Chemical Composition, Total Phenolic Content, and Antioxidant Activity of the Essential Oils Extracted from the Needle of Ten Pinus Taxa
Abstract
This study investigated the chemical compositions of essential oils (EOs) from ten taxa belonging to the Pinus genus. The studied taxa that grow wildly in China, and the EOs were extracted by steam distillation; the chemical compositions were isolated and characterized by GC-MS. Eighty-one components, representing over 92.10% of the EOs, were identified. The main constituents of EOs were α-pinene (6.44%–53.00%), β-caryophyllene (2.43%–24.64%), β-pinene (0.00%–22.32%), δ-cadinene (2.56%–17.56%), germacrene D (0.74%–11.38%), and camphene (0.78%–10.48%). Furthermore, we determined the total phenolic content (TPC) of the EOs, with the values from 26.50 to 60.01 mg eq GAE/mL EO. DPPH free radical scavenging activity (DPPH), ferric reducing antioxidant power (FRAP), and ABTS radical cation scavenging activity (ABTS) were used to evaluate the antioxidant capacity of the EOs, and the obtained values were ranged from 499.15 to 1,272.75 mmol eq Trolox/mL EO, 1,255.67 to 3,857.93 mmol eq Trolox/mL EO, and 370.81 to 1,677.19 mmol eq Trolox/mL EO, respectively. The results showed that all of the EOs studied had strong antioxidant activity, and these pine plants could be used as natural antioxidants in functional food and pharmaceutical industries.
1. Introduction
Active oxygen species and nitrogen free radicals are produced during immunization activities and are caused by environmental stress, for instance, sunlight, smoke, and pollution [1]. These active substances can cause harmful effects on DNA, RNA, proteins, and lipids, resulting in cell damage. Antioxidants mop up these harmful free radicals and reduce or prevent the damage they cause. Studies have shown that many plants produce antioxidant compounds that protect cells from oxidative stress and reactive oxygen species degradation during metabolic processes. Therefore, finding and identifying effective natural antioxidants for the food and pharmaceutical industries is significant [2]. At present, essential oils (EOs) as functional components are more and more popularly used in the food, beverage, and cosmetics areas.
EOs are important secondary metabolites in plants, containing various compounds, volatile at room temperature, insoluble in water, and generally extracted by steam distillation [3]. EOs presented more biological properties, such as antioxidant [4, 5], antimicrobial [6, 7], anti-inflammatory [8, 9], and antiproliferative [10, 11] and have been widely used in food [12], agriculture [13], cosmetic [14], and pharmaceutical industry [15]. EOs is highly anticipated as a substitute for synthetic antioxidants, and finding efficient EOs from popular plants is currently a research hotspot.
Pinus species are an essential source of essential oils. It belongs to the Pinaceae, and there are approximately 130 species in the whole genus, mainly distributed in the Northern Hemisphere [16]. According to statistics, there are about 39 species of pine plants in China, of which 23 are native species. So far, there have been many reports on the composition of pine EOs. Ioannou et al. detected 161 volatile organic components in needle extracts of 46 pine species using GC–FID and GC–MS [17]. Jeon and Lee reported chemical compositions of seven pine EOs with high contents of monoterpene hydrocarbons and sesquiterpene [18]. Dambolena et al. reported the EOs composition of Pinus wallichiana, Pinus monticola, and Pinus strobus and analyzed their antifungal and antifumonisin activities [19]. Kurti et al. identified 112 compounds from five Pinus species by HS-SPME-GC-MS and assayed their antioxidant and antimicrobial activities. The results indicated that the components of oxygenated monoterpenes and sesquiterpenes were responsible for the antioxidant and antimicrobial activity [20]. Mitić et al. detected 116 compounds from four Pinus species and compared their antimicrobial and insect larvicidal activity. The study showed that Pinus sylvestris EOs contains higher oxygenated terpenes such as α-murrolol, α-cadinol, and 13-epi-manool oxide, which might significantly contribute to the antimicrobial efficiency [6]. All the results indicated that the EOs of pine needles contain a complex mixture of monoterpenes, sesquiterpenes, diterpenoids, and their derivatives.
The compositions of plant EOs are determined by genotypes and affected by soil, climate, extraction, and analysis methods to a great extent [21]. Although there have been many reports on the composition of pine EOs, little information is available about the constituents and antioxidant activity of EOs from China, such as Pinus bungeana, P. strobus, Pinus densata, Pinus koraiensis, Pinus armandii, Pinus yunnanensis, and Pinus sylvestris var. mongolica. In this research, the chemical constituents and antioxidant activities of EOs from ten Pinus taxa, including P. bungeana, Pinus tabulaeformis var. mukdensis, P. strobus, P. densata, Pinus tabulaeformis, P. koraiensis, P. armandii, Pinus massoniana, P. sylvestris var. mongolica, and P. yunnanensis, were compared. EOs was extracted by steam distillation and identified via GC-MS. Moreover, the antioxidant activities of those EOs were evaluated accurately and systematically by a variety of indicators, such as TPC (total phenolic content), DPPH (DPPH free radical scavenging activity), FRAP (ferric reducing antioxidant power), and ABTS (ABTS radical cation scavenging activity).
