Effect of Immobilizing Bacillus megaterium on the Compressive Strength and Water Absorption of Mortar
Abstract
The world’s growing population and industrialization have led to increased construction activities. This has increased the amount of waste aggregates which can be recycled in construction and cut the cost of infrastructure development. This study, therefore, reports the experimental findings for the effect of immobilizing Bacillus megaterium on the compressive strength and water absorption of laboratory prepared test mortar. Bacterial solution used in this work had a concentration of 1.0 × 107 cells/mL. The impact of recycled mortar impregnated with bacteria was studied after curing the specimens in water, saturated lime water, and 1.5% sulfuric acid. Compressive strength for test specimens cured in the three media was determined at the 2nd, 7th, 28th, and 56th day of curing. SEM analysis was done for mortars cured in acidic media and saturated lime water after curing for 28 days. The test results indicated that curing in water and saturated water improved the compressive strength, while the acidic medium lowered it. Recycled mortar is, therefore, an ideal material for immobilizing Bacillus megaterium before introduction into fresh concrete/mortar. The use of recycled mortar is a good strategy to reduce wastes from construction activities, save on the cost of construction materials, and enhance environmental conservation.
1. Introduction
Development of infrastructure is rising everyday all over the world due to increasing population and industrialization. There are many materials for use in structural development, but the most widely used in building and construction is mortar/concrete [1]. Some of the structures where concrete/mortar is used in large volumes include multistorey buildings, waste disposal sewer systems, railway lines, and roads [2]. Mortar/concrete use is extensive since it is relatively inexpensive, readily available, has high compressive strength, and can be conveniently casted into desired shapes and sizes [3, 4]. Concrete structures are, however, faced with tensile stresses. This might be due to tensile loading, plastic shrinkage, errors in design, poor workmanship, and expansive chemical reactions [5, 6]. The tensile stresses lower the strength of the structures, shorten the service life, and expose the concrete to aggressive environmental conditions [5, 7]. Ingress of deleterious ions such as chlorides and sulfates into structures causes premature deterioration, and this also leads to reduction of the service life [8].
A lot of money is used annually to maintain and repair deteriorated mortar/concrete structures with an estimation of $276 billion in the USA [9]. Much efforts in research have been employed to curb the problems faced in construction industry and prolong the service life of the cement-based structures [10–12]. Currently, conventional repair methods which require constant monitoring and repair are being used. They include injection of epoxies, surface coatings, and use of fibers [13]. The use of microbially induced calcite precipitation (MICP) is a major development that has been applied in structural remediation. The MICP process involves the use of microorganisms which induce the precipitation of inorganic calcium carbonate [14]. In cement-based materials, MICP has been widely used in remediation of degraded structures, mechanical performance improvement, and porosity reduction [15–17]. Despite MICP being important in enhancing the service life of cement-based structures, there is a challenge of harsh environmental conditions against the microorganisms. The concrete matrix is dense, has low porosity less than 1%, pore sizes smaller than 1 μm, and a high pH about 12 to 13.5 [18]. In addition to the above conditions, the concrete matrix has limited supply of oxygen and moisture, and these make it hard for microorganisms to remain active long enough [19].
Despite the challenges faced in the use of MICP, several studies have successfully introduced different microorganisms into the mortar/concrete and reported their findings. Jonkers et al. [15] incorporated Bacillus pseudofirmus and B. cohnii directly into mortar specimen during mixing. After 9 days of curing, about 1% of the total spores incorporated was retrieved. The study further reported that as the specimen aged, the number of viable cells decreased. The incorporation of the microorganisms into the cement matrix, however, reduced the compressive strength. Similarly, Erşan et al. [20] reported similar findings. According to the above findings, there is a possible depletion of the nutrients in the cement paste environment which causes hibernation of the microorganisms [21]. The direct incorporation of microorganisms into the harsh environment of cement-based material has led to innovations by researcher to protect them under these conditions.
Despite having decrease in compressive strengths after direct incorporation of microorganisms, there are studies which have reported an increase in the same. Mutitu et al. [1] reported enhancement of compressive strength after direct incorporation of Lysinibacillus sphaericus. Direct incorporation of Bacillus cohnii into fresh paste by Wangui et al. [22] was reported to increase compressive strength of mortar specimens. The two studies above attributed the increase in compressive strength to involvement of the active microorganisms in hydration process forming calcium silicate hydrate which enhances strength development. Research on encapsulation of these microorganisms by immobilizing them on materials that act as covers has been widely conducted, and several materials have been found to provide protection.
