[Retracted] Degree-Based Molecular Descriptors of Guar Gum and Its Chemical Derivatives
Abstract
The most abundant polycarbonates that are found in food are polysaccharides. A long chain of monosaccharide with glycosidic linkages forms polymeric carbohydrates. These carbohydrates with water in the process of hydrolysis produces sugar monosaccharides or oligosaccharides. The examples of polysaccharides include starch, galactogen, and glycogen. They contribute various applications mainly in food storage, pharmaceutical industry, and petroleum extraction. In this work, a polysaccharide known as guar gum is studied and also ten degree-based topological indices, namely, Zagreb indices, Randic index, general Randic index, forgotten index, ABC index, GA index, GH index, Sombor index, and SS index are computed. The chemical derivatives of guar gum such as HPG, CMG, and CMHPG are studied, and topological indices are determined. Finally, numerical and graphical comparison of all the above said ten indices are made for guar gum and its chemical derivatives.
1. Introduction
The cells in living organisms produce natural polymers called biopolymers. They possess monomers, reacts with other monomers to form a larger polymer chain. Biopolymers based on the monomers used are classified into three types such as, polynucleotides, polypeptides, and polysaccharides. Polysaccharides have significant applications in recovery of petroleum in every phase of recovery, from well drilling in wastewater treatment due to its versatile functions that include thickening, cross linking, and adsorption. The fluid properties are corrected using biopolymers as additives, helps in cost of petroleum recovery, and enhances the efficiency. A type of hydrocarbon fluid that is extracted from the oil well is petroleum. The oil wells are drilled with deepness of 20 000 feet to extract petrol in the crude form. The process of oil production has various stages starting from the primary stage where the early stage in production is driven by fluid and rock expansion and gravity drainage which results in recovery of 10% of original oil in place. It is followed by variation in the reservoir pressure, and the recovery increases incrementally.
Biopolymers are utilized as additives in the extraction of petroleum. They will enhance the efficiency and safety of the recovery process. The biopolymers such as xanthan gum, scleroglucan, and their products are useful in the drilling and production process as thickness agents while guar gum and its derivatives are used in hydraulic fracture in the transportation of petroleum. The monomeric sugars attached to O-glycosidic linkages form a larger structure resulting in the biopolymer polysaccharide. Prior to the use of biopolymers in the petroleum industry, it was widely used in the food and pharmaceutical industries. As biopolymers have enormous applications, it is extracted or fermented on a large scale as they are cost-effective.
The guar gum, a type of polysaccharide, is also termed as guaran. It is extracted from beans of guar and has been significantly used as a thickening agent in food and industrial applications. It is off-white, free-flowing powder that is dehusked, hydrated, and milled mechanically. The biggest guar producing countries include India, Pakistan, Sudan, and the USA. 80% of world’s guar production is in Rajasthan and India because of its best suitable climatic conditions especially for growing guar seeds.
The guar gum has been extensively used as emulsifier, thickening agent, and stabilizer in the food, beverage, cosmetics, and pharmaceutical industries. Also, it is widely used as hydrofracking liquid in the petroleum industry. The extensive use of guar in the petroleum industry impacts in a large transport volume of guar from India to the USA.
The direct extraction of the guar gum from plant material using fermentation is comparatively cheaper than polysaccharide biopolymers. A span of chemical derivatives of guar gum with modifications have been synthesized, improves the solubility, and rheological properties in hydrofracking fluid.
Hydroxypropyl guar (HPG), carboxymethyl guar (CMG), and carboxymethyl hydroxypropyl guar (CMHPG) are the chemical derivatives synthesized using guar gum. HPG is synthesized using isopropyl alcohol, propylene oxide, and guar gum while CMG uses two-step reactions with sodium hydroxide with guar gum to form alkoxides and CMHPG is formed with the reaction of guar alkoxides and chloroacetic acid [1].
In chemical graph theory, a mathematical formula that can be applied to any graph refers to a topological index or numerical invariant [2–6]. In the literature, various topological indices discussed based on degree and its neighborhood degree for numerous graph structures helps in analysing physicochemical properties of a molecule [7–9]. This tool is very efficient as it is cost-effective and requires less time [10–14].
