[Retracted] Computing the Energy and Estrada Index of Different Molecular Structures
Abstract
Graph energy is an invariant that is derived from the spectrum of the adjacency matrix of a graph. Graph energy is actually the absolute sum of all the eigenvalues of the adjacency matrix of a graph i.e. , and the Estrada index of a graph G is elaborated as , where, λ1, λ2, …, λn are the eigenvalues of the adjacency matrix of a graph. In this paper, energy E(G) and Estrada index EE(G) of different molecular structures are obtained and also established inequalities among the exact and estimated values of energies and Estrada index of TUC4C8 nanosheet and naphthalene.
1. Introduction
Chemical graph theory is a branch of mathematical chemistry and mathematical chemistry gives a natural way to understand the mathematical structures which actually act behind the existing chemical concepts to inaugurate the novel mathematical ideas and mathematical techniques in chemistry. The chemical graph theory is the intersection of graph theory and chemistry. In chemical graph theory, one can pick the molecular structure of different compounds and transform them into a molecular graph and then apply different graph invariants such as the energy of a graph. This graph invariant is much more popular in both mathematicians and chemists and was invented in 1978 by Ivan Gutman [1].
When graph energy was introduced in 1978, no one was attracted to this invariant. Even its chemical roots belong to 1940. Mathematicians were not interested initially but when the 20th century was started, a dramatic change occurred and graph energy got great respect from the whole world when the number of countries such as Pakistan, Australia, Austria, Brazil, Canada, and many more produced numbers of papers in graph energy. The definition of graph energy is motivated by already existing results for the Huckel molecular orbital total π-electron energy [2, 3]. The energy of a graph defined here totally coincides with the total π-electron energy computed using Huckel molecular orbital theory. The energy of a graph will be zero if the graph is totally disconnected.
About 20 years ago Estrada introduced a new index called the Estrada index. The Estrada index is denoted by EE(G) and is defined as [4]. The application of the Estrada index is to measure the folding degree of long-chain proteins [5–8]. To measure the degree of long-chained molecules such as proteins, weighted graphs were used. This index is also used in complex networks to measure the centrality measures of these structures such as social, communication, and metabolic[9–11]. For detailed surveys on the Estrada index, readers can check [4, 12–22].
The motivation of the paper was to find the energy and Estrada index of nanostructures. Matrices are used nowadays for dealing with different types of problems in daily life. We are dealing with square matrices which are very useful in different applications such as in the solving system of equations, by using its determinant to find the area and orthogonal vectors. In this paper, we find the energy of a graph by finding the eigenvalues of its adjacency matrix. Let G be a simple undirected graph in which V denotes the vertex set containing v1, v2, …, vn as atoms and E denotes the edge set e1, e2, …, en as bonds. The adjacency matrix A(G) of a graph G is a square matrix that can be obtained by the adjacent and nonadjacent vertices in such a way if (i, j)- entry of a matrix is 1 whenever the vertices vi and vj are adjacent, otherwise 0. The eigenvalues of the adjacency matrix of order n are λ1, λ2, …, λn and the set of these eigenvalues is called a spectrum of the graph and since the adjacency matrix is symmetric so all the eigenvalues are real.
2. Methodology
- (1)
We draw a chemical structure in HyperChem [23]. HyperChem is a molecular editor, which offers a wide range of molecular and quantum mechanics calculations.
- (2)
The Adjacency matrix is found using TOPOCLUJ [24].
- (3)
By using MATLAB, the eigenvalues of the adjacency matrix are found [25].
- (4)
A polynomial of your desired order is constructed which best fits the data using the cf toolbox [26].
3. Energy and Estrada Index of TUC4C8 Nanosheet
- (1)
Single-walled carbon nanosheets(SWCTs): Single-walled carbon nanosheets consist of single graphene. It does not have any complex structure because of impurity. It bends easily [27].