Liu et al. [23] immobilized Bacillus pasteurii onto recycled aggregates and compared its crack healing capacity against direct incorporation of the bacteria, bacteria immobilization on diatomaceous earth, and immobilization on expanded perlite. Specimen with bacteria immobilized on recycled aggregates and expanded perlite healed close crack widths of 0.28 mm and 0.32 mm, respectively. Specimen prepared by direct introduction of bacteria had the lowest crack width healing. Alazhari et al. [24] immobilized bacteria spores onto coated expanded perlite and encapsulated nutrients for the bacteria separately. The results obtained showed that 20% substitution of the fine aggregates with expanded perlite carrying the healing agents can enhance self-healing. Bacterial spores have also been immobilized on to diatomaceous earth for self-healing in concrete [16, 17]. The diatomaceous earth immobilized bacteria were reported to have higher ureolytic activity compared to un-immobilized bacteria. Other protective carriers include expanded clay particles [7, 13], hydrogel [25, 26], polyurethane membrane, and silica gels [16, 17]. Other materials that are used include the use of glass, natural fibers, gelatin capsules, paraffin to enclose water, wax, and polyurethane [7, 27, 28]. These methods, however, encounter some challenges, including lowering workability and failure to repair failed concrete/mortar [10, 29].
Previous studies have only reported direct introduction of Bacillus megaterium into mortar/concrete or immobilizing in either recycled aggregate or activated/nonactivated earth. Whereas the recycled aggregate is available in large quantities and thus its viability of use, it is coupled with a lot of variation from one source of production to the other. Recycled mortar is not as variable as recycled aggregate. Recycled mortar provides a closure simulation of the concrete into which the bacteria would be introduced. Further, the recycled mortar was used due to its high compatibility with cementitious materials. The results obtained from this research are intended to provide a method for introducing bacteria that has acclimatized to enhance bacteria activity. The results would also provide an alternative approach of reusing recycled laboratory testing mortar from cement industries. Most cement industries use the laboratory testing mortars as inactive additive to cement. This is despite the use of standard sand, a key laboratory expense in most laboratories. Further, the resultant recycled mortar is standardized in its preparation requirements, thus providing a consistent immobilizing material for bacteria.
2. Materials and Methods
2.1. Materials
Ordinary Portland cement (OPC 42N) and Portland Pozzolana cement (PPC 32.5N) were used in this study. Cement samples were purchased from the local Kenyan market. Standard sand was sourced from Xiamen ISO Standard Sand Company Limited in China and conformed to ISO 679 : 2009 and EN 196-1. Recycled mortar obtained from construction sites in Embu and Kitengela, Kenya, was used. Analytical grade (AR) reagents and chemicals were used in preparation of the media for culturing the bacteria. The chemicals included nutrient broth, calcium lactate, C6H10O6Ca, peptone from casein and other animal proteins, meat extract, agar, sodium hydrogen carbonate (NaHCO3), anhydrous sodium carbonate (Na2CO3), and distilled water.
2.2. Bacillus megaterium Culturing
The Bacillus megaterium microbial solution was prepared using nutrients as per the supplier’s manual. Bacillus megaterium was obtained from the University of Embu Microbiology Laboratory. The liquid medium chosen for culturing the bacteria consisted of 13.00 grams of nutrient broth (5.00 grams of peptone, 5.00 grams of sodium chloride, 1.50 grams yeast extract, and 1.50 grams of HM peptone B) and 3.95 grams of calcium acetate per liter of distilled water. These contents were mixed to obtain liquid medium per stock culture. The mixture was first autoclaved for 15 minutes at a temperature of 121°C for sterilization. This mixture was then cooled to room temperature. After cooling, a 1 M Na-sesquicarbonate solution (1.0 mL in 10.0 mL) prepared by mixing 4.2 grams NaHCO3 with 5.3 grams anhydrous Na2CO3 and made up to 1 liter using distilled water was added to the stock culture to adjust the pH to about 8.5.
The Bacillus megaterium spore powder sample was added to culture media in a laminar flow chamber. These cultures were then incubated on a shaker incubator at 130 shakes per minute maintained at 30°C for 72 hours. An optical density test was conducted using a spectrophotometer set at 600 nm for determining the quantity of culture solution required to mix. The microbial solution concentration was observed using the spectrophotometer. The microbial solution concentration (1 × 107 cells/mL) was maintained throughout the mortar samples preparation.
