Plant Growth Promoting and Abiotic Stress Tolerant Chickpea (Cicer arietinum L.) Rhizobial Isolates from Some Areas of South Wollo Zone, Ethiopia
Abstract
Chickpea (Cicer arietinum L) is an important pulse crop prized for its high protein content and is grown as a food source worldwide, including Ethiopia. However, the yield of chickpea is low due to low soil fertility and the ever-changing abiotic stresses. Therefore, this study aimed at isolation, characterization, and selection of chickpea rhizobia effective in their nitrogen fixation and abiotic stress tolerance potential. As a result, 150 nodule samples were collected from three districts of chickpea-producing areas in South Wollo. The nodules were crushed, and the rhizobia were isolated and characterized by using standard microbiological procedures. Based on the presumptive tests conducted, 103 (68.7%) of the rhizobial isolates were tentatively categorized as Rhizobium species. Regarding phosphate solubilization, only 48 (46.7%) solubilized phosphate with a solubilization index ranging from 2.1 to 2.7 mm. Twenty-four (50%) of the isolates were found to be hydrogen cyanide producers. Among the rhizobial isolates tested under greenhouse conditions, 37 (77.1%) of them induced nodulation on their host plant (chickpea). Their symbiotic effectiveness evaluation test confirmed that 16(47.1%), 6(17.6%), 26.47%, and 3(8.8%) were highly effective, effective, low effective, and ineffective, respectively. Of the authenticated rhizobial isolates, 12 (35.5%) of them, including WuCR-15, 16, 17, 18, 19, 20, 23, 30, 31, 32, 36, 38, and 48, accumulated higher shoot dry matter than the positive control. Isolates WuCR- 11, 17, and 36 showed resistance to low and high extreme abiotic stresses of pH, temperature, and salt. Consequently, rhizobial isolates, WuCR- 11, 17, and 36, which were effective and competent in all the tested parameters, were recommended as good rhizobial candidates for applications under greenhouse and field conditions.
1. Introduction
Chickpea (Cicer arietinum L.), a member of the Leguminosae family, is a popular pulse crop recognized for its high protein content [1]. It is a staple basic food crop in many tropical and subtropical Afro-Asian countries, and it is one of the world’s primary pulse crops, typically grown in marginal and semi-arid environments [2]. It is the third most significant pulse crop in the world [3]. Chickpeas were initially cultivated about 7,000 years ago in the Middle East. India is the largest producer of chickpeas worldwide, which accounts for 64% of global production [4]. Apart from India, Australia (12.35%), Myanmar (3.25%), and Ethiopia (2.92%) are the major chickpea-producing countries in the world [5]. Chickpeas are a self-pollinated crop with two cultivars: Desi and Kabuli. The Desi-type cultivar, which accounts for over 85% of global production, is mostly grown in India, Ethiopia, Mexico, and Iran, and the Kabuli chickpea, on the other hand, is grown in Afghanistan, North Africa, Southern Europe, and the United States [6]. Kabuli seeds are larger and cream-colored, with a thin seed coat, whereas Desi seeds are smaller and reddish-brown with a thick seed coat [7].
Ethiopia is Africa’s leading producer of chickpeas and has the largest producing area [8]. It is grown on a total area of 200, 066.05 ha, with an annual production of 2, 538, 713.21 qt [9]. Chickpea is a multi-purpose crop that is widely grown in Ethiopia’s highlands and semiarid regions. The country is also regarded as a secondary hub of chickpea variety. Chickpea is currently grown in four regions of Ethiopia: Amhara; Oromia; the Southern Nations, Nationalities, and Peoples’ Region (SNNPR); and Tigray. The Amhara and Oromia areas contribute 93% of Ethiopia’s total chickpea production, while the SNNPR and Tigray regions produce 3.5% and 3%, respectively [10]. Among the Chickpea producing regions of Ethiopia, North Gonder, South Gonder, North Shoa, East Gojam, South Wollo, North Wollo, West Gojam, and Gonder Zuria are the major ones accounting for over 80% of the country’s chickpea production [11].
Rhizobium is the most common and widely distributed microorganism that can fix N2 on the roots of over 20,000 Fabaceae species [12]. Rhizobia are Gram-negative bacteria that produce root nodules on legume plants and live inside them as intracellular symbionts, turning ambient nitrogen into ammonia for assimilation by the plant in return for plant-derived organic acids [13, 14]. Because of chemical communication that induces the production of specialized structures, such as nodules in which bacteria are harbored, the Legume Rhizobium symbiosis is dependent on the specialization of plant and bacterial species. Mesorhizobium ciceri and Mesorhizobium mediterranean have been found as rhizobia that particularly nodulate chickpea [15]. Sinorhizobium medicae has been discovered to grow nodules on chickpea more recently, although this symbiosis is unsuccessful [16].
Rhizobia can boost plant phosphorus nutrient usage by mobilizing inorganic and organic phosphates, in addition to their positive nitrogen-fixing action with legumes. Phosphorus is always strongly bound with aluminum, iron, calcium, and magnesium in acid or alkaline soils, forming insoluble compounds or sparingly soluble phosphates that are unavailable for plant absorption [17]. Phosphate-solubilizing Rhizobia could create organic acids and enzymes that convert sparingly soluble phosphates into compounds that plants could easily absorb, improving the status of the available phosphorus in the soil [18]. The application of nitrogen-fixing and phosphate-solubilizing microorganisms could reduce the usage of chemical fertilizers and subterranean water pollution, improve the soil’s ecological environment, and boost crop production and quality [19].
