are required to complement evidence from clinical trials, by providing data on PWE who are more diverse in terms of clinical characteristics than those recruited for clinical trials and additional information on the individualized treatment strategies employed in clinical practice [9–11]. To date, few clinical practice studies have specifically investigated the use of PER as monotherapy [12–14].
The PERaMpanel pooled analysIs in effecTiveness and tolerability (PERMIT) study is the largest pooled analysis of PER clinical practice data conducted to date, including data from approximately 5200 PWE treated with PER for focal or generalized epilepsy [15]. The large size of PERMIT has allowed meaningful subgroup analyses to be conducted. The objective of this study was to investigate the effectiveness, safety, and tolerability of PER when used as monotherapy for focal or generalized epilepsy in everyday clinical practice, using data from the PERMIT study.
2. Methods
2.1. Study Design
PERMIT was a pooled analysis of real-world data from 44 prospective, retrospective, and cross-sectional studies and work groups in which PWE with focal and generalized epilepsy were treated with PER, details of which have been previously published [15, 16]. Effectiveness was assessed over 12 months of PER treatment and additionally at the “last visit,” which was the final observation of each individual, regardless of when it occurred (i.e., last observation carried forward). The safety and tolerability of PER were evaluated throughout treatment (i.e., not at specific timepoints). Each study included in PERMIT was approved by its own independent ethics committee at the time it was originally conducted. If necessary, letters were sent to these ethics committees to inform them about the PERMIT study, but additional ethics committee approval was not required for inclusion in PERMIT [15]. All participants gave their informed consent prior to inclusion in the studies, according to the protocol. A post hoc subgroup analysis was conducted of PWE who were treated at baseline with PER monotherapy for focal-onset or generalized-onset seizures, either as first-line monotherapy or following conversion to monotherapy after initial treatment with PER as adjunctive therapy.
2.2. Study Population
The studies included in PERMIT employed broad inclusion/exclusion criteria, to be representative of PWE encountered in clinical practice [15]. The current analysis included all PWE treated with PER monotherapy at baseline.
2.3. Study Assessments
Retention was assessed after 3, 6, and 12 months of PER treatment. Long-term retention (defined as >12 months) was also assessed for studies that reported it. Effectiveness was assessed by seizure type (focal-onset or generalized-onset) after 3, 6, and 12 months and at the last visit. Effectiveness assessments comprised seizure freedom rate, responder rate, and the proportions of PWE with unchanged or worsening seizure frequency. Seizure freedom was defined as no seizures since at least the prior visit (either 3 or 6 months, depending on the timepoint at which seizure freedom was assessed). Since the studies included in PERMIT were heterogeneous in terms of design and the information they reported, this was the most pragmatic and feasible definition of seizure freedom. Response was defined as ≥50% seizure frequency reduction from baseline (i.e., prior to PER initiation) [17]. Since the definition of “baseline” differed between studies included in PERMIT, baseline seizure frequency was standardized as the number of seizures per month. Safety and tolerability were assessed by evaluating adverse events (AEs), AEs leading to discontinuation, psychiatric AEs, and psychiatric AEs leading to discontinuation. Information relating to PER dosing and subsequent use of concomitant ASMs was also assessed.
2.4. Subgroup Analyses
The effectiveness, safety, and tolerability of PER were additionally assessed by age group (<18, 18–64, and ≥65 years), for the subgroup of PWE with tumor-related etiology, and for the subgroups of PWE treated with PER as first-line monotherapy versus conversion to monotherapy. Safety and tolerability were assessed by gender (male and female).
2.5. Statistical Analyses
The statistical methodology employed in PERMIT has been published previously [15]. The full analysis set (FAS) comprised all PWE treated with PER monotherapy. The retention population included PWE from the FAS whose PER status was known at some point during the first 12 months after starting treatment (including those with ongoing PER treatment at 12 months, those who stopped PER prior to 12 months, and those lost to follow-up/end of study follow-up prior to 12 months). The effectiveness population included PWE from the FAS who had at least one effectiveness measurement available. The tolerability population included PWE from the FAS for whom data on AEs were available.