2.3. Preparation of Protective Carriers and Immobilization of Bacteria
Recycled mortar used in this study had been prepared in accordance to Kenyan standard (KS EAS 2168-1 : 2020) as outlined in Section 2.6. The mortars were basically prepared in the usual manner for laboratory quality control and disposed after testing. The mortars were prepared at Savannah Cement Company in Kenya. The mortars were crushed using a jaw plate crusher and sieved through sieves of different sizes. The particle size of the recycled mortar used was maintained between 500 μm and 1 mm. The recycled mortar was placed in a beaker covered with an aluminum foil and autoclaved for sterilization. For impregnation, 7.5 grams of bacterial solution was weighed using an analytical balance and added to 20 grams of the recycled mortar in a plastic container. The contents were shaken gently to achieve homogeneity. The resulting mixture was oven dried for 24 hours at 35°C. After drying, the recycled mortar was stored in closed plastic containers for 28 days before use in the mortar preparation. In mortar preparation, BE was used during labelling for samples which had the recycled mortar impregnated with bacterial incorporated in them.
2.4. Preparation of Agar Plates
To prepare 1 liter of agar media, 13 grams of nutrient broth and 15 grams of aga-agar powder were weighed on a clean aluminum foil and transferred into a 1-liter conical flask. 1000 mL of water was added to the contents in the flask, and the mixture was stirred to dissolve the broth and agar powder. The flask was then covered, and the media autoclaved for 15 minutes at a temperature of 121°C as per the nutrient broth supplier’s manual. The flask was taken out of the autoclave, and the media was allowed to cool. Approximately, 20 mL of the media was poured into sterile petri dishes, and it was allowed to set.
2.5. Determination of the Presence of Bacteria in Recycled Mortar
This was done through colony counter method on agar plates. 1 gram of recycled mortar impregnated with Bacillus megaterium was weighed into an Eppendorf tube, and 1 mL of nutrient media added. This Eppendorf tube was labelled 1. The tube was shaken for three minutes to achieve proper mixing. Into other five Eppendorf tubes labelled 2 to 6 and placed on a rack, 900 μL of the nutrient media was placed. 100 μL was transferred using a pipette from tube 1 into tube 2 and shaken for mixing. Serial dilution was performed in decreasing order from tube 1 to 6.
2.6. Mortar Preparation and Mortar Prisms Molding
Mortar mix for the prisms was fabricated according to Kenyan standard (KS EAS 2168-1 : 2020) [30]. Compaction molds of 40 mm × 40 mm × 160 mm dimensions were used to cast the test specimens. The water to cement ratio was 1 : 2, while the cement to sand ratio was 1 : 3. Four mortar categories were prepared for both OPC and PPC. First, control mortar prisms were prepared by placing 225 grams of water, 1350 grams of standard sand, and 450 grams of cement in a mixing basin of an automatic programmable mixer. The mixer was allowed to run for four minutes to allow the contents to mix well. This category was cast and cured in water and was labelled as OPC H-H and PPC H-H. The second to fourth categories were prepared by adding 20 grams of recycled mortar impregnated with Bacillus megaterium. The subsequent categories are labelled as provided in Table 1.