2. Materials and Methods
2.1. Plant Materials
The needles of P. bungeana, P. tabulaeformis var. mukdensis, P. strobus, P. densata, P. koraiensis, P. armandii, P. massoniana, P. tabulaeformis, P. yunnanensis, and P. sylvestris var. mongolica were collected from their wild-growing populations in China (Table 1). After drying in a cool place, the needles were cut into 1 cm pieces and extracted by steam distillation for 4 h (150 g samples with 1,500 mL distilled water). The EOs were extracted with ether, dried with sodium sulfate, and recovered by a vacuum rotary evaporator at room temperature. The obtained essential oils were stored at –20°C for later use. The yields were calculated by dividing the volume of the EOs by the dry weight of the samples (% v/w).
| Taxa | Origin | Latitude (°N)/longitude (°E) | Elevation (m) |
|---|---|---|---|
| P. bungeana | Taigu, Shanxi | 37.7372/112.6757 | 793 |
| P. tabulaeformis var. mukdensis | Harbin, Heilongjiang | 45.7240/126.6403 | 159 |
| P. strobus | Yingkou, Liaoning | 40.2059/122.1278 | 11 |
| P. densata | Deqin, Yunnan | 28.5303/98.8233 | 2832 |
| P. koraiensis | Baishan, Jilin | 41.7339/128.1802 | 1313 |
| P. armandii | Liping, Shaanxi | 32.8442/106.6032 | 1287 |
| P. massoniana | Ningde, Fujian | 26.9224/119.1260 | 606 |
| P. tabulaeformis | Taigu, Shanxi | 37.4244/112.5784 | 803 |
| P. yunnanensis | Yuxi, Yunnan | 24.1683/102.3289 | 2018 |
| P. sylvestris var. mongolica | Tahe, Heilongjiang | 52.3204/124.7718 | 445 |
2.2. Identification of the Chemical Components
GC-MS was performed in split injection mode (split ratio 1:20) on a Trace GC-MS system (Trace 1300GC, ISQ MS, Waltham, MA, USA). The capillary column adopted an OM-5MS (30 m × 0.25 mm, 0.25 μm film thicknesses). Helium was carrier gas, and the flow rate was 1 mL/min. Diluted samples (1/500 in dichloromethane, V/V) were injected with 1.0 μL injection volume using Thermo Scientific™ TriPlus™ RSH autosampler (Thermo Fisher Scientific, Milan, Italy). The heating program was set at 50°C for 3 min, then programmed to 260°C at 2°C/min, and subsequently held at 260°C for 5 min. The mass spectrometer operated in EI mode at 70 eV (30–500 amu). Compounds of EOs were identified, comparing the mass spectra fragmentation and RI with those in databases and kinds of literature [17, 22–25]. The relative contents of each compound were determined by the area normalization method.
2.3. TPC
TPCs in EOs were tested by the Folin–Ciocalteu method [26]. Briefly, added 10 µL EOs into 2 mL of Eppendorf test tube and then added deionized water (200 µL) and Folin–Ciocalteu reagent (50 µL) and thoroughly shaken. After 6 min, 500 µL of 7% sodium carbonate and 400 µL of deionized water were added. After mixing, the mixture was subjected to a dark reaction for 90 min and measured absorbance at 760 nm. The control was deionized water, and each treatment was repeated three times. Results showed the value of gallic acid (mg eq GAE/mL EO).
2.4. DPPH
This assay detects the scavenging activity of the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical in the presence of hydrogen donating antioxidants in oil samples. Refer to the method of Brand-Williams et al. [27]. A total of 39.432 mg DPPH dissolved in methanol solution, constant volume to 100 mL, diluted 10 times when used, and with the final concentration of 0.1 mM. Briefly, added 10 µL EOs to 190 µL DPPH methanol solution. We measured the absorbance at 517 nm after 20 min of dark reaction. The control was replaced by methanol with the same volume of essential oil. Each treatment was repeated three times. The results showed micromole Trolox equivalent per milliliter of EO (µmol eq Trolox/mL EO).
2.5. FRAP
Oil samples (reductant) reduce Fe3+-TPTZ to Fe2+-TPTZ, resulting in blue color. Refer to the method of Benzie and Strain [28]. TPTZ (2, 4, 6-tripyridyl-s-triazine) solution is prepared as follows: 0.3 mol/L acetate buffer 25 mL, 10 mmol/L TPTZ working solution 2.5 mL, and 20 mmol/L Fecl3 solution 2.5 mL composition. This assay added 10 μL EOs into 300 μL TPTZ solution, and the mixture reacted at 37°C for 10 min. The absorbents were measured at 593 nm. The same volume of deionized water was used to replace the essential oil. Each processing was repeated three times. The results showed micromole Trolox equivalent per milliliter of essential oil (μmol eq Trolox/mL EO).