There was great heterogeneity in the objectives of each study included in the pooled analysis and therefore in the information reported. As previously described, PERMIT attempted to combine reported information in the most complete way possible [15]. Missing data were not imputed, except in cross-sectional studies, in which the last visit datum was captured to include in the established cut-off points (3, 6, or 12 months). When an observation timepoint did not match the established cut-off points, the following allocations were made: observations performed between 1.5 and 4.5 months were allocated to the 3-month visit, those performed between 4.5 and 9 months were allocated to the 6-month visit, and those performed between 9 and 15 months were allocated to the 12-month visit. A “final” variable was created in which the last observation of each individual was included, independently of when it occurred (defined as “last visit”). No hypothesis was defined, and no systematic review of the individual PWE was considered, due to the heterogeneity of individual samples and the different objectives of each study; therefore, individual studies were not treated as clusters.
Quantitative variables were described as mean, standard deviation (SD), median, minimum, and maximum values, together with the number of valid cases and confidence intervals (CIs) or interquartile range (25th percentile to 75th percentile). Qualitative variables were described as absolute frequencies and percentages. Data were not available for all PWE at every timepoint; therefore, for each variable, the total number of PWE for whom the datum in question was available was stated and used as the denominator for frequency analyses. Retention (on PER treatment) was studied within the first 12 months of follow-up using the Kaplan-Meier methodology. Between-group comparisons of PWE by age group (<18, 18–64, and ≥65 years) and gender (male and female) were conducted using the chi-squared test, if sample size permitted. The significance level was set at 5%, and the statistical package SPSS 25.0 was used for all analyses.
3. Results
PERMIT collected information from 5200 PWE, and the final FAS included 5193 PWE [15]. Of the PWE included in PERMIT FAS, 268 were treated with PER monotherapy at baseline for focal-onset or generalized-onset seizures. Retention was assessed for 168 PWE, effectiveness for 183, and safety and tolerability for 197 (Figure 1).
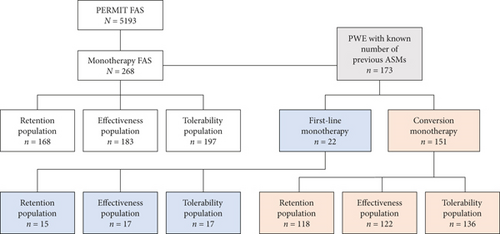
3.1. Study Population
Overall, 51.1% were women, the mean (SD) age was 44.0 (21.5) years, and the mean epilepsy duration was 13.2 (13.9) years (Table 1). Learning disability and psychiatric comorbidity were present in 11.4% and 13.8% of PWE, respectively. Seizure types at baseline were focal-onset only (75.0%), generalized-onset only (24.5%), and both focal-onset and generalized-onset (0.5%). Median (range) monthly seizure frequencies were 0.8 (0.3–30.0) for total seizures, 1.0 (0.3–30.0) for focal-onset seizures, and 0.3 (0.3–1.7) for generalized-onset seizures. The mean (SD) number of ASMs PWE were treated with before initiating PER monotherapy was 2.7 (2.8), and the most frequently used previous ASMs (≥20% of PWE) were levetiracetam (50.0%) and lamotrigine (23.4%).
| Characteristic | Full analysis set N = 268 |
|---|---|
| Gender | |
| N† | 266 |
| Female, n (%) | 136 (51.1) |
| Male, n (%) | 130 (48.9) |
| Age | |
| N† | 204 |
| Mean (SD) (years) | 44.0 (21.5) |
| Median (range) (years) | 41.0 (7.0–96.0) |
| Age category | |
| N† | 204 |
| <12 years, n (%) | 3 (1.5) |
| ≥12–<18 years, n (%) | 18 (8.8) |
| ≥18–64 years, n (%) | 140 (68.6) |
| ≥65 years, n (%) | 43 (21.1) |
| Age at epilepsy onset | |
| N† | 145 |
| Mean (SD) (years) | 30.7 (24.1) |
| Median (range) (years) | 21.0 (0.0–90.0) |
| Duration of epilepsy | |
| N† | 145 |
| Mean (SD) (years) | 13.2 (13.9) |
| Median (range) (years) | 8.0 (0.1–65.0) |
| Etiology‡ | |