| Cement type | Contained encapsulated bacteria incorporated (BE) | Mix media | Curing media | Label |
|---|---|---|---|---|
| OPC | No | Water (H) | Water (H) | OPC (H-H) |
| OPC | No | Bacterial solution (B) | Water (H) | OPC (B-H) |
| OPC | Yes | Water (H) | Water (H) | OPC BE (H-H) |
| OPC | Yes | Water (H) | Saturated lime water (LW) | OPC BE (H-LW) |
| OPC | Yes | Water (H) | Acid (A) | OPC BE (H-A) |
| OPC | Yes | Bacterial solution (B) | Water (H) | OPC BE (B-H) |
| OPC | Yes | Bacterial solution (B) | Saturated lime water (LW) | OPC BE (B-LW) |
| OPC | Yes | Bacterial solution (B) | Acid (A) | OPC BE (B-A) |
| PPC | No | Water (H) | Water (H) | PPC (H-H) |
| PPC | No | Bacterial solution (B) | Water (H) | PPC (B-H) |
| PPC | Yes | Water (H) | Water (H) | PPC BE (H-H) |
| PPC | Yes | Water (H) | Saturated lime water (LW) | PPC BE (H-LW) |
| PPC | Yes | Water (H) | Acid (A) | PPC BE (H-A) |
| PPC | Yes | Bacterial solution (B) | Water (H) | PPC BE (B-H) |
| PPC | Yes | Bacterial solution (B) | Saturated lime water (LW) | PPC BE (B-LW) |
| PPC | Yes | Bacterial solution (B) | Acid (A) | PPC BE (B-A) |
The second category was cast and cured in water and was labelled as OPC BE (H-H) and PPC BE (H-H). The third category was cast in water and cured in saturated lime water and was labelled as OPC BE (H-LW) and PPC BE (H-LW). The last category was cast in water and cured in 1.5% sulfuric acid and was labelled as OPC BE (H-A) and PPC BE (H-A). Categories 2 to 4 were repeated with bacterial solution as the mix media, but the respective curing regimes were maintained. In this case, new labelling was done, OPC BE (B-H) and PPC BE (B-H) for category two, OPC BE (B-LW) and PPC BE (B-LW) for third category, and OPC BE (B-A) and PPC BE (B-A) for the last category. Thereafter, using a trowel, the mortar paste was scooped from the automatic programmable mixing basin and placed in a compaction mold (40 mm × 40 mm × 160 mm dimensions) of a jolting compaction machine. Leveling of the paste was done with a mold trowel in each of the three chambers of the mold after every jolting cycle until a good finish was achieved at the surface. The mold with the mortar paste was then placed in a humid chamber maintained at 95% humidity and 27.0°C for 24 hours. After 24 hours, the obtained mortar prisms were removed from the molds and placed with the 40 × 40 mm side as the base and submerged in basins containing the curing media. The freshly prepared mortars were cured for 2, 7, 28, and 56 days.
2.7. Determination of Compressive Strength
The compressive strength for all the test cement mortars was determined in accordance with the KS EAS 181 (2017). The test was done on the 2nd, 7th, 28th, and 56th day of curing the mortar prisms. Three mortar prisms for each curing regime were picked from the curing station and wiped off any deposits using dry woven cloth. They were then placed on the testing machine after their identities were noted, and a load of 50 N/s was applied vertically on the prism. The obtained halves of prisms were then pulverized and smoothly crushed by load application at 2400 N/s. The average strength of the three prisms were calculated and considered as the final compressive strength. The load was increased smoothly at a constant rate of over the entire mortar prism until fracture.
2.8. Water Sorptivity Determination
3. Results and Discussion
3.1. Bacterial Growth in Recycled Mortar
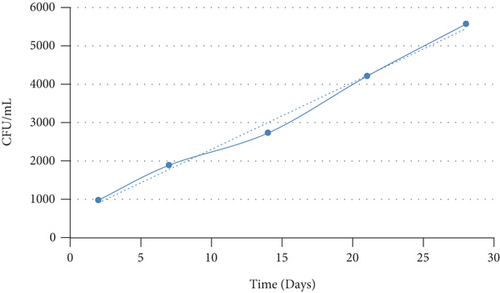
The colonies per plate were counted using the colony counter machine. Triplicates were obtained every time counting was done, and the average values were calculated. The count was done on the 2nd, 7th, 14th, 21st, and 28th day after impregnating the recycled mortar with Bacillus megaterium bacterial solution.
The number of colonies increases from the 2nd to the 28th day as shown in Figure 1. The increase can be attributed to the availability of feed contained in the bacterial solution in which the Bacillus megaterium was cultured. Initially, the initial colonies count on the 2nd day cannot be approximated to be equivalent to the initial concentration of the bacterial solution. This is because after incorporation into the recycled mortar, the bacteria spores have to adapt to the new environment which can cause death of some spores, while other spores tend to hibernate [21]. Once the bacteria adapt to the recycled mortar environment, they start feeding on the available nutrients [14]. As time moves on, the number of viable bacterial cells increase which is subject to decreasing when the nutrients are depleted over time [14, 31].
3.2. Compressive Strength
3.2.1. Compressive Strength for OPC Specimens
The compressive strength results for control test mortar specimens molded using water and Bacillus megaterium bacterial solution as the mix media and cured at different ages of 2, 7, 28, and 56 days are presented in Figure 2. The compressive strengths for all the mortar category specimens together with the control mortar increased up to the 28th day of curing. This was in line with the expectations due to the increase in hydration rate with increase in the curing age.