In the present scenario, numerical invariants play a remarkable role in the investigation of physicochemical properties and biological activities of chemical compounds through quantitative structure-activity relationship/quantitative structure-property relationship/quantitative structure-toxicity relationship (QSAR/QSPR/QSTR) studies [15–18].
The drastic growth of the studies on the topological theory widened the thinking of chemists about the chemical behaviour of the compounds by examining its molecular graph. The indices help the chemists to understand its characteristics, which help in the applications related to bioinformatics and proteomics. The study of topological indices help the researchers work on the compounds and its chemical network making it possible to elaborate their inquests related to drugs, medicine, medical research, and experimental science in the QSAR/QSPR analysis [19, 20].
In this work, a polysaccharide known as guar gum and its chemical derivatives HPG, CMG, and CMHPG are modelled as molecular graphs [21, 22]. The topological indices are computed assuming the atoms as vertices and their bonds as edges. Let G = (V, E) be a simple graph with V and E denoting the vertices and edges, respectively. For graph terminologies and notations, refer [23–25].
2. Methodology
Initially, the molecular structure of guar gum and its chemical derivatives are modelled as molecular graphs, and vertex, edge partitions are determined. The popular degree-based topological indices are computed for the above said molecular graphs. Subsequently, graphical comparison of the ten defined indices for the four chemical graphs are made. In this procedure, the methods used are vertex partition, edge partition, and combinatorial computing.
3. Results and Discussions
3.1. Results for the Molecular Graph of Guar gum
From Figures 1 and 2, the details of degrees of vertices and their edges are tabulated in Table 1 for the molecular graph of guar gum.
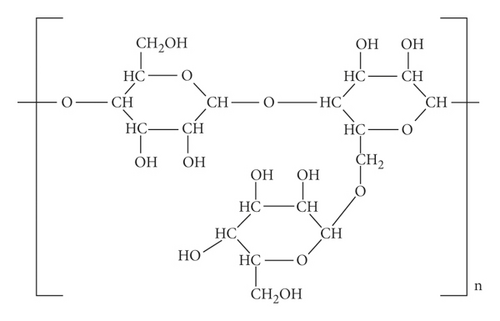
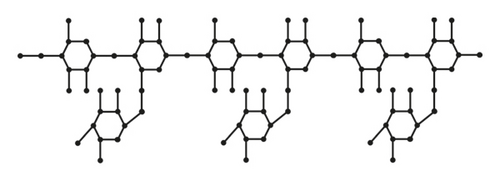
| (dν, dω) where νω ∈ E(G) | No. of edges |
|---|---|
| E1 = (1,2) | 1 |
| E2 = (1,3) | 7n + 1 |
| E3 = (2,2) | 2n |
| E4 = (2,3) | 14n − 1 |
| E5 = (3,3) | 9n |
Theorem 1. Consider a molecular graph G for guar gum, then,
Theorem 2. Consider a molecular graph G for guar gum, then,
Theorem 3. Consider a molecular graph G for guar gum, then,
Theorem 4. Consider a molecular graph G for guar gum, then,
Theorem 5. Consider a molecular graph G for guar gum, then,
Theorem 6. Consider a molecular graph G for guar gum, then,
Theorem 7. Consider a molecular graph G for guar gum, then,
Theorem 8. Consider a molecular graph G for guar gum, then,
Theorem 9. Consider a molecular graph G for guar gum, then,
3.2. Results for the Molecular Graph of HPG and CMG
During the modelling of the chemical derivatives of guar gum such as HPG and CMG into molecular graphs, it was noticed that the vertex and edge partitions were found to be similar, and hence, the results for the abovementioned chemical derivatives are computed together as follows.