| N† | 188 |
| Structural, n (%) | 80 (42.6) |
| Vascular, n (%) | 29 (15.4) |
| Tumor, n (%) | 18 (9.6) |
| Trauma, n (%) | 6 (3.2) |
| Genetic, n (%) | 54 (28.7) |
| Unknown, n (%) | 50 (26.6) |
| Infectious, n (%) | 4 (2.1) |
| Learning disability | |
| N† | 132 |
| Yes, n (%) | 15 (11.4) |
| No, n (%) | 117 (88.6) |
| Psychiatric comorbidity | |
| N† | 181 |
| Yes, n (%) | 25 (13.8) |
| No, n (%) | 156 (86.2) |
| Type of psychiatric comorbidity | |
| N† | 181 |
| Depression, n (%) | 15 (8.3) |
| Anxiety, n (%) | 6 (3.3) |
| Mood disorder, n (%) | 3 (1.7) |
| Hyperactivity, n (%) | 1 (0.3) |
| Obsessive-compulsive disorder, n (%) | 1 (0.3) |
| Others, n (%) | 2 (0.6) |
| Seizure type | |
| N† | 220 |
| Focal-onset only, n (%) | 165 (75.0) |
| Generalized-onset only, n (%) | 54 (24.5) |
| Both focal-onset and generalized-onset, n (%) | 1 (0.5) |
| Seizure frequency (month) | |
| Total seizures | |
| N† | 66 |
| Mean (SD) | 1.9 (4.1) |
| Median (range) | 0.8 (0.3–30.0) |
| Focal-onset seizures | |
| N† | 53 |
| Mean (SD) | 2.2 (4.6) |
| Median (range) | 1.0 (0.3–30.0) |
| Generalized-onset seizures | |
| N† | 13 |
| Mean (SD) | 0.6 (0.4) |
| Median (range) | 0.3 (0.3–1.7) |
| Number of previous ASMs | |
| N† | 173 |
| Mean (SD) | 2.7 (2.8) |
| Median (range) | 2.0 (0–15) |
| Number of previous ASMs | |
| N† | 173 |
| 0, n (%) | 22 (12.7) |
| 1, n (%) | 55 (31.8) |
| 2, n (%) | 36 (20.8) |
| 3, n (%) | 18 (10.4) |
| 4, n (%) | 9 (5.2) |
| 5, n (%) | 9 (5.2) |
| ≥6, n (%) | 24 (13.9) |
| Most frequently used§ previous ASMs | |
| N† | 64 |
| Levetiracetam, n (%) | 32 (50.0) |
| Lamotrigine, n (%) | 15 (23.4) |
- †Number of PWE for whom datum in question was available; ‡International League Against Epilepsy 2017 classification; §≥20% of PWE. ASM: antiseizure medication; PWE: people with epilepsy; SD: standard deviation.
3.2. Treatment
The mean (SD) PER monotherapy dose was 3.0 (1.4) mg/day (median, 2.0; range, 2–8; n = 79) at baseline. At the last visit, the mean (SD) PER monotherapy dose was 5.5 (2.2) mg/day (median 5.0; range, 2–12; n = 144); 50.0% (72/144) of PWE were treated with a final PER dose of ≤4 mg/day, and 76.4% (110/144) were treated with a final PER dose of ≤6 mg/day. A fast titration schedule (2 mg/week) was used in 77.7% (80/103) of PWE, and a slow titration schedule (<2 mg/week) was used in 22.3% (23/103). PER was the first ASM received by 12.7% (22/173) of PWE. Over 50% of PWE had been treated with one (31.8% (55/173)) or two (20.8% (36/173)) previous ASMs, and 13.9% (24/173) had been treated with ≥6 previous ASMs. At the last visit, 29.4% (48/163) of PWE were being treated with concomitant ASMs and 70.6% (115/163) were on PER monotherapy. The type of concomitant ASMs used at the last visit was known for 63 PWE, among whom the most frequently used (≥5% of PWE) were levetiracetam (14.3%) and valproate (6.3%).
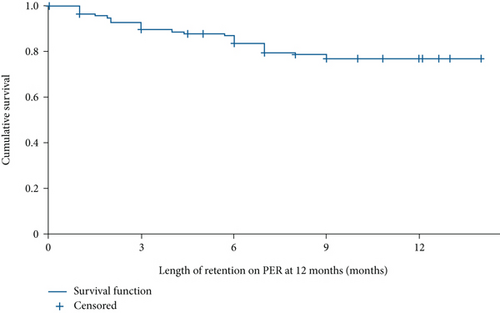
3.3. Retention (Retention Population)
Retention rates were 91.1% (153/168), 87.3% (138/158), and 73.3% (96/131) at 3, 6, and 12 months, respectively. The mean (95% CI) time under PER treatment was 11.8 (11.2–12.5) months (Figure 2). Reasons for PER discontinuation at 12 months were AEs (n = 18; 13.7%), lack of efficacy (n = 6; 4.6%), seizure worsening (n = 1; 0.8%), and other reasons (n = 5; 3.8% (patient decision: not otherwise specified, n = 2; disease progression: tumor, n = 2; and financial problems, n = 1)). Reasons for discontinuation were unknown for five PWE (3.8%). Over the longer term (>12 months), the retention rate was 59.5% (78/131). Reasons for PER discontinuation over the longer term were AEs (n = 24; 18.3%), lack of efficacy (n = 15; 11.5%), seizure worsening (n = 1; 0.8%), and other reasons (n = 5; 3.8% (patient decision: not otherwise specified, n = 2; disease progression: tumor, n = 2; and financial problems, n = 1)). Reasons for discontinuation over the longer term were unknown for eight PWE (6.1%).