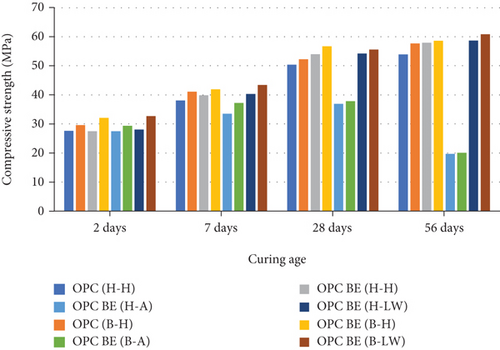
From the findings shown in Figure 2, the compressive strength for OPC with bacteria introduced as mix water (OPC (B-H)) shows a higher compressive strength at early days of curing (2 and 7 days) compared to OPC with encapsulated bacteria (OPC BE (H-H). However, at later days, the compressive strength gain for the latter is higher than the former. The later days’ strength gain could be attributed to inactivity of the bacteria pores in encapsulated mortar whose activity increases as curing progresses as compared to introduction of active bacteria in mix water. Similar finding were obtained by Qureshi et al. [32] and Shahid et al. [33]. Shahid et al. [33] encapsulated nonpathogenic Bacillus strains, i.e., Bacillus subtilis, Bacillus anthracis, and Bacillus pasteurii, into sodium alginate beads. The incorporation of the encapsulated bacteria into concrete cubes was reported to increase the compressive strengths. Lucas et al. [34], also reported 40% increase in the compressive strength of concrete samples prepared by adding bacteria encapsulated in expanded clay. Immobilizing Sporosarcina pasteurii into zeolites also increased the compressive strength of mortar specimens by 10% [9]. The improvement in the compressive strengths can be attributed to the microbial activity of the bacteria used. This is through deposition of CaCO3 within the mortar matrix pores and on the cell surface of the microorganisms [35–37].
The compressive strength of mortar specimens casted with water and cured in 1.5% sulfuric acid, [OPC BE (H-A)], and [OPC BE (B-A)] decreased after the 28th day of curing. The increase in compressive strength up to 28 days of curing can be attributed to formation of more CaCO3 by the encapsulated bacteria. It can also be as a result of the continued hydration with the age of samples which leads to the formation of CH and C-S-H hydration products [38]. This prevented the mortar specimen from adverse effect of the acid and hence the improvement in the compressive strength. Up to the 28th day, there could also have been limited ingress of the sulfuric media into the mortar specimens. On the other hand, decrease in the compressive strength after the 28th day can be as a result of advanced reaction between sulfuric acid and calcium hydroxide. This leads to the formation of secondary gypsum and ettringite as shown in equations (2) and (3). These two products have expansive properties and hence cause deterioration of the mortar and the decrease in the compressive strength.
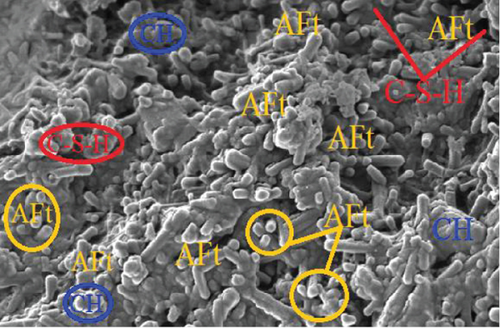
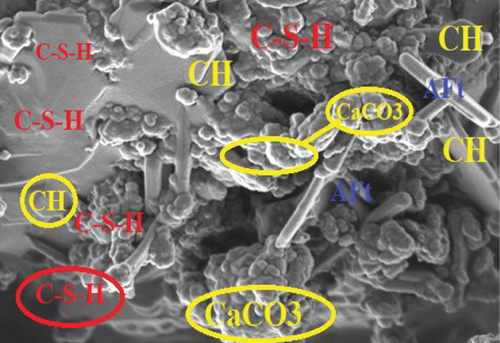
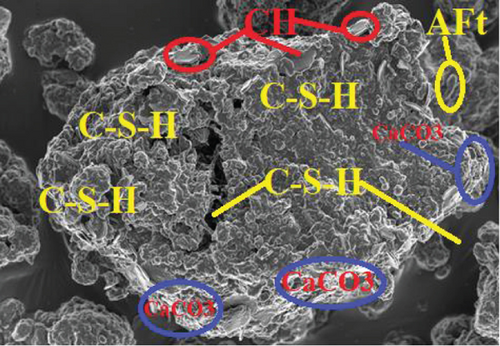
Comparing the compressive strength by mortar prisms in the three-curing media with the control, those in saturated lime water exhibited the highest compressive strength gain. On the 2nd day, all mortar specimen had compressive strength which were almost the same. On the 7th, 28th, and 56th days, the compressive strength varied. This is because at the initial curing period, the hydration rate is increasing with curing period, and therefore, enhancement of the strength is not advanced. The water [OPC BE (H-H) and OPC BE (B-H)] and saturated lime water [OPC BE (H-LW) and OPC BE (B-LW)] cured mortar prisms showed an increase in the compressive strength even after 28 days up to the 56th day of curing.