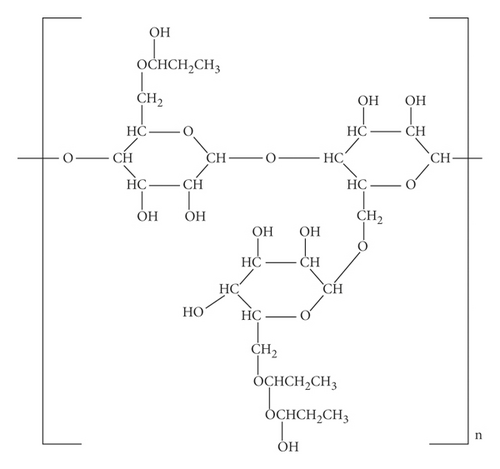
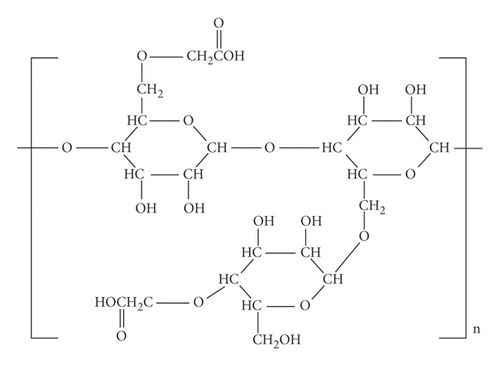
From Figures 3 and 4 the details of degrees of vertices and their edges are tabulated in Table 2 for the molecular graph of HPG and CMG.
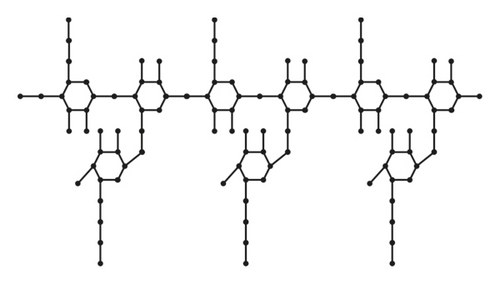
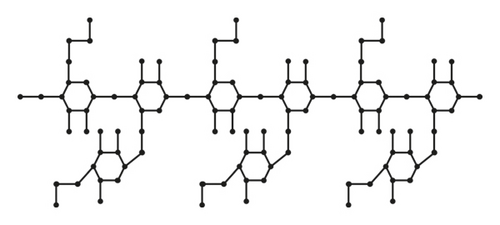
| (dν, dω) where νω ∈ E(G) | No. of edges |
|---|---|
| E1 = (1,2) | 2n + 1 |
| E2 = (1,3) | 7n + 1 |
| E3 = (2,2) | 4n |
| E4 = (2,3) | 14n − 1 |
| E5 = (3,3) | 12n |
Theorem 10. Consider a molecular graph G for hydroxypropyl guar and carboxymethyl guar, then,
Theorem 11. Consider a molecular graph G for hydroxypropyl guar and carboxymethyl guar, then,
Theorem 12. Consider a molecular graph G for hydroxypropyl guar and carboxymethyl guar, then,
Theorem 13. Consider a molecular graph G for hydroxypropyl guar and carboxymethyl guar, then,
Theorem 14. Consider a molecular graph G for hydroxypropyl guar and carboxymethyl guar, then,
Theorem 15. Consider a molecular graph G for hydroxypropyl guar and carboxymethyl guar, then,
Theorem 16. Consider a molecular graph G for hydroxypropyl guar and carboxymethyl guar, then,
Theorem 17. Consider a molecular graph G for hydroxypropyl guar and carboxymethyl guar, then,
Theorem 18. Consider a molecular graph G for hydroxypropyl guar and carboxymethyl guar, then,
3.3. Results for the Molecular Graph of CMHPG
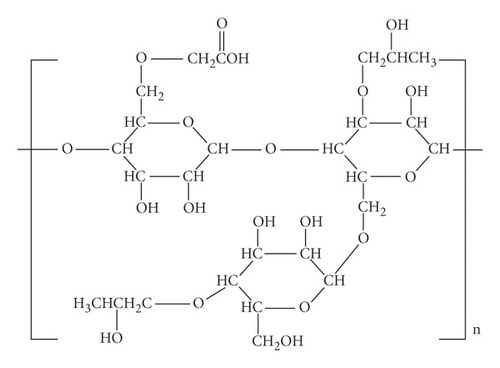
From Figure 5, the details of degrees of vertices and their edges are tabulated in Table 3 for the molecular graph of carboxymethyl hydroxypropyl guar (CMHPG).