2.6. ABTS
The blue-green ABTS cation radical was formed by losing an electron from a nitrogen atom. The nitrogen atom quenches the hydrogen atom in oil samples and decolorizes the solution. Refer to the method of Re et al. [29]. ABTS+ was generated by reacting 5 mL 7 mM ABTS solution and 88 μL 140 mMK2S2O8 and letting this mixture react in the dark for 12 h. The ABTS+ was prepared 1 day in advance and must be used on the same day. Diluted with ethanol to absorbance at 734 nm was 0.70 ± 0.02. Added 10 mL EOs into 990 μL ABTS radical cation to avoid light for 6 min and measured the absorbance at 734 nm. The same volume of deionized water in control replaced the essential oil. Each processing was repeated 3 times, and the results were shown as micromole Trolox equivalent per milliliter of essential oil (μmol eq Trolox/mL EO).
2.7. Statistical Analysis
One-way ANOVA followed by Duncan’s multiple-range test at p < 0.05 was employed to show differences in Pinus taxa essential oil antioxidant by SPSS (ver. 20.0, SPSS Inc., Chicago, USA). Two-tailed Pearsons’ correlation analysis was adopted to examine for correlation between candidate total phenolic content and antioxidant index and between different antioxidant indexes.
3. Results and Discussion
3.1. Chemical Composition
Yields of EOs (yields, v/w, and dry needles) from 10 Pinus taxa were 0.25% to 1.53%. P. koraiensis (1.53%) had the highest yield, while P. densata (0.25%) and P. yunnanensis (0.25%) were the lowest (Table 2). The yields of some taxa were higher than reported earlier. The results of Bo et al. showed that the yield of essential oil from P. sylvestris and P. massoniana were 0.43% and 0.50%, respectively [30]. In our previous study, the yield from P. massoniana, P. tabulaeformis, and P. tabulaeformis var. mukdensis was 0.53%, 0.51%, and 0.47%, respectively (as in this study, materials were collected from different sites) [5]. These differences may be due to different climates, collection time, extraction methods, and other factors [31, 32].
| No. | Compounds | RIa | RI (lit.)b | Relative peak area (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pb | Ptm | Ps | Pd | Pk | Pa | Pm | Pt | Py | Psm | ||||
| 1 | Santene | 882 | 884 | — | tr | 0.11 | — | tr | tr | — | — | — | — |
| 2 | Tricyclene | 921 | 921 | 3.15 | 0.19 | 1.2 | 0.1 | 0.23 | 0.71 | 0.14 | 0.96 | 0.29 | 0.26 |
| 3 | α-Thujene | 925 | 925 | 0.18 | 0.02 | 0.15 | — | tr | tr | tr | 0.26 | tr | 0.11 |
| 4 | α-Pinene | 931 | 932 | 51.57 | 53.00 | 23.47 | 6.44 | 9.36 | 35.46 | 32.71 | 25.11 | 15.84 | 9.36 |
| 5 | Camphene | 952 | 945 | 10.48 | 1.52 | 7.44 | 0.78 | 2.89 | 3.82 | 2.27 | 3.74 | 1 | 1.48 |
| 6 | Sabinene | 968 | 970 | tr | — | 0.15 | — | — | tr | — | 0.8 | — | 0.99 |
| 7 | β-Pinene | 978 | 978 | 6.27 | 22.32 | 1.88 | 0.24 | 2.25 | 4.32 | 11.2 | 8.28 | 2.02 | — |
| 8 | Myrcene | 982 | 982 | 2.36 | 0.29 | 3.07 | 0.11 | 1.79 | 1.38 | 0.4 | 2.17 | 1.06 | 0.24 |
| 9 | α-Phellandrene | 1,000 | 1,003 | tr | tr | 0.25 | tr | 0.67 | tr | tr | tr | tr | tr |
| 10 | δ-3-Carene | 1,007 | 1,008 | — | — | 4.53 | tr | tr | — | — | tr | 2.22 | 5.49 |
| 11 | α-Terpinene | 1,015 | 1,014 | 0.1 | tr | 0.38 | tr | 0.42 | tr | tr | 0.13 | tr | tr |
| 12 | p-Cymene | 1,020 | 1,022 | tr | tr | 0.13 | tr | 0.21 | tr | tr | tr | tr | tr |
| 13 | Limonene | 1,025 | 1,024 | 2.12 | 1.08 | 5.55 | 0.85 | 8.78 | 2.35 | 4.97 | 2.31 | 2.16 | 0.51 |
| 14 | (Z)-β-Ocimene | 1,032 | 1,032 | 0.23 | — | tr | — | tr | tr | 0.01 | tr | — | — |
| 15 | (E)-β-Ocimene | 1,047 | 1,044 | 0.01 | — | tr | — | — | tr | 0.03 | tr | — | 0.1 |
| 16 | γ-Terpinene | 1,056 | 1,055 | 0.16 | tr | 0.18 | tr | 0.12 | tr | 0.05 | 0.22 | tr | 0.1 |