Incorporating recycled mortar with B. megaterium in the specimens enhanced the compressive strengths of those cured in water and saturated lime water as compared to the control. Curing mortar specimen in sulfuric acid exposed it to harsh environment which deteriorated it and led to the decrease in compressive strength when compared to the control mortar. In wastewater and sewer systems, there are sulfate-oxidizing bacteria which produce sulfuric acid through sulfate metabolization [39, 40]. Examples of these bacteria include Thiobacillus thiooxidans, Thiobacillus neapolitanus, and Thiobacillus intermedius. Therefore, curing the specimens in 1.5% sulfuric acid media mimicked the effect which is caused by exposing structures to wastewater and sewer systems. The decrease in the compressive strength for prisms cured in sulfuric acid can be attributed to internal cracking [41]. The sulfuric acid getting into the mortar first reacts with the calcium hydroxide (CH) to form secondary gypsum (CaSO4.2H2O). The reaction between the secondary gypsum and calcium aluminate hydrate (C3A) leads to the formation of ettringite (3CaO.Al2O3.3CaSO4.32H2O) which has expansive properties and leads to particle disintegration and hence degradation [42]. The use of sulfuric acid in mortar and concrete materials was found to cause degradation depth of up to 0.4 mm after 5 days. Further in the study, De Belie et al. [42] reported that the rate of degradation of cement paste of concrete with inert materials by sulfuric acid is very fast because the acid does not react with the inert materials. This is in agreement with the findings of the current study considering that Bacillus megaterium was immobilized on to recycled mortar which is inert. Sulfate attacks can also be manifested as a progressive loss of strength of the cement paste due to loss of cohesion between the hydration products and loss of adhesion between the hydration products and the aggregate particles in the mortar [43].
3.2.2. Compressive Strength for PPC Specimens
The compressive strength test results for PPC mortar specimens are presented in Figure 4. The obtained compressive strengths obtained for both mortar specimens prepared using water and bacterial solution and cured in three different media vary in a similar manner to those of OPC mortar presented in Figure 2. At 2 days of curing ,all mortars have almost the same compressive strengths. On the 7th day, the specimens cured in water, PPC EN (H-H), and PPC EN (B-H) have a higher strength compared to the other categories. At 28 days, there was a variation in the strengths obtained. Specimens cured in saturated lime water, PPC BE(B-LW) had the highest gain in compressive strength while those cured in 1.5% sulfuric acid, PPC BE(H-A) and [PPC BE(B-A) had the least compressive strengths. The decrease in the compressive strength at 56 days for these two samples can be attributed to the formation of ettringite as shown in equations (2) and (3).
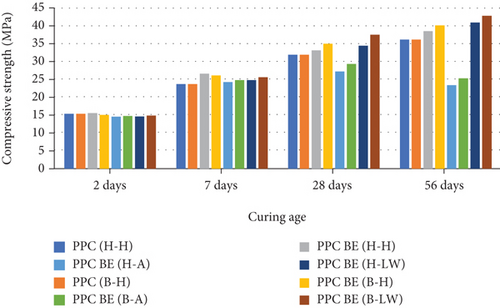
The results presented in Figure 4 show higher compressive strength development for PPC BE (H-H) than PPC (B-H) up to the 7th day of curing. Similar findings were obtained by Wang et al. [16], Wang et al. [17] although investigating the effect of Bacillus sphaericus immobilized on to diatomaceous earth and recycled aggregate [23]. The authors reported that immobilized bacteria develop more activity at later age of curing due to enhanced bacteria growth during the encapsulation process. The encapsulated bacteria will initially take time before enhancing strength at early stages of mortar cubes curing since they are transiting from their pore state to active bacterial state [25, 26, 44].