South Wollo zone is one of the major chickpea growing areas in Ethiopia. Although chickpea is grown widely, only a few studies have been done about symbiotic effectiveness and characterization of rhizobia isolated from chickpea [20, 21] in the area. Moreover, Ref. [22] conducted rhizobial isolation and characterization phenotypically and symbiotically. However, they are limited to some areas, only indicating that there is insufficient information about rhizobia-chickpea symbiosis and their characterization under laboratory and greenhouse. Therefore, the main aim of this study was to isolate, characterize, and select chickpea rhizobia effective in their nitrogen fixation and abiotic stress tolerance potential.
Moreover, the study was also aimed at characterizing the rhizobial isolates for having phosphate solubilization and hydrogen cyanide production properties.
2. Materials and Methods
2.1. Study Sites and Nodule Sample Collection
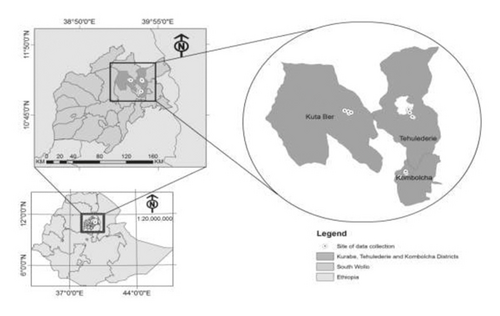
The study sites of this research comprise three districts of South Wollo, including Kutaber, Kombolcha, and Tehulederie (Figure 1) that were known as major chickpea growing areas with no previous history of chickpea rhizobial inoculation. Nodules that looked pink or red in color were collected randomly from the selected Chick pea grown farms in the study areas [23]. The collected nodules were placed inside vials containing a desiccant (Silica gel) and brought to Wollo University Biology laboratory at 4°C until isolation was conducted.
2.2. Isolation of Chickpea Rhizobium Species
The dehydrated or desiccated root nodules were surface sterilized using 70% ethanol for 10 seconds, followed by a 3% (v/v) solution of sodium hypochlorite for 4 minutes after being immersed in sterile distilled water overnight using Petri-dishes with labels. The surface-sterilized nodules were then rinsed in sterile distilled water five times to completely remove the sterilizing chemicals [24]. The nodules were then transferred into sterile Petri-dishes and crushed in a drop of normal saline solution (0.85% NaCl) inside a laminar airflow hood with an alcohol-flamed sterile glass rod [23]. Finally, 0.1 mL (loop-full) of each suspension was streaked onto a Congo red Yeast Extract Mannitol Agar (YEMA-CR) plate and incubated at 28 ± 2°C for 3–4 days. Isolates that did not absorb the congo red were selected for future purification work.
2.3. Purification of Isolates
A single, well-separated rhizobial colony was selected and placed into 6 mL of sterilized yeast extract mannitol broth by using a sterile inoculating loop [23]. The test tubes were then vortexed and shaken for 48 hours at room temperature on a rotary shaker. After two days, a loop of culture suspensions from each test tube was taken and streaked on sterile yeast extract mannitol agar (YEMA) and cultured at 28 ± 2°C for 3–4 days. The colony’s purity and consistency were rigorously checked by repeated re-streaking.
2.4. Preservation of the Isolates
After repeated purification, a single well-isolated colony was selected and streaked into a YEMA slant containing 0.3% (w/v) CaCO3 in a culture test tube, where it was cultured for 3–4 days at 28 ± 2°C [24]. The culture slants were moved and kept inside a refrigerator set at 40°C once sufficient growth was noticed. For future investigation, the pure cultures were kept at −20°C in an Eppendorf tube containing 20% glycerol.
2.5. Presumptive Test of the Isolates
According to Ref. [23], the isolates’ growth was measured using Peptone Glucose Agar (PGA), Gram staining, and YEMA-CR media.
2.5.1. Congo Red Absorption
Congo red stock solution was prepared by dissolving 0.25 g Congo red in 100 mL sterilized distilled water. Ten (10) milliliters of Congo red was added to a liter of YEMA media and sterilized. Finally, a loop full of test isolates was streaked on the medium, covered with aluminum foil, and incubated at 28 ± 2°C for 3–4 days. The absorption of Congo red by the rhizobial isolates, which indicated contaminant growth, was recorded [24].
2.5.2. PEPTONE-GLUCOSE Test
The growth of the rhizobia on peptone glucose agar was determined by using the protocol described by Ref. [25]. The media composition comprised (g/l): glucose (5), peptone (10), agar (15), bromocresol purple (BCP) (10 mL), and distilled water (1000 mL). The pH was adjusted to pH 7.0-7.1 by using 1N NaOH and HCl. The stock solution was prepared by dissolving 1 g of BCP in 100 mL of ethanol. A loop full of bacterial cells grown in yeast extract mannitol broth (YEMB) was streaked on peptone glucose medium and incubated at 28 ± 2°C for 3–4 days. After incubation, the absence of growth or appearance of poor growth indicated rhizobia.