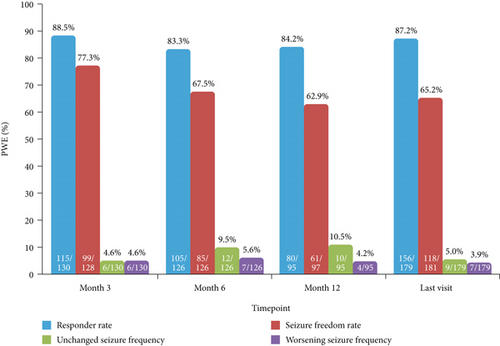
3.4. Effectiveness (Effectiveness Population)
At 12 months, responder and seizure freedom rates for total seizures were 84.2% (80/95) and 62.9% (61/97), respectively, and the proportions of PWE with unchanged and worsening seizure frequency were 10.5% (10/95) and 4.2% (4/95), respectively (Figure 3). At the last visit, responder and seizure freedom rates were 87.2% (156/179) and 65.2% (118/181), respectively, and the proportions of PWE with unchanged and worsening seizure frequency were 5.0% (9/179) and 3.9% (7/179), respectively (Figure 3).
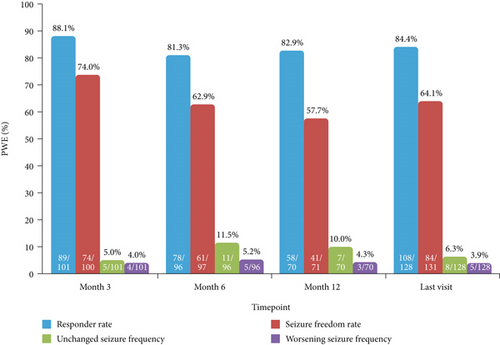
In PWE with only focal-onset seizures at baseline, responder and seizure freedom rates at 12 months were 82.9% (58/70) and 57.7% (41/71), respectively, and the proportions of PWE with unchanged and worsening seizure frequency were 10.0% (7/70) and 4.3% (3/70), respectively (Figure 4). At the last visit, responder and seizure freedom rates in these PWE were 84.4% (108/128) and 64.1% (84/131), respectively, and the proportions of PWE with unchanged and worsening seizure frequency were 6.3% (8/128) and 3.9% (5/128), respectively (Figure 4).
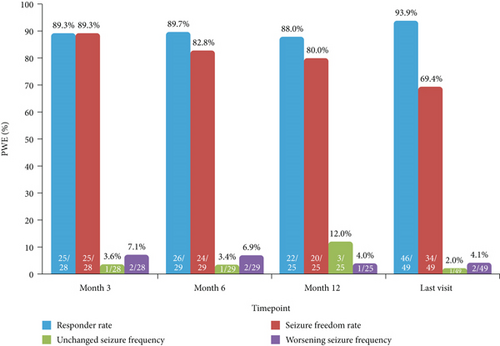
In PWE with only generalized-onset seizures at baseline, responder and seizure freedom rates at 12 months were 88.0% (22/25) and 80.0% (20/25), respectively, and the proportions of PWE with unchanged and worsening seizure frequency were 12.0% (3/25) and 4.0% (1/25), respectively (Figure 5). At the last visit, responder and seizure freedom rates in these PWE were 93.9% (46/49) and 69.4% (34/49), respectively, and the proportions of PWE with unchanged and worsening seizure frequency were 2.0% (1/49) and 4.1% (2/49), respectively (Figure 5).