| 17 | Terpinolene | 1,086 | 1,086 | 0.1 | 0.14 | 6.85 | tr | 8.73 | 4.8 | 1.02 | 2.02 | 0.29 | 0.46 |
| 18 | Linalool | 1,098 | 1,095 | — | — | — | — | tr | — | tr | — | — | — |
| 19 | α-Campholenal | 1,122 | 1,122 | — | tr | tr | tr | tr | tr | tr | tr | tr | — |
| 20 | trans-Pinocarveol | 1,135 | 1,135 | tr | 0.21 | tr | tr | — | tr | tr | 0.1 | 0.1 | tr |
| 21 | Camphor | 1,140 | 1,141 | tr | tr | tr | tr | tr | tr | tr | tr | tr | — |
| 22 | Camphene hydrate | 1,145 | 1,145 | tr | tr | tr | — | tr | tr | tr | tr | — | tr |
| 23 | trans-Pinocamphone | 1,158 | 1,158 | tr | tr | — | — | — | — | tr | tr | — | — |
| 24 | Pinocarvone | 1,161 | 1,160 | tr | tr | tr | tr | — | tr | — | tr | tr | — |
| 25 | Borneol | 1,165 | 1,165 | 0.12 | 0.1 | 0.68 | 0.12 | 0.15 | 0.15 | 0.11 | 0.39 | 0.42 | 0.11 |
| 26 | Terpinen-4-ol | 1,174 | 1,177 | tr | tr | tr | tr | tr | tr | tr | 0.13 | tr | tr |
| 27 | α-Terpineol | 1,183 | 1,186 | tr | tr | tr | — | tr | tr | tr | tr | — | — |
| 28 | Myrtenol | 1,195 | 1,194 | — | tr | — | tr | — | — | — | tr | tr | — |
| 29 | Fenchyl acetate | 1,218 | 1,217 | — | tr | — | — | — | — | — | — | — | — |
| 30 | Thymol, methyl ether | 1,132 | 1,232 | — | — | tr | — | 0.1 | tr | — | tr | — | — |
| 31 | Bornyl acetate | 1,287 | 1,285 | tr | 0.56 | 7.36 | 0.19 | 3.03 | 3.08 | 1.86 | 7.67 | 0.63 | 2.25 |
| 32 | trans-Pinocarvyl acetate | 1,298 | 1,297 | — | — | — | — | — | — | — | tr | — | — |
| 33 | Methyl geranate | 1,323 | 1,323 | — | — | — | — | — | tr | — | — | — | — |
| 34 | δ-Elemene | 1,338 | 1,339 | tr | — | tr | tr | 0.11 | — | tr | — | tr | tr |
| 35 | α-Cubebene | 1,345 | 1,345 | 0.2 | 0.24 | 0.33 | 0.28 | 0.76 | 0.11 | 0.25 | tr | 0.26 | 0.61 |
| 36 | α-Ylangene | 1,358 | 1,357 | 0.11 | tr | 0.15 | 0.1 | 0.26 | tr | tr | tr | 0.11 | tr |
| 37 | α-Copaene | 1,366 | 1,366 | 0.3 | 0.18 | 0.54 | 0.34 | 0.77 | 0.33 | 0.14 | 0.25 | 0.32 | 0.62 |
| 38 | β-Bourbonene | 1,370 | 1,369 | 0.11 | 0.1 | tr | tr | tr | tr | tr | 0.15 | 0.17 | 0.14 |
| 39 | β-Cubebene | 1,386 | 1,387 | 0.1 | tr | 0.13 | tr | tr | tr | — | tr | — | — |
| 40 | β-Elemene | 1,388 | 1,389 | tr | tr | tr | 0.22 | tr | 0.14 | 0.68 | 0.59 | 0.94 | 1.52 |
| 41 | β-Caryophyllene | 1,420 | 1,417 | 9.42 | 2.43 | 8.96 | 24.64 | 11.72 | 15.51 | 14.1 | 24.45 | 18.77 | 4.54 |
| 42 | α-trans-Bergamotene | 1,431 | 1,432 | tr | 0.11 | 0.32 | 1.91 | 0.93 | 0.34 | 0.98 | 0.22 | 1.37 | 1.87 |
| 43 | E-Muurola-3,5-diene | 1,450 | 1,451 | — | tr | tr | — | tr | tr | — | — | — | 0.08 |
| 44 | α-Humulene | 1,453 | 1,452 | 1.6 | 0.38 | 1.4 | 5.00 | 2.17 | 2.62 | 2.28 | 4.12 | 3.48 | 0.77 |
| 45 | (E)-β-Farnesene - | 1,455 | 1,455 | tr | 0.1 | 0.19 | 0.45 | 0.56 | 0.17 | 0.18 | 0.1 | 0.6 | 0.9 |
| 46 | γ-Muurolene | 1,478 | 1,478 | 1.87 | 0.42 | 2.62 | 3.01 | 6.02 | 0.64 | 2.27 | 0.31 | 3.58 | 3.05 |
| 47 | Germacrene D | 1,485 | 1,484 | 3.16 | 3.68 | 6.22 | 11.38 | 5.41 | 5.14 | 0.74 | 0.93 | 1.5 | 0.89 |
| 48 | β-Selinene | 1,488 | 1,488 | tr | — | 0.1 | — | — | 0.15 | — | 0.66 | — | — |
| 49 | β-Ionone | 1,490 | 1,491 | — | 0.11 | — | 0.17 | tr | tr | tr | tr | tr | tr |
| 50 | epi-Cubebol | 1,495 | 1,494 | — | — | — | — | 2.84 | — | 2.82 | — | 3.54 | 4.73 |
| 51 | β-Cadinene | 1,496 | 1,495 | 0.48 | 0.87 | 1.69 | 6.39 | — | 3.85 | — | 2.28 | — | — |
| 52 | α-Muurolene | 1,501 | 1,500 | 0.45 | 0.66 | 0.75 | 2.08 | 1.84 | 0.98 | 0.63 | 0.59 | 1.63 | 3.31 |
| 53 | Germacrene A | 1,506 | 1,508 | — | — | — | — | tr | — | tr | — | tr | |
| 54 | γ-Cadinene | 1,512 | 1,513 | 0.98 | 1.77 | 2.52 | 5.14 | 5.59 | 2.34 | 2.66 | 1.53 | 4.63 | 9.04 |
| 55 | δ-Cadinene | 1,525 | 1,522 | 2.56 | 4.82 | 5.67 | 11.07 | 11.19 | 6.29 | 6.36 | 3.97 | 10.39 | 17.56 |
