An Approach for Modeling, Simulation, and Optimization of Catalytic Production of Methyl Ethyl Ketone
Abstract
The current exploration manifests the progress of a one-dimensional reactor for the production of methyl ethyl ketone (MEK) as a commercial-industrial solvent with a relatively rapid evaporation rate and high solvation ability. MEK has been extensively utilized in colorings, printing, artificial leather, and base oils. One of the methods for the production of MEK is catalytic dehydrogenation of 2-butyl alcohol in the temperature range of 650–750 K utilizing spherical ZnO catalyst. Considering the high cost of fossil fuels to achieve optimal energy consumption, thermal coupling with the Fischer–Tropsch reaction was employed. Eventually, an evolutionary genetic algorithm was adopted to optimize the reactor to maximize MEK production. MATLAB software was utilized for the modeling and optimization. The modeling results were verified by industrial data. Moreover, they indicated a 37 and 55.4% increase in the production rate of thermal coupling and optimal thermal coupling reactors, respectively.
1. Introduction
Considering the ongoing depletion of fossil fuel resources and the escalation of global warming, hydrogen seems to be the future energy carrier. Hydrogen has been considered as an alternative renewable carrier of energy. Regarding the day-by-day increase in hydrogen demand, various methods have been developed to increase hydrogen production. Catalytic modification of natural gas and other hydrocarbons is one of the major routes for hydrogen production. The use of this method will result in the emission of greenhouse gases (GHGs) as well as corrosive ones such as CO2 and CO. One of the approaches for the storage and transfer of hydrogen is the dehydrogenation of high-content organic hydrocarbons. One of the objectives of this study is to simulate a two-reaction thermal coupling reactor for simultaneous production of hydrogen and methyl ethyl ketone (MEK) utilizing the concept of process intensification (PI). Since 1970, PI has been utilized as a new approach in the design of chemical engineering processes, reactors have been regarded as the core-foundation-pillars of the chemical industry [1, 2]. Reactors can reduce process costs and energy consumption. Fischer–Tropsch (FT) process is highly exothermic and comprises a series of exothermic reactions. Thermal coupling of this reaction can reduce the cost of cooling processes and the reaction heat can be utilized in the endothermic reaction of MEK production. This method relies on increasing the simultaneous production of energy and substances. Innovations in catalytic reactor technology are often preferred as the starting point of this trend. In this regard, the thermal multipurpose reactor is a new concept in PI. Thermal coupling of endothermic and exothermic reactions is a branch of multipurpose reactors. In this type of reactor, an exothermic reaction is considered as a heat source of an endothermic reaction. Recently, extensive investigations have been devoted to the catalytic dehydrogenation reaction of 2-butanol and MEK production [3–5]. The coupling of endothermic and exothermic reactions has been identified as an effective method to progress the performance of processes. In particular, the literature is replete with excellent examples of thermally coupled reactors [6–8]. Accordingly, the conventional reactors in the MEK production have been replaced with the new multifunctional reactors. In pursuit of these, the current study aims to integrate the MEK production and 2-butanol dehydrogenation process in a novel thermally coupled reactor concept in which the required heat for the dehydrogenation reaction is supplied by the FT reaction by gas to liquid (GTL) method as an exothermic reaction. Remarkably, this concept leads to a significant decrease in operational costs and energy consumption due to the elimination of certain equipment. It is worthy to note that the mentioned coupled reactor decreases the size of the reactor. Moreover, the thermal coupling can increase the reaction rate as well as the reactor equilibrium conversion. Interestingly, simultaneous production of different products as well as preventing the formation of unwanted products can be considered as another benefit of the current reactor. Finally, an evolutionary genetic algorithm was used to optimize the reactor for maximizing MEK production. In the second section, the previous works in the field of production and modeling of MEK and dehydrogenation of 2-butanol are discussed. The modeling and numerical methods are stated in the third section; while the evaluation and its results are presented in the fourth section.
2. Literature Review
Numerous studies have addressed the production and modeling of MEK production as well as 2-butanol dehydrogenation which are summarized in Table 1. In [9], the dehydrogenation of 2-butanol to methyl ethyl ketone (MEK) was explored in a membrane reactor including Pd-Ag film (2.2 m thickness) deposited on the inside of an α-alumina tube and packed with a 14.4 wt.% Cu on SiO2 catalyst. Conducting experiments at temperatures of 190 to 240°C specifies a better performance of the membrane reactor of 2-butanol conversions than the plug flow reactor. Significantly, the 2-butanol conversion increased with an augmentation in the sweep gas flow rate, but reduced with an upsurge in the 2-butanol feed flow rate. It is worthy to note that in all the tests performed, the selectivity for MEK production was above 96%.