3.5. Safety and Tolerability (Tolerability Population)
AEs were experienced by 45.2% (89/197) of PWE (Table 2). The most frequently reported (≥5% of PWE) were dizziness/vertigo (16.8%), irritability (11.2%), somnolence (9.1%), and depression (6.6%). AEs led to discontinuation of 13.7% (18/131) of PWE over 12 months and 18.3% (24/131) of PWE over the longer term (>12 months). The most frequent AEs leading to discontinuation over 12 months (≥5% of PWE) were depression (7.6%), dizziness/vertigo (6.1%), and irritability (6.1%). Psychiatric AEs were reported for 20.8% (41/197) of PWE and 10.2% (17/166) of PWE with psychiatric AEs discontinued. The types of psychiatric AEs in PWE who discontinued were depression (6.0%) and irritability (4.8%). Of the PWE with psychiatric AEs, the mean (SD; range) PER dose was 5.3 (1.7; 2–10) mg/day (n = 33), and 29.7% (11/37) had a history of psychiatric comorbidity. Of the PWE with psychiatric AEs who discontinued, the mean (SD; range) PER dose was 5.1 (2.0; 2–10) mg/day (n = 14), and 50.0% (8/16) had a history of psychiatric comorbidity.
| Total PWE | N = 197 |
|---|---|
| PWE with any AE, n (%) | 89 (45.2) |
| Most frequently reported AEs†, n (%) | |
| Dizziness/vertigo | 33 (16.8) |
| Irritability | 22 (11.2) |
| Somnolence | 18 (9.1) |
| Depression | 13 (6.6) |
| PWE with AEs leading to discontinuation (12 months), n (%) | 18 (13.7)‡ |
| Types of AEs leading to discontinuation (12 months), n (%) | |
| Depression | 10 (7.6)‡ |
| Dizziness/vertigo | 8 (6.1)‡ |
| Irritability | 8 (6.1)‡ |
| Somnolence | 5 (3.8)‡ |
| Instability/ataxia | 4 (3.1)‡ |
| Confusion | 1 (0.8)‡ |
| Dry mouth | 1 (0.8)‡ |
| PWE with any psychiatric AE, n (%) | 41 (20.8) |
| PWE with psychiatric AEs who discontinued§, n (%) | 17 (10.2)¶ |
| Types of psychiatric AEs in PWE who discontinued§, n (%) | |
| Depression | 10 (6.0)¶ |
| Irritability | 8 (4.8)¶ |
- †≥5% of PWE; ‡N = 131; §these PWE had psychiatric AEs, but it was not possible to determine if it was these AEs that led to discontinuation; ¶N = 166; AE: adverse event; PWE: people with epilepsy.
3.6. Subgroup Analyses
3.6.1. By Age Group (<18, 18–64, and ≥65 Years)
Age at baseline was known for 204 PWE, of whom 21 (10.3%) were aged <18 years, 140 (68.6%) were aged 18–64 years, and 43 (21.1%) were aged ≥65 years. There were no statistically significant differences in responder and seizure freedom rates between age groups, and sample sizes were too small to detect statistically significant differences between groups in the proportions of PWE with unchanged or worsening seizure frequency (Supplementary Figure S1). There were no statistically significant differences between age groups in the incidence of AEs and psychiatric AEs, and sample sizes were too small to detect statistically significant differences between groups in the rate of discontinuation due to AEs over 12 months (Supplementary Table S1A).
3.6.2. Tumor-Related Etiology
A total of 18 PWE were known to have tumor-related epilepsy at baseline (Table 1). In these PWE, the 12-month retention rate was 66.7% (6/9), and the responder and seizure freedom rates at the last visit were 88.2% (15/17) and 58.8% (10/17), respectively. AEs were reported by 27.8% (5/18) of PWE with tumor-related epilepsy.
3.6.3. First-Line Monotherapy versus Conversion to Monotherapy
The number of previous ASMs was known for 173 PWE, of whom 22 (12.7%) initiated PER as first-line monotherapy and 151 (87.3%) initiated PER as monotherapy having been treated with 1–15 previous ASMs (Table 1). The numbers of PWE from these subgroups who were included in the retention, effectiveness, and tolerability populations are shown in Figure 1. For the subgroups of PWE treated with PER as first-line monotherapy versus conversion to monotherapy, the 12-month retention rate was 76.9% (10/13) versus 73.7% (70/95), respectively; the responder and seizure freedom rates at the last visit were 94.1% (16/17) versus 83.1% (98/118) and 82.4% (14/17) versus 57.9% (70/121), respectively; and the proportion of PWE with AEs was 35.3% (6/17) versus 46.3% (63/136), respectively. Numbers of PWE in the first-line monotherapy subgroup were too low to allow between-group statistical comparisons.