2.5.3. Gram Reaction Test
The Gram reaction of the rhizobial isolates was determined by using the KOH technique [26]. A loop full of rhizobial isolates grown for 48 h on YEMA was taken and mixed well with 3% KOH on a clean microscope slide. The presence of stringiness that can be raised to 1 cm from the slide indicated Gram-positive rhizobia, whereas the appearance of viscosity property showed Gram-negative rhizobia.
2.6. Phosphate Solubilization
2.7. Morphological Characterization
2.7.1. Colony Morphology
The isolates’ variation in morphological characteristics was determined [25–30]. A loop full of isolates grown for 48 h was inoculated on YEMA media and incubated at 28 ± 2°C for 3–4 days. After incubation, the size, shape, diameter, texture, margin, and pigmentation of the rhizobial colonies were recorded.
2.7.2. Acid/Base Reaction
The ability of the isolates to acid or alkaline in YEMA media amended with bromothymol blue (BTB) (0.025 w/v) was determined (31). A loop of isolates grown for 48 h in YEMB was streaked onto the YEMA- BTB medium and cultured at 28 ± 2°C for 3–4 days. After four days of incubation, the color change of the medium was recorded.
2.8. Authentication of the Isolates and Preliminary Screening of Their Symbiotic Effectiveness in Sand Experiment
The ability of the rhizobial isolates to infect and nodulate on the host plant was carried out under greenhouse conditions by using the desi chickpea variety brought from Sirinka Agricultural Research Centre (23). Pots that were surface sterilized with 95% ethanol and rinsed with 1N sulfuric acid were used for conducting the experiment. Pots of sand were sterilized, of which 3 Kg was added into sterilized pots. Seeds of uniform color, size, and shape were selected. The seeds were then surface sterilized, washed with sterilized distilled water, and placed on sterilized plates containing 0.75(w/v) water agar. They were then cultured for 3–4 days at 28 ± 2°C for germination. Three days after germination, five germinated seeds were dipped into each pot for germination after three days. The seedlings were thinned down to three and inoculated with 1 mL of selected rhizobial grown in YEMB for 72 h. The pots were set up in a randomized design (CRD) with triplicates in a greenhouse with 12 h photoperiods and an average of 25 and 18°C day and night temperature. The sampling includes negative control without treatment and positive control without bacterial inoculation receiving 10 mL of 0.05% KNO3 once a week. All plants were irrigated with 10 mL of N-free medium nutrients once a week (Table 1) and 100 mL of water every two days [29].
| Stock solutions | Chemicals | g/L |
|---|---|---|
| 1 | CaCl2.2H2O | 294.1 |
| 2 | KH2PO4 | 136.1 |
| 3 | FeC6H5O7.3H2O | 6.7 |
| MgSO4.7H2O | 123.3 | |
| K2SO4 | 87.0 | |
| MnSO4.H2O | 0.338 | |
| 4 | H3BO3 | 0.247 |
| ZnSO4.7H2O | 0.288 | |
| CuSO4.5H2O | 0.100 | |
| CoSO4.7H2O | 0.056 | |
| Na2MoO2.2H2O | 0.048 | |
2.9. Relative Effectiveness of the Isolates
2.10. Physiological Characterization of Isolates
2.10.1. Temperature, pH, And Salt Tolerance of the Rhizobial Isolates
The abiotic tolerance of the isolates to extreme temperature, pH, and salt concentrations was determined. The potential of the rhizobial isolates to grow and tolerate several temperature ranges such as 5, 10, 15, 20, 25, 35, 40, 45, and 50°C was evaluated [1]. A loop full of rhizobial isolates grown on YEMA media were streaked and incubated by adjusting at the specific temperature for 3–4 days. The growth and tolerance of the isolates were recorded. Regarding pH tolerance of the isolates, a loop full of the isolates was streaked on YEMA media adjusted at a different pH of 4.0, 4.5, 5.0, 5.5, 8.0, 8.5, 9.0, 9.5, and 10.0 [32, 33]. The pH of the media was adjusted by adding 1N HCl and NaOH to pH 7.0 before autoclaving. Similarly, the salt tolerance of the isolates was determined by inoculating each isolate on YEMA media containing 1–10% NaCl concentrations [23]. In all the physiological tolerance tests, the rhizobial inoculated and streaked plates were incubated at 28 ± 2°C for 3–4 days. After incubation, the growth and tolerance of the isolates to the tested tolerance tests were recorded.
2.11. HCN Production
All isolates were tested for HCN production using a slant YEMA medium inserted with filter paper strips dipped in picric acid and 2% sodium carbonate [34]. The test tubes were sealed by using parafilm to prevent the escape of gases produced. The test tubes were incubated at 28 ± 2°C for 3–5 days. The color change of the filter paper from deep yellow to orange, then to brown, indicated the production of HCN.
2.12. Data Analysis
One-way ANOVA was used to analyze and interpret the recorded data. Duncan’s multiple range test (DMRT) was used to evaluate and contrast the experimental treatments with their controls using SPSS ver. 20. The Pearson correlation coefficient was used to examine the correlation between different characteristics. The dendrogram was constructed by using 28 phenotypic parameters that were recorded as 1 for the presence of growth and 0 for the absence of growth.