| Authors | Year | Subject |
|---|---|---|
| Keuler et al. [9] | 2002 | Dehydrogenation of 2-butanol in Pd-Ag membrane reactor |
| Lightard et al. [10] | 2002 | Sustainable catalyzed dehydrogenation of alcohols by hydrogen gas circulation |
| Liu et al [11] | 2006 | Investigation of synthesis technology and catalyzed hydration of butylene for MEK production |
| Fang et al. [12] | 2009 | Using a new mesoporous catalyst (Cu-Zn-Al2O3), they investigated the dehydrogenation of alcohol |
| Multer et al. [13] | 2012 | Production of MEK by a hybrid biochemical method using biomass |
| Dehkordi at al. [14] | 2022 | Degradation of dissolved RDX, NQ, and DNAN by cathodic processes in an electrochemical flow-through reactor. |
| Katoch et al. [15] | 2021 | A review on genetic algorithm: past, present, and future. |
| Marvast et al. [16] | 2005 | Fischer–Tropsch synthesis: modeling and performance study for Fe-HZSM5 bifunctional catalyst. |
| Ebrahimi et al. [17] | 2022 | Crosstalk between ferroptosis and the epithelial-mesenchymal transition: implications for inflammation and cancer therapy. |
Fatholahi et al. in [10] manifests the catalytic dehydrogenation of alcohols into aldehydes and ketones in the presence of transition metal catalysts like Ru(OCOCF3)2(CO) (PPh3)2. One of the advantages of using Robinson’s catalyst is the conversion of secondary alcohol to the corresponding ketones with high selectivity in relatively short reaction times. Moreover, the catalyst could be recovered three times without main loss in catalytic activity.
Another methodology for the synthesis of methyl ethyl ketone (MEK) is designed and suggested in [11]. Accordingly, secondary butanol (SBA) is produced from catalytic hydration of butylene. Subsequently, MEK synthesis was carried out by dehydrogenation of SBA. Successfully, the designed and optimized methodology has also shown highly promising results with high catalyst activity, good selectivity, and long lifespan.
In [12], a series of meso-structured Cu-Zn-Al2O3 has been synthesized and characterized using XRD, N2 physical adsorption, and TPR procedures. In the next step, their catalytic activity was investigated for dehydrogenation of 2-butanol to methyl ethyl ketone (MEK) at atmospheric pressure. Interestingly, the results corroborated that the mesoporous Cu-Zn-Al2O3 catalysts were promising in the production of MEK and disapproving for the formation of by-products than the catalysts synthesized by coprecipitation technique.
Research in the area of biomass conversion to fuels and chemicals has recently gained much importance in sustaining the progress of biochemical and chemical processes. In [13], production of MEK by a hybrid biochemical method using biomass was explored. In this circumstance, 2,3-butanediol was synthesized through the microbial fermentation (the bacteria Klebsiella oxytoca (K.O) was used). In the second step, using dehydration over a solid acid catalyst, MEK was produced from the conversion of 2,3-butanediol. It is worth noting that dehydration kinetics were dependent on the concentration of 2,3-butanediol with a slightly higher reaction order than one (1.22).
3. Modeling Approaches
MEK is currently produced on commercial scales using a two-stage N-butylene method involving hydration of butylene to produce 2-butyl alcohol (SBA) followed by dehydrogenation SBA and production of MEK. Depending on the catalyst, there exist three main steps: indirect hydration of sulfate, direct hydration of resin, and direct hydration of acid. This method is benefited from high MEK selectivity (about 95%), facile separation of product and dehydrogenation of the vapor phase, high 2-butanol conversion, and lower energy consumption. This process has low economic advantages relative to the dehydrogenation of 2-butanol; the main factor is, however, accessibility and cost of n-butane. Compared to all processes, the dehydrogenation of 2-butanol has higher advantages in addition to its cost-effectiveness. Therefore, this method was selected.
3.1. Kinetics of Dehydrogenation Reaction
T is in Kelvin in all the equations.
Figure 1 illustrates a schematic representation of 2-butanol dehydrogenation for the production of MEK.

3.2. Dehydrogenation Reaction
Kinetics of 2-butyl alcohol catalyzed dehydrogenation was examined to produce MEK at atmospheric pressure and various temperatures (650–750 K) in the presence of spherical ZnO catalysts. Thanks to its nature, this catalyst makes it possible to directly evaluate the surface involved in the reaction and define the ratio of surface to the feed (known as S/F) instead of the weight ratio of the feed (W/F) which is common in catalysis studies. The feed composition varied from 2-butyl alcohol to a mixture with high MEK content. In these studies, the mass transfer effects were considerable and should be considered as an indicator of the catalyst surface condition. Investigation of the effect of feed composition on the initial reaction rate showed that dehydrogenation was the controlling stage which involved a single-site mechanism. Moreover, these results were used to determine the values of the height of the reactor unit (HRU) to relate the mass rate with temperature. HRU provides a simple approach for calculating the depth required to reach a proper conversion percentage [21, 22].
3.3. Kinetics of Fischer–Tropsch Reaction
During this process which involves gas to liquid conversion, hydrogen and carbon dioxide are mixed in various stoichiometric coefficients to form heavier hydrocarbons [23–25]. Figure 2 schematically illustrates the reaction [19].