3.6.4. By Gender (Male and Female)
Information on gender was available for 195 PWE from the tolerability population, of whom 103 (52.8%) were female and 92 (47.2%) were male. The incidence of AEs was higher in females versus males (57.3% vs. 31.5%; p < 0.001), as was the incidence of psychiatric AEs (27.2% vs. 13.0%; p = 0.015) and the rate of discontinuation due to AEs over 12 months (19.4% vs. 6.9%; p = 0.039) (Supplementary Table S1B). The presence of psychiatric comorbidities at baseline was higher in females than in males, although this was not statistically significant (18.1% vs. 9.2%; p = 0.083).
4. Discussion
This subanalysis of the PERMIT study demonstrated that PER is effective and generally well tolerated when used as monotherapy to treat PWE with focal and generalized epilepsy in everyday clinical practice. After 12 months, over 70% of PWE were retained on PER, and responder and seizure freedom rates were 84.2% and 62.9%, respectively. In PWE with only focal-onset seizures at baseline, responder and seizure freedom rates at 12 months were 82.9% and 57.7%, respectively, and corresponding values for those with only generalized-onset seizures at baseline were 88.0% and 80.0%, respectively. Responder and seizure freedom did not differ significantly between PWE aged <18, 18–64, and ≥65 years. Although the number of PWE with a tumor-related etiology was low, the 12-month retention rate and the responder and seizure freedom rates at the last visit were similar to those in the overall study population, and the proportion of these PWE reporting AEs (27.8%) was lower than that in the overall population (45.2%). In the overall study population, the most frequently reported AEs (dizziness/vertigo, irritability, and somnolence) were generally consistent with PER’s known safety profile [3]. Psychiatric AEs are commonly associated with PER treatment [3] and, in the current study, were experienced by one-fifth of PWE. In clinical trials, which predominantly investigated PER as adjunctive therapy, commonly reported psychiatric disorders comprised aggression, anger, anxiety, and confusional state [3], and it is therefore perhaps noteworthy that depression emerged as one of the most frequently reported psychiatric AEs in PWE treated with PER as monotherapy in clinical practice. However, depression was the most common type of psychiatric comorbidity at baseline, and it is important to note that PWE with clinically significant psychiatric conditions were excluded from the Phase III clinical trials [18]. Safety and tolerability did not differ significantly between PWE aged <18, 18–64, and ≥65 years, but PER was significantly less well tolerated in female versus male PWE, consistent with previous studies of other ASMs [19, 20] and classes of medication [20], which indicate potential gender differences in terms of drug pharmacokinetics and pharmacodynamics, health-seeking behavior, and/or effects of endocrine function and sex hormones [20–23]. The higher incidence of psychiatric AEs observed in female versus male PWE may reflect the fact that psychiatric comorbidities at baseline were more common in females than in males, although the difference was not statistically significant.
The responder and seizure freedom rates observed in PWE treated with PER monotherapy in the current study were substantially higher than in the overall PERMIT population (in which 94.4% of PWE received PER as adjunctive therapy at baseline), where the 12-month responder and seizure freedom rates were 58.3% and 23.2%, respectively [15]. In Phase III trials in which PWE with refractory focal-onset seizures received adjunctive treatment with PER (up to 12 mg/day), responder rates over 13 weeks of maintenance treatment ranged from 20.6% to 36.1% and seizure freedom rates ranged from 1.5% to 5.0% [18, 24, 25]. In a Phase III trial in which individuals with IGE received adjunctive treatment with PER (up to 8 mg/day), the responder and seizure freedom rates over a 13-week maintenance period were 64.2% and 30.9%, respectively [26]. The high responder and seizure freedom rates observed with PER monotherapy in the current study (particularly in PWE with generalized seizures) indicate that individuals selected for monotherapy treatment in PERMIT were either less refractory to treatment or had less severe epilepsy or were being treated earlier in their disease course (e.g., new-onset/treatment-naïve PWE) than those who received PER as adjunctive therapy in clinical trials and in PERMIT. This notion is supported by results from a single-arm, open-label, Phase III study of PER monotherapy in PWE with primarily newly diagnosed focal epilepsy, in which the rate of seizure freedom during a 26-week maintenance period at the last evaluated dose (4 or 8 mg/day) was 74.0% [8]. Notably, in this trial, PWE who experienced a seizure when treated with PER 4 mg/day during a 26-week maintenance period could be uptitrated to 8 mg/day and reassessed in an additional 26-week maintenance period [8]. A post hoc analysis of the trial demonstrated that a third of PWE who experienced seizures during the titration period of the 4 mg phase of the study went on to achieve seizure freedom during the maintenance period at 4 mg/day [27]. This led the authors to conclude that, in clinical practice, it may be inappropriate to discontinue or switch from PER monotherapy based solely on seizure response before an effective dose has been reached, since the half-life of PER is approximately 105 hours and it may take 2–3 weeks before steady-state plasma PER concentrations are attained [27]. In the current study, PER monotherapy was initiated using slow titration (<2 mg/week) in less than a quarter of PWE (22.3%). It is therefore possible that the tolerability of PER monotherapy observed in the current study may have been improved if slow titration had been utilized more often [15, 28], and in some cases where tolerability issues result in downtitration, slow re-uptitration may improve subsequent tolerability. In addition, over three-quarters of PWE (76.4%) were treated with a final PER dose of ≤6 mg/day, indicating that a relatively low dosage is effective for most PWE when PER is used as monotherapy (in comparison with adjunctive therapy). Greater use of slow titration may additionally facilitate the use of higher dosages in PWE who require it.