- (2)
Multiwalled carbon nanosheets(MWCTs): Multiwalled carbon nanosheets consist of multigraphene. It has a complex structure due to its high purity. It cannot be bent easily [28, 29].
Now, we construct the quadratic polynomial which best fits the curve. We will also find the difference between Eexact(T), Eestimated(T), and EEexact(T), EEestimated(T) of TUC4C8 nanosheet 1 as shown in Figure 1.
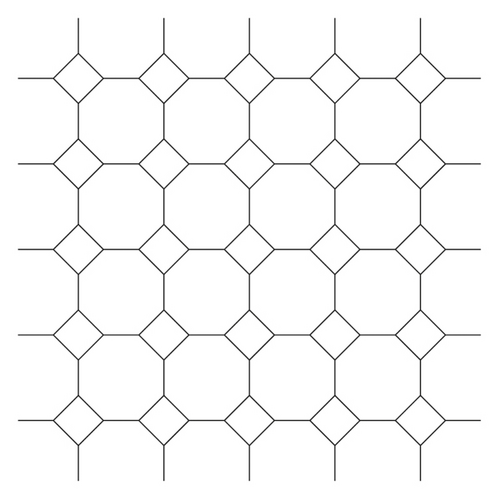
Proposition 1. Let T be the graph of TUC4C8 nanosheet, then the energy E(T) and the Estrada index EE(T) of T are given below:
The equations (1) and (2) characterize the energy E(N) and Estrada index EE(N), respectively. We have established the inequality Eexact(N) > Eestimated(N), where Eexact and Eestimated show the exact and estimated value of energy. This inequality poses that the exact value Eexact is always greater than Eestimated. So, the error among Eexact and Eestimated is always positive.
But on the other hand, the exact value EEexact(N) is always smaller than EEestimated(N) which describes the error between EEexact and EEestimated is negative.
In Table 1, m is an integer from 1 to 10 while n is fixed, and in Tables 2 and 3 quadratic equations are constructed.
| (m, n) | Energy (E(N)) | Estrada index (EE(N)) |
|---|---|---|
| (1, n) | −0.001n2 + 7.741n + 1.9175 | −0.0001n2 + 16.5749n + 3.0418 |
| (2, n) | −0.0011n2 + 13.6178n + 3.7202 | 0.0012n2 + 30.0994n + 6.00914 |
| (3, n) | −0.0012n2 + 19.5378n + 5.5891 | −0.0013n2 + 43.6375n + 9.1248 |
| (4, n) | 0.0053n2 + 25.294n + 7.583 | −0.004n2 + 57.1646n + 12.172 |
| (5, n) | −0.0182n2 + 31.313n + 9.2495 | −0.0105n2 + 70.6945n + 15.215 |
| (6, n) | −0.1515n2 + 38.655n + 9.625 | −0.0316n2 + 84.2794n + 18.1878 |
| (7, n) | −0.5176n2 + 49.0138n + 7.2347 | −0.0853n2 + 98.0099n + 20.9764 |
| (8, n) | −1.2827n2 + 64.7168n + 0.0926 | −0.1968n2 + 112.015n + 23.4188 |
| (9, n) | −2.6562n2 + 88.725n − 14.2985 | −0.3985n2 + 126.4621n + 25.305 |
| (10, n) | −4.8907n2 + 124.633n − 38.947 | −0.73n2 + 141.557n + 26.377 |
| (n) | Energy (E(N)) |
|---|---|
| n2 | −18 × 10−3m4 + 191 × 10−2m3 − 696 × 10−2m2 + 102 × 10−1m − 507 × 10−2 |
| n | 264 × 10−2m4 − 2985 × 10−1m3 + 1.1526m24.1125m + 2.748 |
| 1 | −213 × 10−2m4 + 2228 × 10−1m3 − 7712 × 10−1m2 + 2.8762m − 389 × 10−1 |
| (n) | Estrada index EE(N) |
|---|---|
| n2 | −3 × 10−4m4 + 3.6 × 10−3m3 − 1.6 × 10−2m2 + 2.86 × 10−2m − 1.6 × 10−2 |
| n | 1.6 × 10−3m4 − 2.01 × 10−2m3 + 8.74 × 10−2m2 + 13.379m + 3.127 |
| 1 | −2 × 10−3m4 + 2.5 × 10−2m3 − 1.081 × 10−1m2 + 3.2289m − 1.02 × 10−1 |
Error among the Eexact and Eestimated are shown in Table 4 and (Table 5).