3.4. Effective Parameters of the Reaction
S/F is simpler and is used instead of W/F. For porous catalysts, W/F depends on the catalyst type, preparation method, and particle size. On the other hand, S/F is more basic as it only depends on the reaction nature and is independent of the S/F ratio [8, 26]. The application of the mechanical stirrer makes it possible to reach higher conversion rates and hence higher temperatures [26]. Since feed rate is not effective in controlling this system, it can be considered a small value to reach high conversion percentages at lower temperatures. Therefore, more than one temperature may be employed [26]. In an agitated reactor mass, the transport effects can be removed by increasing the flow rate [29].
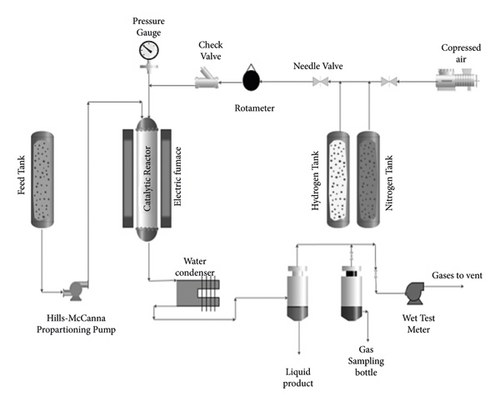
3.5. Experimental Apparatus
Figure 3 schematically represents the experimental setup of this study. This setup included a small continuous pilot encompassing a stainless steel tube. The reaction feeding was supplied by an electric furnace. The liquid mixture of 2-butyl alcohol and MEK was pumped from a calibrated feed tank [29]. The input hydrogen, nitrogen, and air were also regulated by a needle valve. The flow intensity was measured by a rotameter. To assess the production rate, the reactor effluent was first passed through a condenser and then a phase separator in which the incompressible gases can be collected on the water and salt or passed into the hygrometer [26, 30].
3.6. Selecting the Proper Model to Describe the Reactor
A one-dimensional model in the axial direction is generally used to model MEK production reaction as it is endothermic and the reaction site length is larger than the catalyst tube. Therefore, a one-dimensional steady-state was utilized for the simulation. Furthermore, the mass-energy equilibrium equations will be discussed.
3.6.1. Mass-Energy Balance Equations
To model MEK production, a one-dimensional model in the axial direction is generally used as the reaction is endothermic and the reaction site length is larger than the catalyst tube. Therefore, a one-dimensional steady-state approach was utilized for the simulation. The mass-energy balance equations are as follows:
3.6.2. Mass-Energy Balance Equations
- (i)
The transport mechanism is in the axial direction and in the form of plague
- (ii)
The pressure drop is negligible along the reactor
- (iii)
Axial, thermal, and mass scattering were neglected
- (iv)
The process is steady and one-dimensional;
Equations of FT reaction (exothermic).
Equation of the dehydrogenation part (endothermic).
3.6.3. Mass and Energy Balance in the Thermal Coupling Condition
3.6.4. Mass Balance in the 2-butanol Dehydrogenation
3.7. Auxiliary Equations
In this section, auxiliary equations of the model are presented for the prediction of the physical properties as well as heat transfer.
3.7.1. Heat Transfer Coefficient
3.7.2. Heat Capacity
3.7.3. Viscosity
3.7.4. Thermal Conduction Coefficient of the Fluid
3.7.5. Standard Formation Enthalpy
Standard formation enthalpy refers to the amount of enthalpy of a reaction in which one mole of the compound is formed under standard conditions (for pure material; the pressure of 1 atm, T = 25°C).
3.8. One-Dimensional Modeling of the Reaction
As Pe = 10 for the fixed-substrate reactors and Pe of this modeling varied from 93–124.5, one-dimensional modeling is a proper choice for this design.
3.9. Optimization of Thermal Coupling Reactor for the Production of MEK
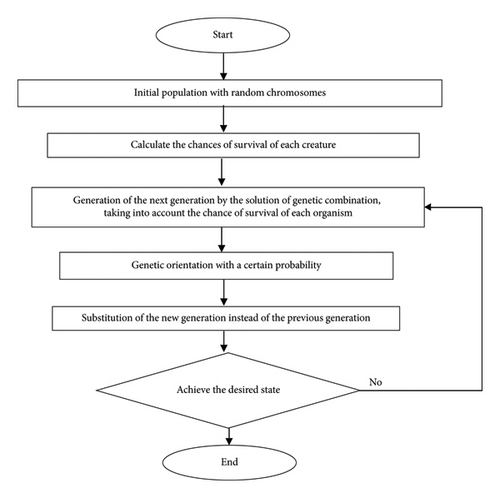
In this section, the genetic algorithm is used as a powerful tool to optimize the thermal coupling reactor. A genetic algorithm is a random search algorithm inspired by the nature [31, 32]. A GA tries to discover the most appropriate solution to a problem. The GA technique has repeated the natural survival for the best generation to form feature subsets (chromosomes) with the best performance in producing new feature chromosomes. As the population progresses from one generation to another, inappropriate solutions disappear, and appropriate ones are used. Each individual creates a chromosome in this method, and its colony is called a population. The fit function is applied to provide new solutions and used as operators such as duplication and mutation. To improve the optimized global network parameters, the GA uses three basic operators: selection, crossover, and mutation. Selection compares chromosomes in the population and chooses them to take part in the reproduction process. Selection also occurs with a given probability on the basis of the fitness function. Moreover, the recombination or crossover is carried out after finishing the selection process. It combines with predefined probability, features of two selected parent chromosomes, and forms similar children. Mutation characteristic is applied to create alternate subsets for each chromosome [32, 33]. Generally, mutation refers to the creation of a new chromosome from one and only one individual with predefined probability.