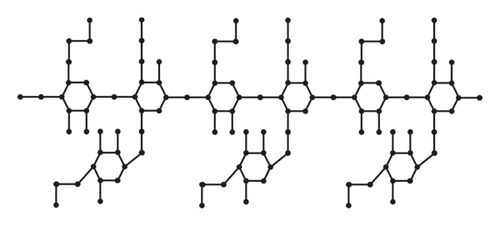
| (dν, dω) where νω ∈ E(G) | No. of edges |
|---|---|
| E1 = (1,2) | 3n + 1 |
| E2 = (1,3) | 6n + 1 |
| E3 = (2,2) | 5n |
| E4 = (2,3) | 15n − 1 |
| E5 = (3,3) | 12n |
Theorem 19. Consider a molecular graph G for carboxymethyl hydroxypropyl guar, then,
Theorem 20. Consider a molecular graph G for carboxymethyl hydroxypropyl guar gum, then,
Theorem 21. Consider a molecular graph G for carboxymethyl hydroxypropyl guar, then,
Theorem 22. Consider a molecular graph G for carboxymethyl hydroxypropyl guar, then,
Theorem 23. Consider a molecular graph G for carboxymethyl hydroxypropyl guar, then,
Theorem 24. Consider a molecular graph G for carboxymethyl hydroxypropyl guar, then,
Theorem 25. Consider a molecular graph G for carboxymethyl hydroxypropyl guar, then,
Theorem 26. Consider a molecular graph G for carboxymethyl hydroxypropyl guar, then,
Theorem 27. Consider a molecular graph G for carboxymethyl hydroxypropyl guar, then,
4. Numerical Comparisons
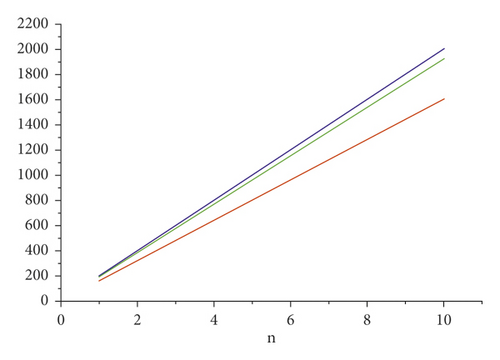
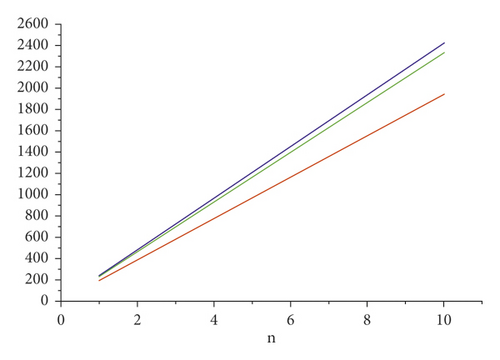
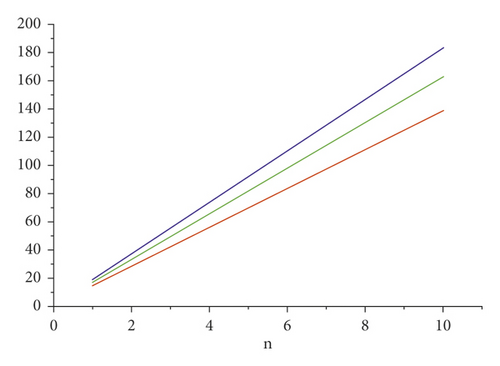
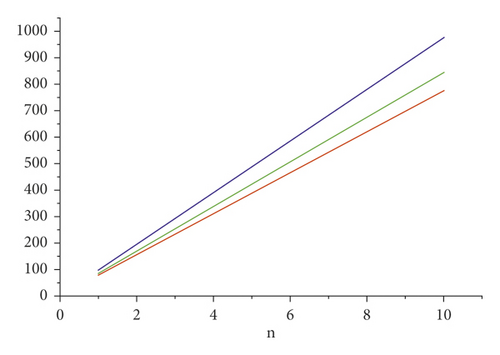
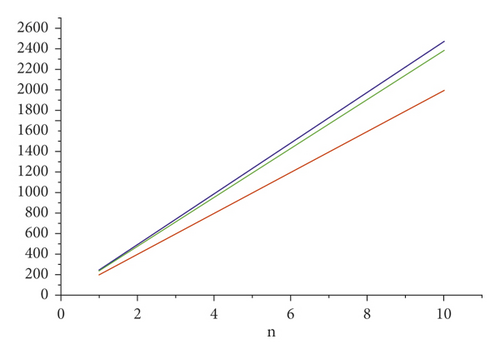
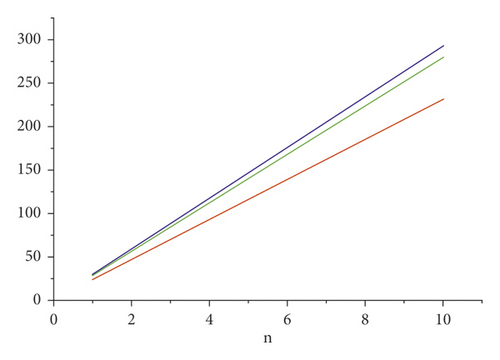
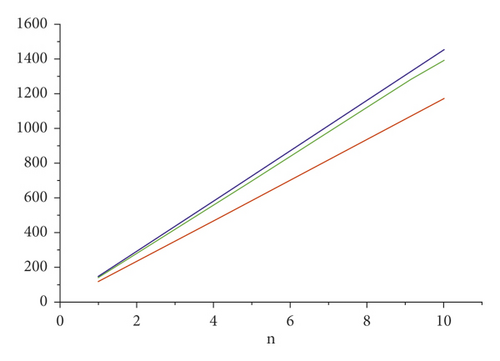
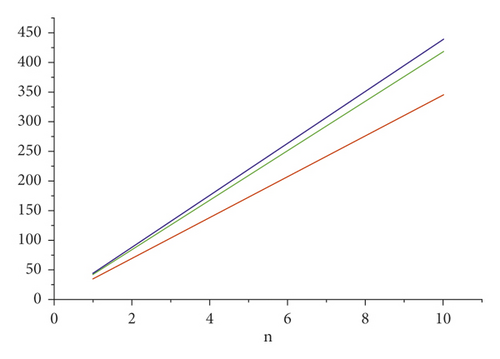
For the comparison of guar gum, HPG and CMG, and CMHPG, indices values are computed for n = 1 to 10 and are as depicted in Tables 4, 5, and 6. It is observed that, by increasing the value of n, the order of values will also increases as shown in Figures 6, 7, 8, 9, and 10.
| n | M1 | M2 | R | RR | F | ABC | GA | GH | SO | SS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 162 | 193 | 14.636 | 78.114 | 432 | 23.846 | 31.61 | 198.442 | 118.243 | 35.008 |
| 2 | 322 | 387 | 28.396 | 155.531 | 862 | 46.876 | 62.39 | 397.422 | 234.693 | 69.429 |
| 3 | 482 | 581 | 42.156 | 232.948 | 1292 | 69.906 | 93.17 | 596.402 | 351.143 | 103.85 |