| 56 | trans-Cadina-1,4-diene | 1,534 | 1,533 | 0.12 | 0.1 | 0.24 | 0.32 | 0.52 | 0.16 | 0.15 | tr | 0.3 | 0.5 |
| 57 | α-Cadinene | 1,537 | 1,537 | 0.11 | 0.17 | 0.22 | 1.46 | 1.16 | 0.24 | 0.93 | 0.12 | 1.38 | 1.23 |
| 58 | α-Calacorene | 1,545 | 1,544 | tr | tr | tr | tr | tr | tr | — | 0.2 | tr | 0.1 |
| 59 | Elemol | 1,550 | 1,549 | — | — | tr | tr | tr | tr | tr | tr | tr | tr |
| 60 | (E)-Nerolidol | 1,561 | 1,561 | — | — | — | — | — | — | — | — | 0.13 | 0.1 |
| 61 | β-Calacorene | 1,565 | 1,564 | tr | tr | tr | tr | tr | — | — | — | tr | tr |
| 62 | Germacrene D-4-ol | 1,575 | 1,574 | 0 | — | tr | 0.13 | tr | tr | tr | tr | tr | tr |
| 63 | Spathulenol | 1,580 | 1,578 | — | 0.42 | 0.24 | 1.3 | 0.23 | 0.25 | 0.18 | 0.64 | 1.23 | 4.75 |
| 64 | Caryophyllene oxide | 1,585 | 1,583 | 0.2 | 0.14 | 0.32 | 1.68 | 0.18 | 0.19 | 0.23 | 1.1 | 1.96 | 0.16 |
| 65 | Gleenol | 1,588 | 1,586 | — | — | — | — | tr | — | — | — | — | 0.14 |
| 66 | Viridiflorol | 1,590 | 1,592 | — | — | — | 0.17 | tr | tr | tr | tr | tr | tr |
| 67 | Humulene oxide II | 1,607 | 1,606 | tr | tr | tr | 0.28 | tr | tr | tr | 0.14 | 0.27 | tr |
| 68 | 1, 10-di-epi-Cubenol | 1,618 | 1,618 | tr | 0.12 | 0.17 | 0.26 | 0.26 | 0.15 | tr | 0.12 | 0.26 | 0.52 |
| 69 | epi-α-Muurolol | 1,641 | 1,640 | tr | 0.97 | 0.87 | 3.26 | 1.71 | 1.16 | 0.58 | 0.74 | 3.36 | 5.63 |
| 70 | δ-Cadinol | 1,645 | 1,645 | tr | — | 0.12 | 0.59 | 0.28 | 0.15 | 0.1 | 0.1 | 0.66 | 0.79 |
| 71 | α-Eudesmol | 1,651 | 1,652 | — | — | — | — | tr | — | — | — | — | — |
| 72 | α-Cadinol | 1,655 | 1,654 | tr | 1.06 | 0.57 | 3.97 | 1.66 | 1.07 | 0.83 | 0.69 | 4.08 | 6.8 |
| 73 | α-Bisabalol | 1,689 | 1,690 | — | — | — | — | — | tr | — | — | tr | 0.29 |
| 74 | Pentadecanal | 1,718 | 1,718 | — | — | — | — | — | — | — | — | 1.61 | tr |
| 75 | Octadecane | 1,798 | 1,798 | — | — | — | — | — | — | — | — | 0.23 | — |
| 76 | Cembrene | 1,938 | 1,937 | — | 0.1 | tr | — | tr | tr | — | 0.2 | tr | — |
| 77 | Pimaradiene | 1,946 | 1,943 | tr | tr | tr | tr | tr | tr | 1.48 | tr | — | tr |
| 78 | Neocembrene | 1,958 | 1,960 | — | — | — | — | — | — | 0.44 | — | — | — |
| 79 | Manoyl oxide | 1,994 | 1,993 | — | tr | tr | — | — | tr | — | 0.17 | — | — |
| 80 | Palustradiene | 2,006 | 2,005 | — | — | tr | — | — | — | 1.43 | — | — | — |
| 81 | 13-epi-Manoyl oxide | 2,015 | 2,016 | — | — | — | — | — | — | 0.75 | — | — | — |
| Total | 98.62 | 98.38 | 97.72 | 95.34 | 95.08 | 98.05 | 95.96 | 98.66 | 92.79 | 92.10 | |||
| Monoterpene hydrocarbons | 76.73 | 78.56 | 55.10 | 8.52 | 35.24 | 52.84 | 52.58 | 46.00 | 24.88 | 19.10 | |||
| Oxygenated monoterepenes | 0.12 | 0.31 | 0.68 | 0.12 | 0.25 | 0.15 | 0.11 | 0.62 | 0.52 | 0.11 | |||
| Sesquiterpene hydrocarbons | 21.57 | 15.79 | 32.05 | 73.79 | 49.09 | 39.01 | 32.10 | 40.27 | 50.01 | 46.63 | |||
| Oxygenated sesquiterpenes | 0.20 | 2.71 | 2.29 | 12.55 | 7.05 | 2.97 | 4.74 | 3.53 | 16.36 | 24.01 | |||
| Diterpene hydrocarbons | — | 0.10 | — | — | — | — | 3.35 | 0.20 | — | — | |||
| Oxygenated diterpenes | — | — | — | — | — | — | 0.75 | 0.17 | — | — | |||
| Others | — | 0.91 | 7.60 | 0.36 | 3.45 | 3.08 | 2.33 | 7.87 | 1.02 | 2.25 | |||
| Yield % v/w (mean ± SD) | 1.31 ± 0.25 | 1.02 ± 0.21 | 0.75 ± 0.12 | 0.25 ± 0.06 | 1.53 ± 0.45 | 0.52 ± 0.11 | 0.93 ± 0.18 | 0.81 ± 0.14 | 0.25 ± 0.05 | 1.44 ± 0.51 | |||
- aRetention indices on OM-5MS column. bLiterature values [23–25]. Pb: P. bungeana, Ptm: P. tabulaeformis var. mukdensis, Ps: P. strobus, Pd: P. densata, Pk: P. koraiensis, Pa: P. armandii, Pm: P. massoniana, Pt: P. tabulaeformis, Py: P. yunnanensis, and Psm: P. sylvestris var. mongolica. Components with percentage ≥0.1% are presented. —: not detected and tr: trac.