The bacterial cell wall is negatively charged and acted as a nucleation site for the Ca2+ ions available from calcium acetate used as feed for the bacteria [22]. In this study, Bacillus megaterium improved the compressive strength of both OPC and PPC mortars which could enhance sustainability of cement-based structures for long period.
For all OPC and PPC mortars cured in water and saturated lime water, the compressive strength at 28 days was above the minimum strength of 42.5 MPa and 32.5 MPa, respectively. Ramakrishnan et al. [49] and Siddique et al. [50] reported similar findings in their studies where there was an increase in the compressive strength. Sharma [51] also reported an increase in the compressive strength of soil samples over time after addition of Bacillus pasteurii. The compressive strength improvement by calcite-producing bacteria, Bacillus megaterium, can be attributed to the deposition of CaCO3 on the cell surface of the microorganism. There could also be plugging in of the calcite (CaCO3) within the pores of the mortar matrix. Saturated lime water can also be attributed to the enhanced CaCO3 precipitation in the mortar specimens cured in it. The deposition of calcite helps in filling voids in the mortar matrix, and this increases the strength of the samples [52].
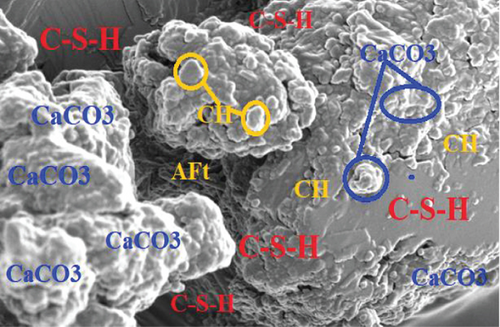
Most of the surface of micrograph (a) is covered by white deposits of calcium carbonate (CaCO3) precipitates. In comparison to micrograph (b), (a) does not have any deposits of ettringite (AFt). The ettringite on micrograph (b) can be attributed to the use of sulfuric acid as the curing media. The high amount of calcium carbonate deposits on (a) can be attributed to the high amount of calcium ions as a result of lime water used as curing media and calcium acetate used as feed for the bacteria. The amount of CaCO3 deposits on micrographs (c) and (d) are not as much as can be observed in (a). From the four micrographs on Figure 3, it can be argued that bacterial activity and maybe carbonation of calcium hydroxide contributed to the deposition of CaCO3 precipitates in micrographs (a), (c), and (d). The presence of CaCO3 in the mortar samples increases the compressive strength as it was discussed in Section 3.2. Similar micrographs have also been reported [53–56]. The authors attributed the precipitation of CaCO3 in cementitious samples to the availability of calcium sources.
3.3. Sorptivity Test Results
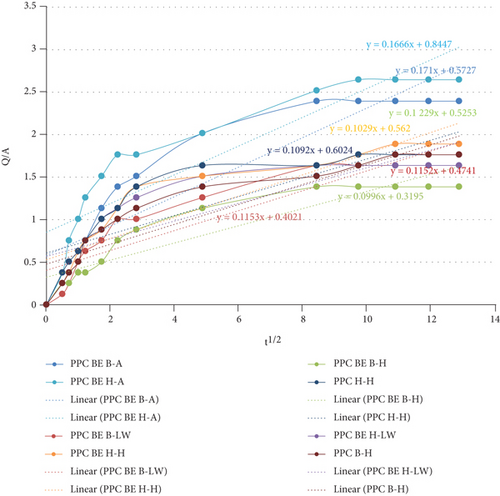
Both OPC and PPC mortar prisms were subjected to water sorptivity test after 28 days of curing for 168 hours. Figure 5 and the graphs show the sorptivity coefficients for OPC and PPC test mortar prisms, respectively. From both figures, it was observed that all mortar prisms had continuous small increase in the amount of water absorbed up to the 96th hour. The amount of water absorbed after this hour remained constant up to the 168th hour. For the OPC mortars in Figure 5, the sample OPC BE (H-A) exhibits the highest water uptake, while the sample OPC BE(B-LW) had the lowest uptake. In Figure 6, the sample PPC BE (H-A) shows the highest gain in water sorption, while sample the PPC BE(B-LW) exhibits the lowest uptake. The constant water sorption after the 96th can be attributed to saturation of the mortar prisms with water or sealing of the mortar pores to an extent that no more water could be absorbed.