3. Result
3.1. Isolation and Purification of Rhizobial Isolates
In this study, chickpea (Cicer arietinum L.) rhizobia were isolated from the root nodule bacteria collected from the three sampling districts of South Wollo, namely, Kutaber, Tehulederie, and Kombolcha. Consequently, among 150 rhizobial isolates streaked on YEMA-CR, 69% (103) did not absorb Congo red, whereas the rest showed absorbance indicating contamination (S1a and S1b). Furthermore, the Gram reaction test conducted by using KOH and the BCP growth test results revealed that 103 (69%) of the rhizobial isolates were tentatively classified as Rhizobium. In addition to these, the isolates changed BTB supplemented YEMA medium into yellow color, indicating their acid production characteristics (S1c). Among the rhizobial isolates [35] taken to the greenhouse for authentication, 37 (77.1%) of them induced nodulation. Concerning cultural characterization of the rhizobial isolates, 35 (72.9%) of the isolates exhibited a milky and mucus-like appearance, while the remaining 13 (27.1%) of the isolates showed a watery and transparent appearance. Moreover, 32 (66.66%) of the isolates formed round shapes, and 16 (33.33%) of the isolates formed flat shapes with an entire circular margin. Among the isolates, 89% and 11% of them exhibited white-milky mucoid and watery-transparent texture with an entire circular shape.
3.2. Symbiotic Effectiveness Test on Sand Culture
The ability of the isolates to induce nodules, accumulate nodule dry weight, shoot dry weight, increase shoot length, and fix nitrogen was presented (Table 2). In the current study, since 37 (71%) of the rhizobial isolates induced nodules on the host plant (chickpea), they were identified as root nodulating chickpea Rhizobium species.
| Isolate code | NN | NDW | SDW | SL | SE | Status |
|---|---|---|---|---|---|---|
| WuCR-1 | 34.67 ± 10.69d-j | 0.15 ± 0.046a-e | 0.84 ± 0.33b-e | 22.00 ± 2.65bc | 20.74 | IE |
| WuCR-2 | 37.00 ± 16.52d-i | 0.12 ± 0.049b-h | 0.69 ± 0.37c-k | 20.33 ± 2.31 b-e | 17.05 | IE |
| WuCR-3 | 26.67 ± 6.03f-k | 0.14 ± 0.030a-f | 0.64 ± 0.14d-l | 18.17 ± 1.26 c-g | 88.94 | HE |
| WuCR-7 | 7.67 ± 3.21lm | 0.04 ± 0.029hi | 0.26 ± 0.04 l | 10.33 ± 0.29 j-l | 35.94 | LE |
| WuCR-8 | 18.67 ± 3.79j-l | 0.07 ± 0.010e-i | 0.30 ± 0.08j-l | 11.50 ± 1.80 i-l | 41.47 | LE |
| WuCR-10 | 18.67 ± 9.61j-l | 0.11 ± 0.067b-h | 0.39 ± 0.03g-l | 16.00 ± 3.61 d-i | 53.46 | E |
| WuCR-11 | 15.33 ± 6.03k-m | 0.12 ± 0.035b-h | 0.38 ± 0.14g-l | 14.17 ± 3.21 f-k | 53 | E |
| WuCR-12 | 12.00 ± 1.73k-m | 0.08 ± 0.015d-i | 0.31 ± 0.02i-l | 11.83 ± 1.53 i-l | 43.32 | LE |
| WuCR-14 | 23.00 ± 4.36g-l | 0.13 ± 0.015a-g | 0.58 ± 0.03d-l | 17.33 ± 1.53 c-h | 80.18 | HE |
| WuCR-15 | 39.67 ± 15.04d-h | 0.13 ± 0.035b-h | 0.78 ± 0.26b-g | 17.33 ± 4.93 c-h | 107.4 | HE |
| WuCR-16 | 27.00 ± 14.11e-k | 0.10 ± 0.068b-h | 0.49 ± 0.21e-l | 15.53 ± 2.34e-j | 67.74 | E |
| WuCR-17 | 59.67 ± 25.50a-c | 0.17 ± 0.081ab | 1.07 ± 0.45bc | 22.17 ± 2.93bc | 148.4 | HE |
| WuCR-18 | 20.00 ± 7.55i-l | 0.11 ± 0.079b-h | 0.81 ± 0.08b-f | 19.33 ± 2.57c-f | 112 | HE |
| WuCR-19 | 37.67 ± 13.87d-i | 0.13 ± 0.010b-g | 0.84 ± 0.22b-e | 20.50 ± 3.04b-e | 116.1 | HE |
| WuCR-20 | 46.00 ± 11.79 cd | 0.13 ± 0.032a-g | 1.12 ± 0.26 b | 21.17 ± 1.61b-d | 154.8 | HE |
| WuCR-21 | 11.33 ± 9.45k-m | 0.06 ± 0.057f-i | 0.35 ± 0.05h-l | 13.67 ± 1.53g-k | 48.85 | LE |
| WuCR-23 | 44.00 ± 6.56c-f | 0.16 ± 0.035a-c | 0.89 ± 0.17b-e | 20.53 ± 0.25b-e | 123.5 | HE |
| WuCR-25 | 12.33 ± 13.32k-m | 0.04 ± 0.044hi | 0.28 ± 0.08 kl | 11.33 ± 3.33i-l | 38.71 | LE |
| WuCR-27 | 7.33 ± 5.13lm | 0.04 ± 0.035hi | 0.25 ± 0.02 l | 11.83 ± 0.58i-l | 35.02 | LE |