According to the GC-MS analyses, 81 components were identified, accounting for 92.10–98.66% of EOs (Table 2). The composition content of EOs varied greatly, and the majority of constituents included α-pinene (6.44–53.00%), β-caryophyllene (2.43–26.64%), β-pinene (0–22.32%), δ-cadinene (2.56–17.56%), germacrene D (0.89–11.38%), camphene (0.78–10.48%), γ-cadinene (0.98–9.04%), limonene (0.85–8.78%), terpinolene (0.06–8.73%), bornyl acetate (tr-7.67%), α-cadinol (tr-6.8%), β-cadinene (0–6.39%), γ-muurolene (0.42–6.02%), δ-3-carene (0–5.49%), epi-α-muurolol (tr-5.63%), and α-humulene (0.38–5.00%). From chemical structure, EOs were mainly terpenoids. Monoterpenes (8.52-78.56%) were the main components in 10 Pinus taxa,,followed by sesquiterpenes (15.79–73.79%), sesquiterpenes oxygenated (0.20–24.01%), and other components (0–7.87%). Diterpene hydrocarbons (0–3.35%) were at relatively low amounts for all samples (Table 2). Studies have shown that if the proportion of monoterpenes to sesquiterpenes was more than 50%, the essential oil has economic value [18]. In this study, the proportion of these four components in EOs of 10 taxa was far more than 50%, accounting for more than 89.53%, showing that these taxa have great economic development potential.
As the content and composition of EOs were affected by many factors, different taxa and origins varied greatly. In Table 2, P. bungeana needle oil contained α-pinene in the highest percentage (51.57%), followed by camphene (10.48%) and β-caryophyllene (9.42%), while Jeon and Lee found β-caryophyllene (27.2%), α-pinene (17.90%), and α-Amorphene (14.80%) as main constituents from Korea [18]. P. tabulaeformis var. mukdensis oil was rich in α-pinene (53.00%) and β-pinene (22.32%). However, quite different chemical constituents have been reported for samples collected from Liaoning province, China, with β-caryophyllene (24.08%) and α-pinene (16.55%) [5]. In P. strobus needle oil, α-pinene (23.47%), β-caryophyllene (8.96%), and camphene (7.44%) were significant constituents. α-pinene was also identified as the dominant compound in a needle oil of P. strobus collected from Korea and Poland origin, while Dambolena et al. found β-pinene (29.10%) as main constituents in Argentina origin [18, 19, 33]. The main compounds of the P. koraiensis were β-caryophyllene (11.72%) and δ-cadinene (11.19%), while Sun and Domrachev et al. identified α-pinene (19.38%) and germacrene D (16.01%) as main constituents of oils from Korea and Russia, respectively [22, 34]. P. armandii leaf oil was characterized by a high abundance of α-pinene (35.46%) and β-caryophyllene (15.51%), while the main components of a Greece sample were γ -muurolene (40.7%) and β-caryophyllene (36.3%) [35]. The major constituents of P. massoniana foliage oil were α-pinene (26.9%) and β-caryophyllene (14.1%). Likewise, Ioannou et al. identified α-pinene as primary metabolites and in an equal percentage, followed by germacrene D (20.70%), β-pinene (16.30%), and β-caryophyllene (11.60%) [17]. P. tabulaeformis needle oil was dominated by α-pinene (25.11%) and β-caryophyllene (24.45%). However, Ioannou et al. found that they were rich in β-caryophyllene (15.90%) and germacrene D (14.50%) collected from Spain [17]. P. yunnanensis needle oil was dominated by β-caryophyllene (18.77%), α-pinene (15.84), and δ-cadinene (10.39%). The major constituents of P. sylvestris var. mongolica foliage oil were δ-cadinene (17.56), α-pinene (9.36), and γ-cadinene (9.04). In P. densata needle oil, β-caryophyllene (24.64%), germacrene D (11.38%), and δ-cadinene (11.07%) were the major constituents. As far as we know, this is the first report of the EOs compositions of P. yunnanensis, P. sylvestris var. mongolica, and P. densata.