Use of an ASM as monotherapy is preferred to a polytherapy regimen, since monotherapy is associated with a lower risk of toxicity and helps optimize treatment adherence [29]. Newly diagnosed epilepsy is typically treated with ASM monotherapy, and almost half of PWE achieve seizure freedom with their first ASM [30]. Correct choice of initial monotherapy is crucial for optimizing the likelihood of treatment success but is dependent on clear diagnosis of epilepsy syndrome or seizure classification (i.e., focal or generalized) at presentation, which is often not possible [ 31, 32]. When a clear diagnosis is not possible, the preferred approach is to use an ASM with broad-spectrum activity [31]. Since PER is effective in treating both focal-onset and generalized-onset seizures, it is considered to have broad-spectrum potential [ 33, 34]. Indeed, neurophysiological studies suggest that PER’s efficacy for both focal- and generalized-onset seizures may be due to its inhibitory action on thalamocortical pathways in types of epilepsy characterized by thalamocortical hyperexcitability [35, 36]. A recent systemic review analyzed clinical data on PER in the treatment of generalized seizures, including a total of 91 reports/studies [37]. Strong evidence was found supporting the efficacy of PER for tonic-clonic seizures in IGE, and observational studies provided evidence of its effectiveness for myoclonic, absence and tonic seizures, and generalized epilepsy syndromes [37]. Furthermore, no evidence suggesting an association between PER treatment and seizure worsening in generalized epilepsies was identified [37]. For this reason, there may be a rationale for selecting PER as monotherapy treatment in newly diagnosed epilepsy. In the current study, only a minority of PWE (12.7%) received PER as their first ASM, since the median duration of epilepsy was 8.0 years, but a larger proportion of PWE were converted to monotherapy, supporting the preferential use of monotherapy over polytherapy. Although the number of PWE treated with PER as first-line monotherapy was too low to allow between-group statistical comparisons, subgroup analysis of PWE treated with PER as first-line monotherapy versus conversion to monotherapy demonstrated numerically higher responder and seizure freedom rates at the last visit in the former versus latter subgroup. Indeed, almost 95% of PWE treated with PER as first-line monotherapy were responders, and over 80% were seizure-free at the last visit. Retention rates were similar between the subgroups, but a lower proportion of PWE treated with PER as first-line monotherapy versus conversion to monotherapy experienced AEs (35.3% vs. 46.3%). These findings indicate that PER may be a rational choice as a broad-spectrum first-line monotherapy in PWE with newly diagnosed epilepsy, although further research is required to confirm these observations. In the overall study population, only a small proportion of PWE experienced seizure worsening when treated with PER monotherapy (at 12 months: 4.2% of the total population, 4.3% of PWE with only focal-onset seizures at baseline, and 4.0% of PWE with only generalized-onset seizures at baseline), further supporting the use of PER as a broad-spectrum ASM in the monotherapy setting.