i and j show the number of components in the exothermic and endothermic parts, respectively.
- (1)
Input temperature of the exothermic part (T0, Exo)
- (2)
2-molar flow rate of the exothermic part ((F0, Exo)
- (3)
Input temperature of the endothermic part (T0, Endo)
- (4)
Input molar flow rate of the endothermic part (F0, Endo)
- (5)
Reactor length (L)
- (6)
Number of reactor tubes (n)
- (7)
The volume of the catalyst in the endothermic part of the reactor (V)
- (8)
The input pressure of the endothermic part of the reactor (P)
4. Evaluation of the Model
-
Simulation of ideal reactors (PFR, CSTR, and Batch reactors) even with bypass or recycling side streams
-
Simulating the connection of these reactors in series or parallel
-
Optimizing the design of reactors
-
Conversion versus temperature diagrams for reversible exothermic reactions.
4.1. Validation of the Model
The model was validated by comparing the simulation results with experimental data for the typical steady-state MEK production reactor with acceptable validity. The results are presented in Table 2.
| Parameters | Experimental data | Simulation results | Relative error |
|---|---|---|---|
| CO conversion | 0.7794 | 0.754 | 3.25 |
| H2 conversion | 0.9283 | 0.98 | 5.56 |
| Output products (gmol/h) | 19.96 | 18.74 | 6.1 |
| Reactor output temperature (°C) | 562 | 566 | 0.712 |
4.2. Sensitivity Analysis
In this section, the influence of the operational conditions and design parameters in the aligned flow regime was assessed on the yield of MEK production and 2-butyl alcohol conversion.
Figure 5 assesses the changes in the conversion percentage of 2-butyl alcohol concerning the inlet temperature of the endothermic part. As the inlet temperature of the endothermic section decreased, the conversion percentage declined sharply. Although this temperature reduction is economically and operationally beneficial, the highest possible temperature should be adopted to avoid a low alcohol conversion rate.
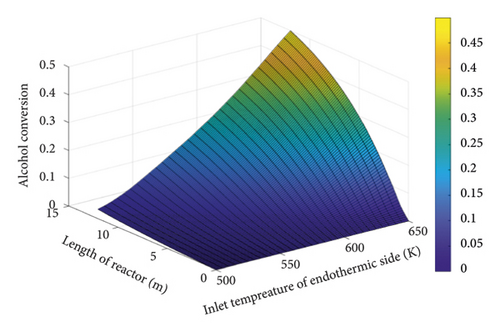
Figure 6 depicts the changes in hydrogen conversion along the reactor length in terms of the exothermic part temperature. As seen, a decline in the temperature of this section from 650 to 500 K decreased the deriving force between these two sections (endothermic and exothermic) prohibiting the reaction onset in the exothermic (FT) part. Thus, the highest possible temperature should be selected in this section such that a rise in the driving force initiates the reaction in the exothermic part, elevating the temperature. This temperature elevation is in favor of production and can enhance hydrogen generation.
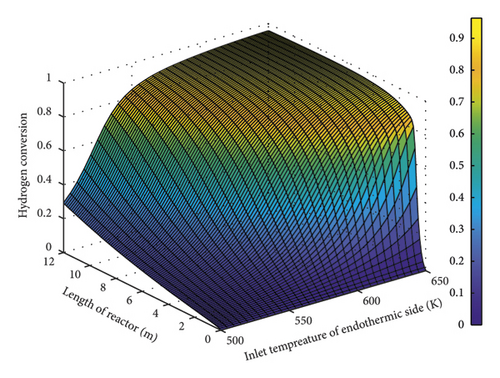
Figure 7 illustrates the variations in the conversion percentage f alcohol according to the molar flow rate on the exothermic part of the reactor. As expected, this volume of catalyst was not sufficient and declined the conversion due to the fixed amount of catalyst (200 mg) and flow rate increase. In this modeling, the molar flow rate was set at an optimal point of 1 mol/g to stabilize the conversion percentage at 30–35%.