3.2. Total Phenolic Content
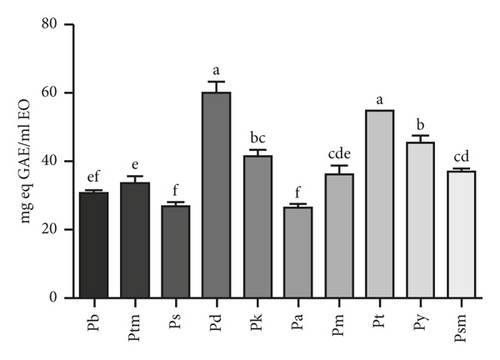
Due to polyphenols can act as electron donors in free radical reactions and directly promoting antioxidant activity, they were usually strongly correlated with antioxidant activity [36]. In this study, TPC was estimated using a Folin–Ciocalteau reagent. The TPCs of EOs studied were 26.50 to 60.01 mg eq GAE/mL EO. P. densata has the highest value of 60.01 mg eq GAE/mL EO, followed by P. tabulaeformis, P. yunnanensis, P. koraiensis, P. sylvestris var. mongolica, P. massoniana, P. tabulaeformis var. mukdensis, P. bungeana, P. strobus, and P. armandii with TPC values of 54.62, 45.47, 41.40, 36.88, 36.35, 33.52, 30.74, 26.83, and 26.50 mg eq GAE/mL EO, respectively (Figure 1). Many studies have revealed that in addition to genotypes, altitude and temperature were primary environmental factors for the content and composition of polyphenols in plants [37–41]. Among them, Kabtni et al. found higher phenol content in plant samples collected at higher elevations [38]. In this study, P. densata and P. yunnanensis had higher collection sites than other taxa, except P. tabulaeformis (Table 1). Besides genotype differences, that may be part of the reasons why the phenolic content of P. densata and P. yunnanensis were higher than the other taxa.
3.3. Antioxidant Activity
Antioxidant activity occurs through multiple mechanisms and requires multiple assayed methods to evaluate [36]. In this work, three methods (DPPH, FRAP, and ABTS) were used to evaluate the antioxidant activity of EOs. The three assays gave very different values in absolute terms (i.e., mmol Eq Trolox/mL EO) but showed the same relative pattern (Table 3).
| Taxa | DPPH (mmol eq Trolox/mLEO) | FRAP (mmol eq Trolox/mL EO) | ABTS (mmol eq Trolox/mL EO) |
|---|---|---|---|
| Pb | 1,065.00 ± 47.98cd | 441.18 ± 13.00e | 1,378.50 ± 137.32fg |
| Ptm | 1,161.66 ± 56.06abc | 418.63 ± 7.56e | 1,741.67 ± 130.30e |
| Ps | 990.31 ± 53.13d | 406.09 ± 3.88e | 1,472.99 ± 53.17f |
| Pd | 1,272.75 ± 21.48a | 1,677.19 ± 104.61a | 3,140.51 ± 89.74b |
| Pk | 1,141.79 ± 73.53c | 1,007.24 ± 83.56c | 2,570.07 ± 109.37c |
| Pa | 499.15 ± 56.76e | 370.81 ± 9.78e | 1,255.67 ± 74.24g |
| Pm | 1,155.45 ± 38.97bc | 564.64 ± 25.82d | 2,403.74 ± 37.92d |
| Pt | 1,263.20 ± 71.51ab | 1,286.11 ± 76.20b | 3,857.93 ± 92.91a |
| Py | 1,128.44 ± 12.61c | 1,007.62 ± 59.50c | 2,501.97 ± 51.06cd |
| Psm | 1,118.03 ± 50.73c | 956.63 ± 55.66c | 1,674.41 ± 15.16e |
- All values are mean ± SD. Different letters within the same columns show significant differences (p < 0.05).