To our knowledge, only three clinical practice studies have to date specifically investigated the use of PER monotherapy in clinical practice, all of which were included in PERMIT [12–14]. There is currently very limited evidence for direct head-to-head comparisons of ASMs as monotherapy in the real-world or pragmatic-use setting, particularly for PWE with generalized-onset seizures. The first Standard And New Antiepileptic Drugs (SANAD) trial was a long-term, unblinded study that assessed the effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for the treatment of focal epilepsy [38] and the effectiveness of valproate, lamotrigine, or topiramate for treatment of generalized and unclassifiable epilepsy [39]. These comparisons provided evidence supporting the use of lamotrigine as first-line treatment for focal-onset seizures and valproate as a first-line treatment for generalized-onset seizures [38, 39]. Since SANAD was conducted before the approval of more recent ASMs, the SANAD II trial has subsequently assessed the effectiveness of lamotrigine versus levetiracetam or zonisamide for focal epilepsy and valproate versus levetiracetam for generalized and unclassified epilepsy [40, 41]. Results from SANAD II demonstrated that lamotrigine was superior to levetiracetam and zonisamide in treating focal epilepsy and that valproate was superior to levetiracetam in treating generalized and unclassified epilepsy [40, 41]. In the 52-week, two parallel-group, unblinded, randomized, Keppra vs. Older Monotherapy in Epilepsy Trial (KOMET), which compared the effectiveness of levetiracetam with extended-release sodium valproate and controlled-release carbamazepine as monotherapy in individuals with newly diagnosed epilepsy, estimated 6-month seizure freedom rates for total seizures were 63.8% for levetiracetam versus 69.2% for extended-release sodium valproate and 57.5% for levetiracetam versus 62.0% for controlled-release carbamazepine [42]. Estimated 6-month seizure freedom rates for focal-onset seizures were 55.5% for levetiracetam versus 61.0% for controlled-release carbamazepine, and estimated 6-month seizure freedom rates for generalized-onset seizures were 66.0% for levetiracetam versus 73.0% for extended-release sodium valproate [42]. In the current study, 12-month seizure freedom rates (which represented the percentage of PWE who had no seizures for ≥6 months) were 62.9% for total seizures, 57.7% for focal-onset seizures, and 80.0% for generalized-onset seizures. However, as KOMET was a randomized controlled trial conducted specifically in individuals with newly diagnosed epilepsy, whereas PERMIT was a pooled analysis of real-world data that included only a minority of PWE with newly diagnosed epilepsy, a comparison of the findings between the two studies should be interpreted cautiously.
This study has some limitations. Firstly, it is a post hoc analysis of PERMIT, which was itself limited in being a retrospective pooled analysis of studies that were heterogeneous in terms of objectives and information reported, as previously acknowledged and discussed [15]. Secondly, the study population did not comprise a “pure monotherapy” cohort for the entire duration of the follow-up, since approximately 30% of PWE were receiving concomitant ASMs at the last visit. Moreover, it was not possible to evaluate the timing, type(s), and dosage of concomitant ASMs introduced during PER treatment, all of which could have influenced the results observed in these PWE.
In summary, PERMIT provides strong evidence that PER is effective and generally well tolerated when used as monotherapy to treat PWE with focal and generalized epilepsy in clinical practice, its effectiveness being particularly favorable in those with generalized-onset seizures. These findings support the use of PER as a broad-spectrum ASM in the monotherapy setting.
Conflicts of Interest
YC has no disclosures. RTD has participated on advisory boards and in industry-sponsored symposia for Eisai, UCB, Bial, Esteve, and GW Pharmaceuticals. AAR received travel support and speaker’s honoraria from Eisai. TW received personal fees from Eisai, GW Pharmaceuticals, and UCB and owns stock in Amgen. MT has received speaker’s honoraria from Eisai, UCB Pharma, and Arvelle Therapeutics. SM has no disclosures. ET reports personal fees from EVER Pharma, Marinus, Arvelle, Angelini, Argenix, Medtronic, Bial-Portela & Cª, NewBridge, GL Pharma, GlaxoSmithKline, Boehringer Ingelheim, LivaNova, Eisai, UCB, Biogen, Genzyme Sanofi, and Actavis. His institution received grants from Biogen, UCB Pharma, Eisai, Red Bull, Merck, Bayer, the European Union, FWF Osterreichischer Fond zur Wissenschaftsforderung, Bundesministerium für Wissenschaft und Forschung, and Jubiläumsfond der Österreichischen Nationalbank. MM was formerly an employee of Eisai Inc. OS was formerly an employee of Eisai Europe Ltd. VV has received honoraria and/or research funds from Angelini, Arvelle, Bial, Eisai, Esteve, GW Pharma, NewBridge, Novartis, Takeda, UCB Pharma, and Zogenix.
Acknowledgments
This work was funded by Eisai Ltd.
Open Research
Data Availability
Data are available from the authors upon reasonable request.