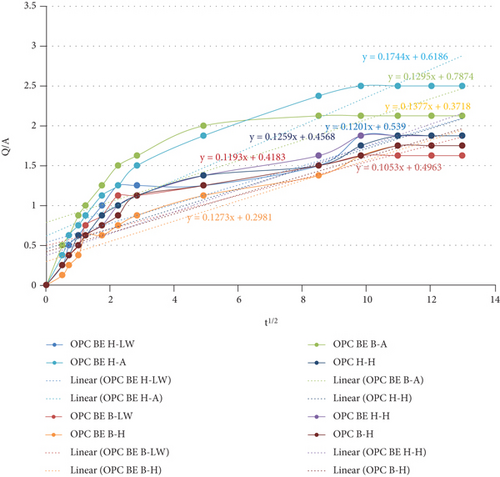
In Figure 5, it can be observed that the water absorption ability of OPC BE (H-H) is high compared to that of OPC (B-H). OPC (H-H) also has a higher absorption than OPC BE (B-H). The lower water absorption ability of OPC (B-H) and OPC BE (B-H) against the other samples can be attributed to the precipitation of calcium carbonate as it can be observed in the SEM micrograph presented in Figure 3(d).
Similar observations were made on the PPC mortar categories. In Figure 6, PPC BE(H-H) and PPC (H-H) had higher water absorption ability as compared to PPC (B-H) and PPC BE(B-H). The cause for lower water absorption can be attributed to CaCO3 precipitation as a result of bacterial addition to the mortar. Other studies have also reported similar result [9, 22, 32, 55]. The authors attributed the decrease in sorptivity to the continuous hydration of the cement particles and improved pore structure reducing capillary suction as a result of calcite precipitation after the bacteria were added. Achal et al. [57] also reported similar findings which he attributed to the deposition of an inert layer of calcium carbonate on the surface and inside the pores. The deposition aids in sealing all the possible channels for passage of air, water, and other pollutants.
Microbial test mortar prisms cured in saturated lime water, samples OPC BE(B-LW) and PPC BE(B-LW) exhibited the lowest water sorption ability. This can be as a result of precipitation of high amounts of calcium carbonate originating from both the bacterial solution [47, 56] and the saturated lime water. Vazinram et al. [58] reported reduction in water absorption as a result of curing concrete specimens in saturated lime water. Acharya and Patro [59] added 7% lime and observed a decrease in the water permeability.
These findings are attributed to the densification of the specimen matrix which reduces the number of pores for holding water particles. The densification can be as a result of calcium carbonate precipitate deposits within the matrix pores [54]. The SEM micrographs presented in Figure 3 show the arrangement of hydration products that are attributed to the ability of the samples to absorb water. Micrograph (a) is dominated by precipitates of calcium carbonate which occupy much of the pore in the mortar matrix and hence reduce the amount of water absorption. Micrograph (b) has ettringite occupying most of the spaces within the hydration products. This opens up more pores within the specimen matrix, and more water is absorbed.
4. Conclusions
- (1)
OPC and PPC with bacteria encapsulated on recycled mortar develop increased compressive strength albeit with time compared to the cement with bacteria introduced directly as mix water
- (2)
PPC with encapsulated bacteria and with additional bacteria as mix water exhibited enhanced compressive strength development at later stages of curing compared to either PPC with encapsulated bacteria or with bacteria introduced as mix water
- (3)
There is a positive relationship between the compressive strength and calcium carbonate precipitation by Bacillus megaterium
- (4)
All OPC and PPC mortars with encapsulated bacteria, prepared with bacteria as mix media and cured using saturated lime water, exhibited the highest compressive strength on the 56th day of curing
- (5)
Exposure of cement-based structures to acidic environment causes degradation and deterioration which lowers the compressive strength and reduces the service life of the structures
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work could not be accomplished without the support offered by various institutions during the experimentation and writing of this paper. The authors wish to acknowledge the institutions for giving access to scholarly repository and library materials, laboratory facilities, and disposables. The institutions include the University of Embu and Savannah Cement Limited, all from Kenya.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.