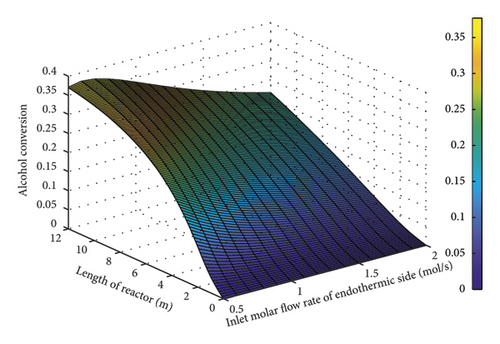
Figure 8 shows the influence of molar flow rate on the exothermic part of the reactor on the conversion percentage of alcohol. As the heat generated in the exothermic part will be transported to the endothermic part, the variables should be set in such a way that the temperature on the exothermic side does not drop and decline the conversion percentage. As seen, a rise in the molar flow rate of the exothermic part (in constant catalyst volume) declined the conversion percentage, reducing the temperature or the produced heat, this will also decrement the heat traveling from the exothermic wall to the endothermic part. The alcohol conversion rate will also be decremented (endothermic part). Therefore, the input molar flow rate was considered as 0.236 mol/s which led to a conversion rate of 30% which is industrially acceptable.
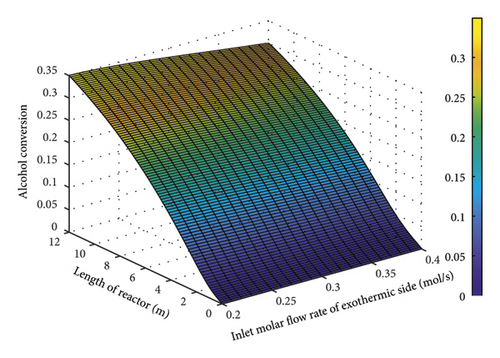
Figure 9 illustrates the changes in CO conversion rate as a function of the molar flow rate of the endothermic part. The lower the molar flow rate in this section, the lower the cooling function. As the amount of heat in this section is enough, a rise in the molar flow rate from 0.5 to 2 mol/s caused no significant changes in the conversion rate. It can be said that the CO conversion rate had no significant dependence on the input molar flow rate of the endothermic part. Therefore, the molar flow rate of this section was set to 1 mol/s.
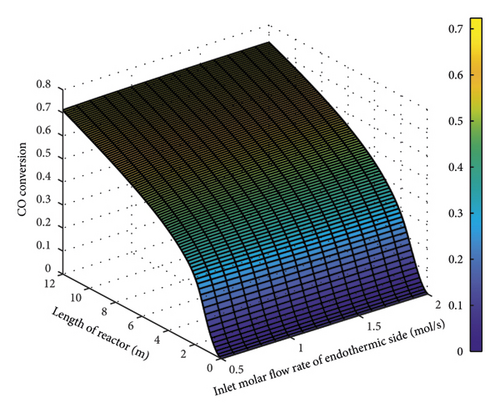
Figure 10 presents the variations in the CO conversion percentage as a function of the input molar flow rate of the exothermic part. As expected, a rise in the molar flow rate of the exothermic part, as well as an insufficient amount of catalyst, declined the conversion percentage. As the maximum CO conversion rate is 72% in industrial applications, in this modeling, the amount of 0.236 was chosen to provide both a high conversion rate and production yield.
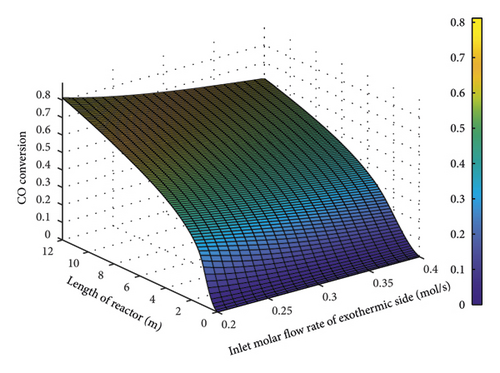
4.3. Optimization Parameters
In the above equations, subscripts 1 and 2 denote exothermic and endothermic parts, respectively. Table 3 lists the optimal values of the parameters after optimization with a genetic algorithm.
| Parameter | TCFTR | OTCFTR |
|---|---|---|
| Reactor length | 12 | 11.86 |
| Number of the reactor tube | 1 | 5 |
| Input temperature of the exothermic part of the reactor | 569 | 569.45 |
| Input molar flow rate of the exothermic part of the reactor | 0.268 | 0.3614 |
| Input molar flow rate of the endothermic part of the reactor | 620 | 625.84 |
| Input molar flow rate of the exothermic part of the reactor | 1 | 1.1514 |
4.4. Aligned Flow Regime Results for Three Modes of CFTR, TCFTR, and OTCFTR
In this section, the results are separately discussed for aligned flow in three modes of conventional FT reaction (CFTR), thermally coupled FT reaction (TCFTR), and optimized thermally coupled FT reaction (OTCFTR).