| WuCR-30 | 22.00 ± 5.57hi | 0.10 ± 0.020b-h | 0.74 ± 0.10b-h | 18.17 ± 0.76c-g | 101.8 | HE |
| WuCR-31 | 41.67 ± 7.37d-f | 0.14 ± 0.015a-f | 0.81 ± 0.05b-f | 20.17 ± 1.26b-e | 112 | HE |
| WuCR-32 | 40.67 ± 10.79d-g | 0.14 ± 0.031a-f | 0.82 ± 0.16b-f | 17.50 ± 0.50c-h | 112.9 | HE |
| WuCR-33 | 11.00 ± 3.61k-m | 0.05 ± 0.010g-i | 0.24 ± 0.02 l | 10.17 ± 1.61 kl | 33.64 | IE |
| WuCR-34 | 15.00 ± 9.85k-m | 0.12 ± 0.062b-h | 0.30 ± 0.11i-l | 12.83 ± 5.13h-k | 41.94 | LE |
| WuCR-35 | 13.33 ± 1.53k-m | 0.12 ± 0.057b-h | 0.40 ± 0.06f-l | 15.63 ± 1.95e-i | 55.3 | E |
| WuCR-36 | 67.00 ± 8.00a | 0.21 ± 0.040a | 1.70 ± 0.73a | 29.33 ± 3.06a | 234.6 | HE |
| WuCR-37 | 15.00 ± 6.56k-m | 0.08 ± 0.040c-h | 0.34 ± 0.14h-l | 13.10 ± 3.50g-k | 47.47 | LE |
| WuCR-38 | 61.00 ± 5.29ab | 0.16 ± 0.025a-d | 0.95 ± 0.13b-d | 24.50 ± 0.50 b | 130.9 | HE |
| WuCR-39 | 15.67 ± 5.69k-m | 0.15 ± 0.035a-e | 0.41 ± 0.28f-l | 14.23 ± 3.16f-k | 56.22 | E |
| WuCR-40 | 21.00 ± 6.24i-l | 0.11 ± 0.064b-h | 0.48 ± 0.18e-l | 18.33 ± 3.88c-g | 66.36 | E |
| WuCR-41 | 45.00 ± 7.94b-e | 0.14 ± 0.012a-e | 0.71 ± 0.05b-j | 20.50 ± 1.32b-e | 97.7 | HE |
| WuCR-42 | 15.00 ± 8.19k-m | 0.08 ± 0.060c-h | 0.25 ± 0.10 l | 13.33 ± 4.80g-k | 34.56 | LE |
| WuCR-47 | 35.67 ± 9.07d-j | 0.11 ± 0.015b-h | 0.61 ± 0.05d-l | 18.07 ± 1.68c-h | 83.87 | HE |
| WuCR-48 | 43.33 ± 3.79c-f | 0.14 ± 0.036a-f | 0.75 ± 0.16b-h | 17.83 ± 2.25c-h | 103.2 | HE |
| + Cont. | 0.00 ± 0.00 m | 0.00 ± 0.000i | 0.72 ± 0.31b-i | 17.17 ± 5.25c-h | 100 | HE |
| − Cont. | 0.00 ± 0.00 m | 0.00 ± 0.000i | 0.26 ± 0.05 l | 7.27 ± 0.25 l | 35.4 | LE |
- Key : The means followed by the same letters on the same column are not significantly different at P < 0.05. WuCR = Wollo University Chickpea Rhizobia, NN = Nodule Number, NDW= Nodule Dry Weight, SDW = Shoot Dry Weigh, SL = Shoot Length, SE = Symbiotic Effectiveness, HE = Highly Effective, E = Effective, LE = Less Effective, IE, ineffective, + Cont. = positive Control, Cont. = Negative Control.
In this study, the result of the tested symbiotic parameters showed a significant difference at P < 0.01 (Table 2). Regarding nodule number, plants inoculated with the rhizobial isolates Wu-36 and WuCR 25 induced the maximum and minimum nodule numbers of 85 and 1 per plant, respectively. Chickpea plants inoculated by rhizobial isolates WuCR—36, 17, and 38— accumulated the highest consecutive nodule dry weights of 0.21 g, 0.17 g, and 0.16 g, respectively. Likewise, WuCR—36, 20, and 17—inoculated plants accumulated a shoot dry weight of 1.70 g, 1.12 g, and 1.07 g, respectively. Plants inoculated by rhizobial isolate Wu-36 performed better in all, i.e., nodule number, nodule dry weight, and shoot dry weight parameters consistently. The maximum and minimum nodule number, nodule dry weight, and shoot dry weight of this study were 85, 86, and 15 higher than the negative control, respectively. Chickpea plants inoculated with rhizobial isolates Wu-36, 38, and 17 recorded the highest shoot length in order. The shoot dry matter of all the rhizobia-inoculated plants was higher than the negative control, and most of them were also higher than the positive control (S2a and S2b). Among the rhizobial isolates, 47.1%, 17.6%, 26.5%, and 8.8% of the isolates were highly effective, effective, lowly effective, and ineffective, respectively. Isolates Wu-36, 20, and 17 scored the highest relative percent symbiotic effectiveness of 234.6, 154.6, and 148.4%, respectively. The findings indicated that isolate Wu- 36 scored top in all the nitrogen-fixing parameters. In general, 22 (64.7%) of the isolates showed a good performance in their symbiotic properties.