| 4 | 642 | 775 | 55.916 | 310.365 | 1722 | 92.936 | 123.95 | 795.382 | 467.593 | 138.271 |
| 5 | 802 | 969 | 69.676 | 387.782 | 2152 | 115.966 | 154.73 | 994.362 | 584.043 | 172.692 |
| 6 | 962 | 1163 | 83.436 | 465.199 | 2582 | 138.996 | 185.51 | 1193.3 | 700.493 | 207.113 |
| 7 | 1122 | 1357 | 97.196 | 542.616 | 3012 | 162.026 | 216.29 | 1392.3 | 816.943 | 241.534 |
| 8 | 1282 | 1551 | 110.956 | 620.033 | 3442 | 185.056 | 247.07 | 1591.3 | 933.393 | 275.955 |
| 9 | 1442 | 1745 | 124.716 | 697.45 | 3872 | 208.086 | 277.85 | 1790.3 | 1049.8 | 310.376 |
| 10 | 1602 | 1939 | 138.476 | 774.867 | 4302 | 231.116 | 308.63 | 1989.3 | 1166.3 | 344.797 |
| n | M1 | M2 | R | RR | F | ABC | GA | GH | SO | SS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 194 | 232 | 17.047 | 84.943 | 512 | 28.676 | 38.495 | 237.685 | 140.993 | 42.317 |
| 2 | 386 | 465 | 33.218 | 169.189 | 1022 | 56.536 | 76.16 | 475.908 | 280.193 | 84.047 |
| 3 | 578 | 698 | 49.389 | 253.435 | 1532 | 84.396 | 113.825 | 714.131 | 419.393 | 125.777 |
| 4 | 770 | 931 | 65.56 | 337.681 | 2042 | 112.256 | 151.49 | 952.354 | 558.593 | 167.507 |
| 5 | 962 | 1164 | 81.731 | 421.927 | 2552 | 140.116 | 189.155 | 1190.6 | 697.793 | 209.237 |
| 6 | 1154 | 1397 | 97.902 | 506.173 | 3062 | 167.976 | 226.82 | 1428.8 | 836.993 | 250.967 |
| 7 | 1346 | 1630 | 114.073 | 590.419 | 3572 | 195.836 | 264.485 | 1667 | 976.193 | 292.697 |
| 8 | 1538 | 1863 | 130.244 | 674.665 | 4082 | 223.696 | 302.15 | 1905.2 | 1115.393 | 334.427 |
| 9 | 1730 | 2096 | 146.415 | 758.911 | 4592 | 251.556 | 339.815 | 2143.5 | 1254.593 | 376.157 |
| 10 | 1922 | 2329 | 162.586 | 843.157 | 5102 | 279.416 | 377.48 | 2381.7 | 1393.793 | 417.887 |
| n | M1 | M2 | R | RR | F | ABC | GA | GH | SO | SS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 202 | 241 | 19.085 | 98.074 | 528 | 29.978 | 40.552 | 246.466 | 146.613 | 44.361 |
| 2 | 402 | 483 | 37.294 | 195.451 | 1054 | 59.14 | 80.274 | 493.47 | 291.433 | 88.135 |
| 3 | 602 | 725 | 55.503 | 292.828 | 1580 | 88.302 | 119.996 | 740.474 | 436.253 | 131.909 |