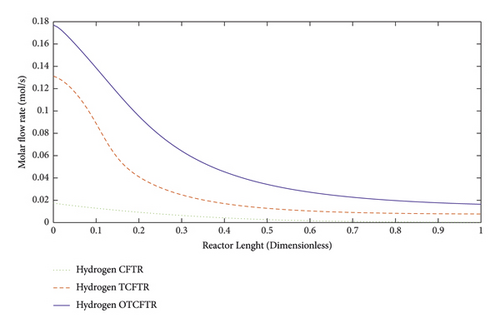
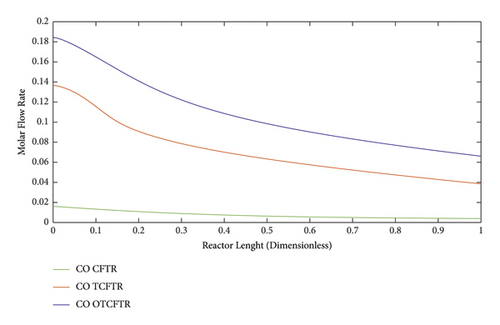
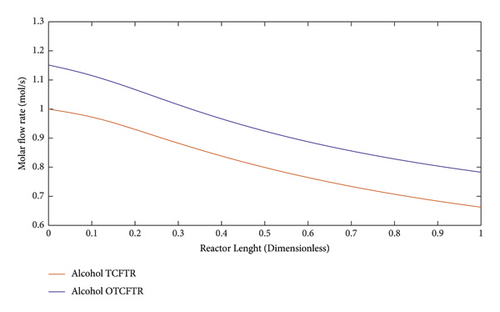
4.4.1. Variations in the Molar Flow Rate of the Materials along the Reactor Length
Figures 11–15 compare the molar behavior of various materials in the exothermic part of the reactor in three modes of CFR, TCFTR, and OTCFTR. Regarding the temperature sensitivity of the reaction, the production rate of the reactor rose by the temperature elevation. Despite some differences in the molar profile of various materials, this difference is due to better temperature control of TCFT and OTCFTR modes, the better results, were, however, obtained at the end of the reactor.

In recent years, hydrogen has been considered a clean fuel due to global warming as well as rising fossil fuel prices. As shown in Figure 10, a considerable amount of hydrogen was produced by this process (above 5 mol/s) which can be used as a clean fuel. In general, after coupling and optimization, the amount of hydrogen was increased by 0.022 mol/s. In the OTCFR mode compared to the TCFR mode, the molar flow rate as the feed of the exothermic part was increased by 0.014 mol/s.
Carbon dioxide is a toxic gas. It is, however, an important precursor in FT reaction for being converted into heavier organic products. According to Figure 11, the same explanation for the decline in the input molar flow rate at the exothermic part for hydrogen holds for the CO.
The exothermic part is associated with the dehydrogenation reaction in which hydrogen is one of the main products. A comparison of the hydrogen production of the coupled and optimized coupled process indicated a rise in hydrogen production after optimization.
2-butyl alcohol is highly used in various industries. Dehydrogenation of this material and production of MEK were studied in this paper. Using fewer amounts of this material at a lower price, the same conversion percentage was achieved for dehydrogenation products which are of crucial significance.
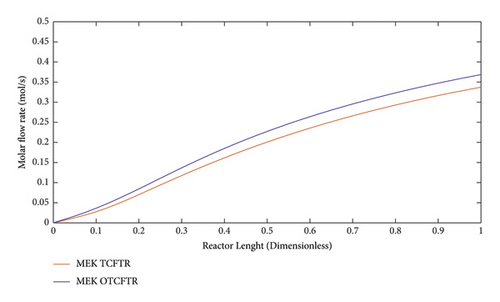
Methyl ethyl ketone is one of the most important and widely used chemicals that is used as a solvent in various industries. This article tried to achieve a cost-effective way by optimizing the production of this material. This diagram shows a rise in the production of this valuable substance after coupling as well as the optimization. Noteworthy, the conversion percentage of methyl ethyl ketone was enhanced to 35% by optimizing the temperature and consuming less energy to perform this exothermic reaction. Figures 16–22 show the changes in molar flow rates of the products of the exothermic reaction (Fischer–Tropsch products).