The Pearson correlation coefficient comparison (Table 3) indicated that nodule number, nodule dry weight, shoot dry weight, shoot length, and symbiotic effectiveness showed a strong positive correlation of 0.798, 0.79, 0.747, and 0.692, respectively, at p < 0.01. The correlation result indicates that the parameters taken were important in determining symbiotic effectiveness.
| Correlations | |||||
|---|---|---|---|---|---|
| NN | NDW | SDW | SL | SE | |
| NN | 1 | 0.798 ∗∗∗ | 0.790 ∗∗∗ | 0.747 ∗∗∗ | 0.692 ∗∗ |
| NDW | 0.798 ∗∗∗ | 1 | 0.653 ∗∗ | 0.637 ∗∗ | 0.493 ∗∗ |
| SDW | 0.790 ∗∗ | 0.653 ∗∗ | 1 | 0.839 ∗∗∗ | 0.787 ∗∗∗ |
| SL | 0.747 ∗∗∗ | 0.637 ∗∗ | 0.839 ∗∗∗ | 1 | 0.700 ∗∗ |
| SE | 0.692 ∗∗ | 0.493 ∗∗ | 0.787 ∗∗∗ | 0.700 ∗∗ | 1 |
- ∗∗ Correlation is significant and ∗∗∗ indicates correlation strongly significant at the 0.01 level (2-tailed). NN = Nodule Number, NDW = Nodule Dry Weight, SDW = Shoot Dry Weight, SL= Shoot Length, SE = Symbiotic Effectiveness.
3.3. Phosphate Solubilization and Hydrogen Cyanide (HCN) Production Test
Among the isolates tentatively confirmed as Rhizobium species, 48 (46.6%) solubilized phosphate, of which 24 (50%) of the phosphate-solubilized rhizobial isolates that showed a solubilization index (SI) of 2.1 mm and above were selected (Figure 2).
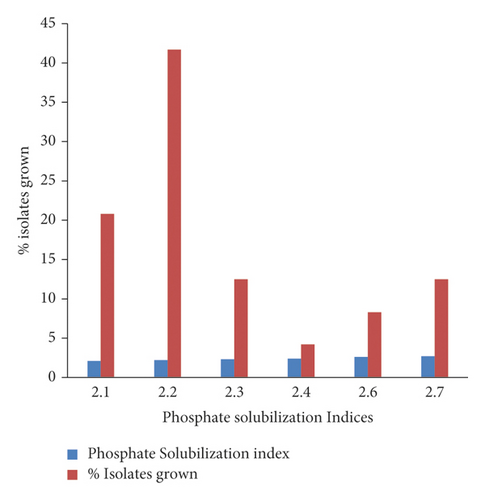
The isolates solubilized phosphate at a PSI ranging 2.1–2.7 mm, where the maximum (2.7 mm) index was recorded by isolates WUCR 5, 10, and 12. Of these isolates, 20.8%, 41.7%, 12.5%, 4.2%, 8.3%, and 12.5% recorded solubilization indices of 2.1, 2.2, 2.3, 2.4, 2.6, and 2.7 mm in order. These findings indicate that rhizobial isolates show variation in their phosphate solubilization efficiency. Regarding HCN production, 13 (27%) of the rhizobial isolates were positive for HCN production. Similarly, the HCN production properties of the rhizobial isolates also revealed the existence of variation among the isolates.
3.4. Physiological Characterization of Isolates
The rhizobial isolates showed variation in their growth and tolerance to different salt concentrations, temperature, and pH ranges (Figure 3–Figure 5).
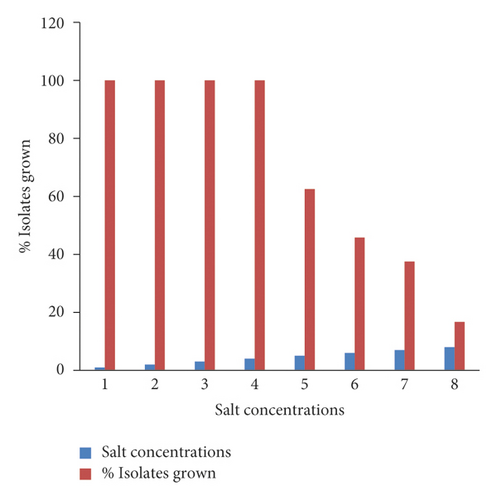
3.5. Salt Tolerance Test
The rhizobial isolates of chickpea showed a wide range of variation in their tolerance to different salt concentrations (Figure 3). All the rhizobial isolates grew at 1–4% salt concentrations. Fifteen (62.5%) and 11 (45.8%) of the isolates grew at salt concentrations of 5 and 6%, respectively, whereas 9 (37.5%) and 4 (16.7%) of them showed tolerance at 7 and 8% salt concentrations, respectively. In this study, the growth of rhizobial isolates reduced as salt concentrations increased. Among all rhizobial isolates, isolates WuCR-3, 6, 7, 10, 17, 23, and 43 grew at all salt concentrations.