The DPPH assay results ranged from 499.15 to 1,272.75 mmol eq Trolox/mL EO. The highest antioxidant activity was found in P. densata, followed by P. tabulaeformis, P. yunnanensis, P. massoniana, P. koraiensis, P. sylvestris var. mongolica, P. tabulaeformis var. mukdensis, P. bungeana, P. strobus, and P. armandii (Table 3). In results of FRAP assay, the rank order was as follows: P. densata (1,677.19 μmol eq Trolox/mL EO) > P. tabulaeformis > P. yunnanensis > P. koraiensis > P. sylvestris var. mongolica > P. massoniana > P. tabulaeformis var. mukdensis > P. bungeana > P. strobus > P. armandii (370.81 μmol eq Trolox/mL EO). The greatest ABTS radical cation scavenging activity was found in P. tabulaeformis (3,857.93 mmol eq Trolox/mL EO), while the P. armandii had the lowest value (1,255.67 mmol eq GAE/mL EO). According to the average of each taxon, ABTS radical cation scavenging activity rank was P. tabulaeformis > P. densata > P. yunnanensis > P. koraiensis > P. massoniana > P. tabulaeformis var. mukdensis > P. sylvestris var. mongolica > P. strobus > P. bungeana > P. armandii. The results of the ABTS assay had a different order from the DPPH. Among the three methods, DPPH and ABTS were based on scavenging synthetic-free radicals, while FRAP measured the reducing power of an antioxidant [27–29]. Although simple, FRAP was often regarded as the measure of total antioxidant capacity [42, 43]. The consistency of DPPH and FRAP results indicated the high reliability of this study. The stoichiometry reactions between the EOs and the ABTS+ and DPPH. were different, which may be one reason for their different results [44]. Other factors like stereoselectivity of the radicals or the solubility of the EOs in different testing systems may also affect antioxidant activities [45, 46].
Generally, among studied EOs, P. densata (DPPH and FRAP) or P. tabulaeformis (ABTS) owed the highest antioxidant activities, while P. strobus had the lowest. These results may be due to their high levels of β-caryophyllene and caryophyllene oxide [47]. Compared that to some popular plants, the majority of tested EOs owed significantly higher antioxidant capacities than Endlicheria arenosa [48]; Cymbopogon nardus [49]; Stachys inflata, Stachys lavandulifolia, and Stachys byzantine [50]; Coreopsis tinctoria [51]; Hertia cheirifolia [52]; Satureja hortensis [53]; and Aniba parviflora [54]. For centuries, pine needles have been used as a drink and traditional medicine in East Asia, especially in China [55, 56]. This study suggested that pine needles would be good natural antioxidants in functional food and pharmaceutical industries.
3.4. Correlation Analysis
Correlation analysis was carried out on the results of different detection methods (Table 4). There was some correlation between DPPH and ABTS (0.66) at the level of 0.05. Meanwhile, there was a strong correlation between ABTS and FRAP (r = 0.82) at the 0.01 level. However, no correlation was found between DPPH and FRAP in this study. Moreover, the results of TPC were positively correlated with FRAP and ABTS at 0.01 level and DPPH at 0.05 level. These results were consistent with previous studies. The higher the content of plant polyphenols, the better the antioxidant activity [57–59]. Therefore, the total phenolic content of plants can reflect their antioxidant capacity to a certain extent.
| DPPH | FRAP | ABTS | |
|---|---|---|---|
| TPC | 0.68 ∗ | 0.96 ∗∗ | 0.92 ∗∗ |
| DPPH | — | ||
| FRAP | 0.60 | — | |
| ABTS | 0.66 ∗ | 0.82 ∗∗ | — |
- ∗Correlation is significant at the 0.05 level. ∗∗Correlation is significant at the 0.01 level.
4. Conclusions
This study studied the chemical constituents and antioxidant activities of EOs from 10 Pinus taxa. Our results showed that 81 chemical compounds were isolated and characterized by GC-MS in total. The composition content of tested EOs varied greatly, and the majority constituents were α-pinene (6.44–53.00%), β-caryophyllene (2.43–26.64%), β-pinene (0–22.32%), δ-cadinene (2.56–17.56%), germacrene D (0.89–11.38%), camphene (0.78–10.48%), γ-cadinene (0.98–9.04%), limonene (0.85–8.78%), terpinolene (0.06–8.73%), bornyl acetate (tr-7.67%), α-cadinol (tr-6.8%), β-cadinene (0–6.39%), γ-muurolene (0.42–6.02%), δ-3-carene (0–5.49%), epi-α-muurolol (tr-5.63%), and α-humulene (0.38–5.00%). P. densata owed the highest phenolic content and antioxidant activity due to its higher collection sites than other taxa. According to correlation analysis, there has a significant and positive correlation between phenolic content and antioxidant activity, which suggests that the content of polyphenols in plants can represent their antioxidant activity. Worthy of note, in our work, all EOs studied had strong antioxidant activities, suggesting that these pine plants would be potential sources in functional food, pharmaceutical, and cosmetics.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors are grateful for the external support from the experimental teaching center, Shanxi Agricultural University, Taigu, China. The authors also appreciate the generosity of Tengfei Wang and Yilin Yao during experimentation. The Starting Fund for Doctoral Research of Shanxi Agricultural University (grant nos. 2015YJ15 and 2015YJ16) funded this research.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.