| 4 | 802 | 967 | 73.712 | 390.205 | 2106 | 117.464 | 159.718 | 987.478 | 581.073 | 175.683 |
| 5 | 1002 | 1209 | 91.921 | 487.582 | 2632 | 146.626 | 199.44 | 1234.5 | 725.893 | 219.457 |
| 6 | 1202 | 1451 | 110.13 | 584.959 | 3158 | 175.788 | 239.162 | 1481.5 | 870.713 | 263.231 |
| 7 | 1402 | 1693 | 128.339 | 682.336 | 3684 | 204.95 | 278.884 | 1728.5 | 1015.5 | 307.005 |
| 8 | 1602 | 1935 | 146.548 | 779.713 | 4210 | 234.112 | 318.606 | 1975.5 | 1160.4 | 350.779 |
| 9 | 1802 | 2177 | 164.757 | 877.09 | 4736 | 263.274 | 358.328 | 2222.5 | 1305.2 | 394.553 |
| 10 | 2002 | 2419 | 182.966 | 974.467 | 5262 | 292.436 | 398.05 | 2469.5 | 1450 | 438.327 |


5. Conclusion
This work focusses on the study of polysaccharides known as guar gum and its chemical derivatives HPG, CMG, and CMHPG. Initially, polysaccharides under the study are modelled as molecular graphs, and vertex, edge partitions are determined. Ten degree-based topological indices, namely, Zagreb indices, Randic index, general Randic index, forgotten index, ABC index, GA index, GH index, Sombor index, and SS index are computed for the above said molecular graphs. It is observed that, even though the molecular structure of the derivatives of guar gum HPG and CMG are different, graph models of these structures remain the same with respect to the vertex and edge partitions. The graphical comparisons of four molecular structures for ten degree-based topological indices are made. The polysaccharide is a class of biopolymers which contributes various applications mainly in food storage, pharmaceutical industry, and petroleum extraction. This work benefits many researchers to study in the field of chemistry and pharmacy.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
All authors contributed equally to this study.
Open Research
Data Availability
The data used to support the findings of this study are cited at relevant places within the text as references.