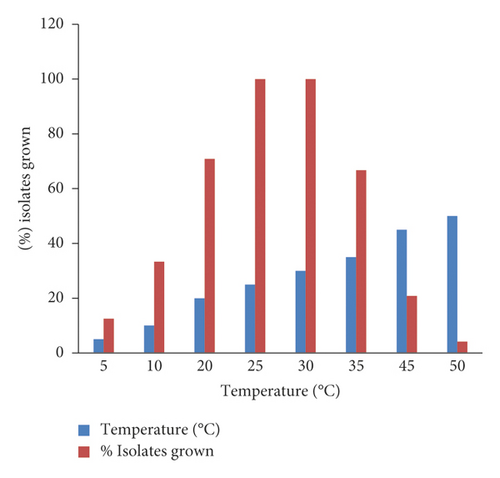
3.6. Temperature Tolerance
The rhizobial isolates of this study showed wide variations in different temperature ranges (Figure 4). All the isolates grew at 25 and 30°C. At lower temperatures of 5 and 10°C, 3 (12.5%) and 8 (33.3%) of the rhizobial isolates showed growth. Likewise, 16 (66.7%), 5 (20.85%), and 1 (4.17%) showed at temperatures of 35, 45 and 50°C, respectively. Among all the rhizobial isolates, only two isolates (WuCR-17 and WuCR-36) grew in all tested temperature ranges (5–50°C). Moreover, only a few isolates grew at extreme temperature ranges.
3.6.1. pH Tolerance
The potential of the isolates to grow and tolerate various pH ranges was presented (Figure 5). All the isolates grew at pH ranges of 7–9 showing good rhizobial colony characteristics. At pH 4 and 5, 5 (20.8%) and 6 (29.2%) of the rhizobial isolates showed growth, whereas 19 (79.2%) of the isolates showed growth at 6 and 10 pH. In this study, most of the rhizobial isolates showed better growth to higher pH than to lower pH. Isolates WuCR-11, 17, 29, 34, 36, and 44 grew at all the tested pH ranges.
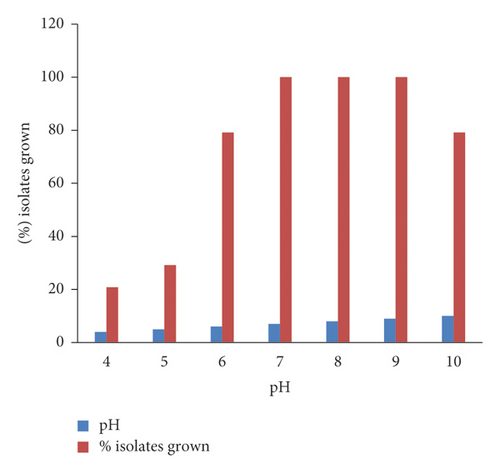
In general, most rhizobial isolates grew and tolerated both lower and upper abiotic stresses. Isolates WuCR-11, 17, and 36 showed the best potential for abiotic tolerance properties, showing growth in all extreme ranges of pH, temperature, and salt concentrations.
4. Discussion
In this study, chickpea (Cicer arietinum L.) root nodule bacteria were collected from three districts of South Wollo, namely, Kutaber, Tehulederie, and Kombolicha. Consequently, 150 bacterial isolates were streaked on YEMA-CR of which 69% (103) did not absorb Congo red. Moreover, the Gram reaction test conducted using KOH, and the PGA result proved that the rhizobial isolates were tentatively categorized as Rhizobium species [25]. Besides, the isolates changed BTB supplemented YEMA medium into yellow color, indicating their acid production characteristics. These same isolates were yellow in color, indicating that they were quick in growth and acid makers, both of which are typical of Rhizobium species [31]. As similarly reported in Ref. [36], the isolates did not show any growth on peptone glucose agar containing BCP, implying that they were Rhizobium species. Among the rhizobial isolates authenticated under greenhouse, 34 (70.8%) of them induced nodulation which is close to the findings of Refs. [37–39], who reported 77% similar cultural characteristics. The remaining isolates failed to form nodules on their host plant which could be due to poor root inoculation or plasmid loss due to long storage in the refrigerator. In general, based on the presumptive tests, cultural characterization, and greenhouse authentication tests conducted, 34 (71%) of the rhizobial isolates were considered as Rhizobium species.
Concerning cultural characterization of the rhizobial isolates, 35 (72.9%) of the isolates exhibited a milky and mucus-like appearance, while the remaining 13 (27.1%) of them showed a watery and transparent appearance. Moreover, 32 (66.66%) of the isolates produced round forms, whereas 16 (33.33%) of the isolates were flat with a complete circular edge. According to Ref. [22], 89% and 11% of isolates had a white-milky mucoid and watery-transparent texture with a full circular form which is nearly similar to the cultural properties of the isolates of the current study.
Out of the culturally characterized and tentatively classed Rhizobium isolates using confirmatory and greenhouse authentication tests, 48 (46.6%) of them solubilized tricalcium phosphate (TCP) adjusted on PVK media. This result coincided with Ref. [22], who reported 42% of TCP solubilizing Chickpea rhizobia. The isolates of this study showed a phosphate solubilization index ranging from 2.1 to 2.7, which is nearly similar to Ref. [39], which reported a maximum solubilization index of 2.85 by chickpea rhizobial isolates indicating that the isolates of the current study can be applied as candidates of phosphate solubilizing rhizobia.
In this study, 34 (71%) of the isolates induced nodules and were authenticated as root-nodulating Rhizobium species, which is low as compared to the findings of Refs. [21, 22, 40], who reported 100% authenticated rhizobial chickpea isolates. Regarding nodule number, the maximum and minimum nodule number was recorded from plants inoculated with isolates Wu-36 and Wu-25 with nodule number of 85 and 1 p/plant, respectively, which is close to the result of Ref. [21] who reported maximum and minimum nodule number of 61 and 2 p/plants, respectively. The difference between the maximum and minimum nodule number, nodule dry weight, and shoot dry weight of this study was 85, 86, and 15, respectively, whereas the study conducted by Refs. [21, 22, 40] reported very less results than the current findings indicating the effectiveness of the study of rhizobial isolates.