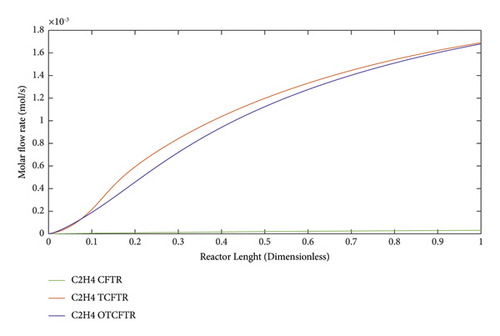
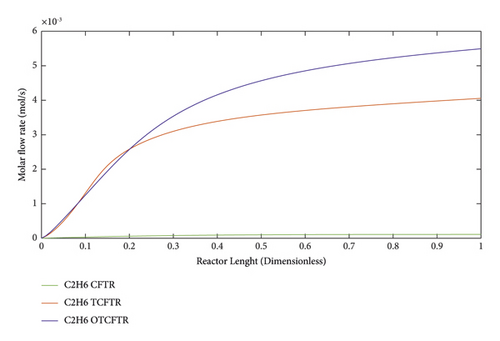
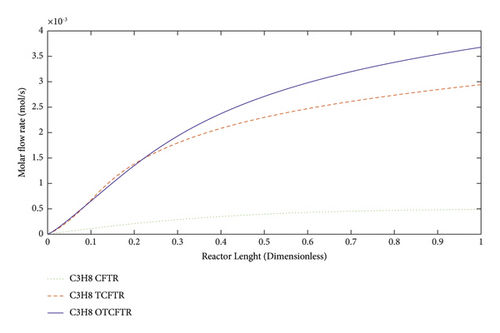
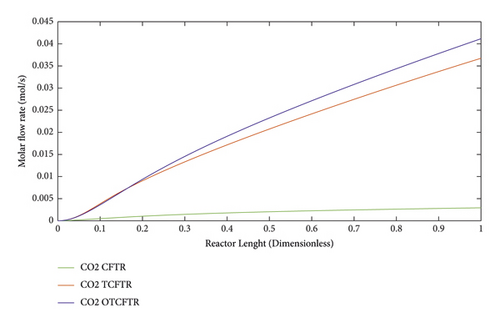
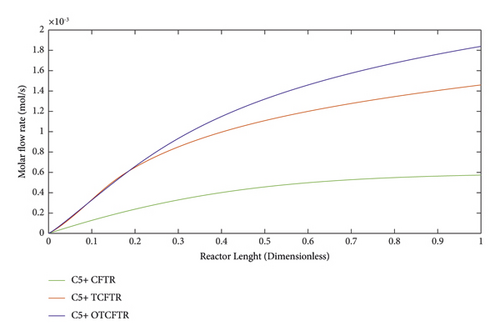
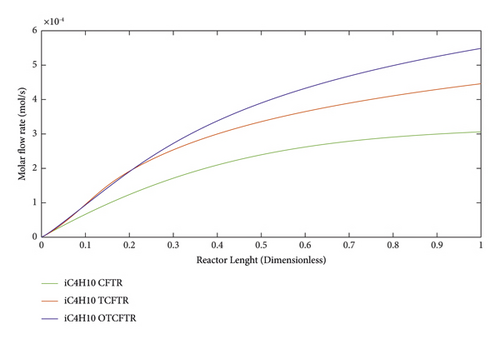
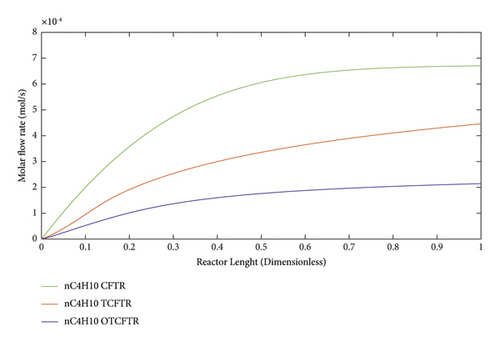
As seen, this material was produced in little amounts before the coupling process. After coupling and optimization, its amount exhibited a considerable increase. It is noteworthy that this increase in production was achieved with less raw material and energy use.
Ethane is a colorless hydrocarbon and one of the derivatives of natural gas. The refinery of this gas will result in ethylene. Ethylene can be used as a fuel after compounding with methane. Ethane is also one of the products of FT reaction. As seen, the production of this material was drastically increased after coupling and optimization as well as temperature regulation.
Propane is a three-carbon alkane and is generally a colorless and odorless gas. It is often compressively liquified for the ease of transportation. This gas is one of the products of oil wells. It can also be produced in this reaction. As seen, MEK production was raised after coupling this exothermic reaction with the endothermic reaction of MEK production. Optimization of this process also considerably enhanced the production of this material.
The above figure shows the changes in the CO during FT reaction after coupling and optimization. The production of this material showed an apparent increase during these processes.
As suggested by the three above figures, the production of all three mentioned materials increased by coupling and optimization. The above figures, respectively, illustrate the changes in the production of gasoline, isobutene, and normal butane.
4.4.2. Temperature Profile along the Reactor Length
Figure 23 compares the temperature profile of the reactor in the endotherm section in TCFTR and OTCFTR modes. Regarding the endothermic nature of the reaction, there is a temperature increase at the beginning of the reactor. By consumption of raw materials, heat exchange, and the amount of heat transferred by the exothermic reaction, the temperature profile showed a linear drop, followed by an increment in the number of products. The heat sink increases. In both OTCFTR and TCFTR modes, the temperature declined at the end of the reactor to reach an equilibrium temperature. Heat is also transferred from the exothermic side resulting in a lower percentage of hydrogen conversion (Figure24).