In the present study, 47.1%, 17.6%, 26.5%, and 8.8% of the isolates were highly effective, effective, lowly effective, and ineffective, respectively. According to a previous study [41], 14.4%, 59%, and 27% of chickpea rhizobia isolates were very effective, lowly effective, and ineffective, respectively. Furthermore, Ref. [21] found that chickpea rhizobial isolates were 7% and 67% highly effective and effective, respectively, indicating the better performance of isolates of this study. Plants inoculated with rhizobial isolates that scored the highest shoot dry weight were effective in nitrogen fixation and considered as good candidate isolates for developing microbial inoculants for field application. On the other hand, plants inoculated with some rhizobial isolates that induced the least number of nodules were lowly effective and ineffective indicating variation in nodulation potential and symbiotic efficiency, which could be due to differences in their evolutionary link, ecological factors, and genetic diversity of rhizobia and their host plants [42]. All the symbiotic parameters showed a moderately to strongly positive correlation with each other at a significance level of 0.01. However, shoot dry matter is a good indicator of relative effectiveness that describes a sound correlation between the nitrogen-fixing capacity of legumes and their shoot dry matter accumulation [33].
In the present study, chickpea rhizobial isolates showed a wide range of variations in their tolerance to different salt concentrations. The rhizobial isolates of this study grew and tolerated all the tested salt concentrations of 1–8%, which is nearly similar to the findings of Ref. [41], who reported chickpea rhizobial isolates that tolerated up to 8% of NaCl concentrations. Among the isolates, 16.7% of them showed growth at 8% salt concentrations which were better than the findings of Ref. [37], who reported 7.4% of chickpea rhizobia grown at 8% salt concentrations. The investigation of such type of rhizobial isolates that tolerated this high amount of salt concentration is categorized as fast-growing rhizobia and could be used as a remedy in areas having high soil salinity problems [43].
The isolates of this study showed wide variations in growth to different temperature ranges of 5–50°C. Consequently, all 12.5%, 18.75%, 66.67%, 20.85%, and 4.17% of the isolates showed growth to 25–35, 5, 10, 20, 45, and 50°C, which were close to the findings of Ref. [44] who reported chickpea rhizobia that tolerated temperature at a range of 10–42°C. Moreover, Refs. [22, 41] reported 17% and 8% of chickpea rhizobial isolates that grew up to 40°C. Among all the isolates, only two strains (Wu17 and Wu36) were grown in all the tested temperature ranges (5–50°C), implying that these rhizobial isolates are endowed with the potential for resisting high- and low-temperature ranges and can be used for developing rhizobial inoculants serving as biofertilizer for tropical and polar areas with low and high soil temperatures.
Regarding pH tolerance, among the isolates, all 87.5%, 18.75%, and 12.5% of the isolates showed growth at pH 5.5–7.5, 8, 9, and 10, respectively, which is nearly similar to Ref. [45], who reported the tolerance of rhizobial isolates to pH 10 and 11. Similarly, Refs. [41, 44] reported rhizobial chickpea isolates grown at a pH level of 9 that was nearly similar to the current study’s findings. Moreover, 22.9% and 54.2% of them grew at pH 4 and 4.5, respectively, which is close to the findings of Ref. [22], who reported 31% of chickpea rhizobia that tolerated a pH of 4.5. In this study, 12.5% and 22.9% of the isolates showed growth at pH 10 and 4. Likewise, Refs. [21, 22] reported nearly similar findings. In general, isolates of this study exhibited tolerance to lower and higher pH levels, indicating that they can be used as important microbial inoculants in alkaline and acidic soils under greenhouse and field conditions.
The isolates of this study showed variations in different salt concentrations and different pH and temperature ranges which are important parameters used as phenotypic markers [46, 47]. In this study, the investigation of the isolates that tolerated both low and high levels of salinity, pH, and temperature is vital at times of the current climate change and could play an important role in boosting crop productivity by increasing their successful symbiotic association with their host plants [48, 49]. Among the tested rhizobial isolates, 13 (27%) produced HCN that coincided with the findings of Ref. [50], who reported the production of HCN by Mesorhizobium species isolated from chickpea. These isolates can be used as biocontrol agents against fungal pathogens like Fusarium wilt by inhibiting their growth by affecting the respiratory system of the pathogen [51–52].
In conclusion, this study screened rhizobial isolates that were highly effective in nitrogen fixation. Moreover, the study also determined that rhizobial isolates have a high potential for resisting extreme abiotic factors such as salt, pH, and temperature. Isolates WuCR-17 and 36 performed best in all the symbiotic nitrogen fixation and abiotic stress tests. Consequently, these isolates can be recommended as good rhizobial candidates to be applied as biofertilizers as a better option for the unaffordable and eco-friendly chemical fertilizer.
Conflicts of Interest
The authors declare that there exists no conflict of interest.
Acknowledgments
This work is done with a little budget financed by Wollo University. This study was supported by Wollo University. The authors are grateful to Sirinka Research Centre for the chickpea variety supply.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