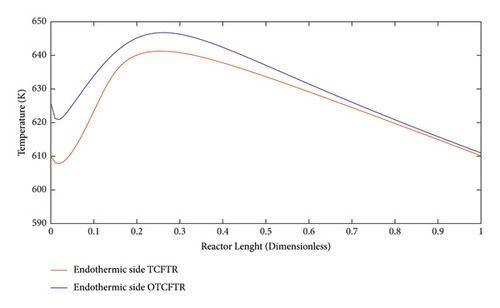
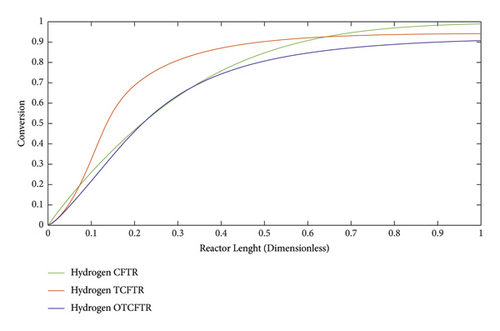
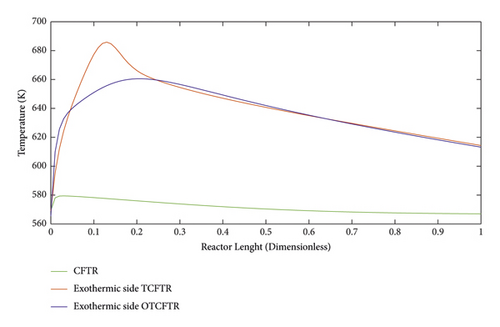
Figure 25 compares the temperature profiles of the exothermic part of the reactor for different modes. Normally, a constant temperature coolant is used to cool the reactor, which also imposes some costs on the process. In this paper, cooling costs were fully removed by an endothermic reaction, and better temperature control was achieved in the reactor. Also, the temperature profile in the reactor significantly decreased along the reactor length. Another advantage of better control of the reactor temperature is the delayed deactivation of the catalysts. OTCFTR mode increased the MEK production and the conversion percentage of 2-butyl alcohol due to the optimization. A slight increase was observed in output temperature compared to the TCFTR mode, which is acceptable.
4.4.3. Analysis of Conversion Percentage of Raw Materials along the Reactor Length
In this section, the conversion percentage of hydrogen and carbon monoxide is analyzed along the reactor length in different modes (Figures 24 and 26). Compared to CFTR, TCFTR, and OTCFTR in addition to higher MEK production led to a decrease in the conversion percentage of hydrogen. By consuming lower amounts of raw materials (reactants), this method reduced the cost of raw material supply and consequently declined the separation costs of the process.
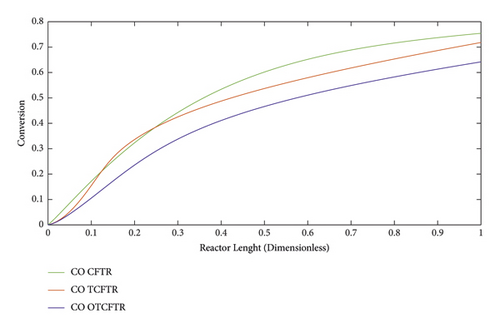
As can be seen, by consuming lower amounts of material due to the smaller conversion percentage of hydrogen, higher product yields were achieved.
In this paper, by minimizing and maximizing various parameters, it was tried to reduce operating costs, consumption of raw materials, and separation costs while enhancing the efficiency of MEK production; and improving this process in the industry.
5. Conclusion
In this work, we presented a novel multifunctional reactor to progress catalytic dehydrogenation reaction of 2-butanol and MEK production. Accordingly, the heat required for thermally coupled reactor and also dehydrogenation of 2-butanol is supplied by the FT reaction through gas to liquid (GTL) method as an exothermic reaction utilizing spherical ZnO catalyst. In this paper, by minimizing and maximizing various parameters, we propose techniques to reduce operating costs, consumption of raw materials, and separation costs while enhancing the efficiency of MEK production, and improving this process in the industry. We employed an evolutionary genetic algorithm to optimize the reactor for the operating conditions of the reaction while maintaining the efficiency and conversion percentage. MATLAB software was also utilized for modeling and optimization. It is worthy to note that the results are separately discussed for aligned flow in three modes of conventional FT reaction (CFTR), thermally coupled FT reaction (TCFTR), and optimized thermally coupled FT reaction (OTCFTR). We found out that after coupling and optimization, the amount of hydrogen increases by 0.022 mol/s. In the OTCFR mode compared to the TCFR mode, the molar flow rate as the feed of the exothermic part was increased by 0.014 mol/s. Notably, the modeling results were verified by industrial data and also indicated a 37% and 55.4% increase in the production rate of thermal coupling and optimal thermal coupling reactors, respectively.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Open Research
Data Availability
Data sharing is not applicable for this manuscript because the results of this research are related to a private company.