| (m, n) | Eexact(T) | Eestimated(T) | Error |
|---|---|---|---|
| (5, 1) | 40.6012 | 40.5443 | 0.0569 |
| (5, 2) | 71.8027 | 71.7646 | 0.0381 |
| (5, 3) | 103.5458 | 103.3415 | 0.2043 |
| (5, 4) | 134.4458 | 134.2103 | 0.2355 |
| (5, 5) | 165.8416 | 165.3595 | 0.4821 |
| (m, n) | EEexact(T) (N) | EEestimated(T) | Error |
|---|---|---|---|
| (4, 1) | 69.3326 | 69.3403 | −0.0077 |
| (4, 2) | 126.4852 | 126.516 | −0.0308 |
| (4, 3) | 183.6298 | 183.689 | −0.0592 |
| (4, 4) | 240.7664 | 240.861 | −0.0946 |
| (4, 5) | 298.895 | 298.04 | −1 |
4. Energy and Estrada Index of Naphthalene
Naphthalene is a very important polycyclic aromatic hydrocarbon and it is useful in indoor and outdoor environments. It is actually a part of crude oil. The construction of naphthalene is based on two fused benzene rings that are connected with each other. Naphthalene is also used in mothballs which are widely used throughout the world. It is also used as an insect repellent product. Naphthalene is vibrantly volatile and insoluble in water, also the part of coal tar. In this section, we find the energy and Estrada index of naphthalene 2 which is given in Figure 2.
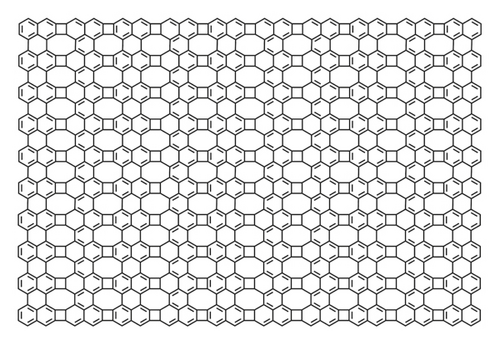
Proposition 2. Let N be the chemical graph of naphthalene. Then, the energy E(N) and Estrada index EE(N) of the chemical graph of naphthalene are given below.
Equation (3) represents the energy and (4) represents the Estrada index, respectively. E(N) shows the energy of naphthalene and EE(N) shows the Estrada index of naphthalene. In the case of naphthalene, we observed this inequality Eexact(N) > Eestimated(N), where Eexact(N) and Eestimated shows the exact and estimated value of energy, respectively.
In the case of the Estrada index, we observed this inequality EEexact(N) < EEestimated(N) , where EEexact(N) and EEestimated reflects the exact and the estimated value of the Estrada index. The detailed analysis has been discussed critically.
In Table 6, m is an integer from 1 to 10 and n is fixed, and in Tables 7 and 8 quadratic equations are constructed.
| (m, n) | Energy (E(N)) | Estrada index (EE(N)) |
|---|---|---|
| (1, n) | 0.0019n2 + 14.5781n − 0.9285 | −0.0117n2 + 28.7981n − 3.5494 |
| (2, n) | 0.0023n2 + 29.7505n − 1.8301 | −0.0413n2 + 62.09n − 2.8202 |
| (3, n) | 0.0031n2 + 44.9205n − 2.7308 | 0.085n2 + 94.4701n − 10.3612 |
| (4, n) | 0.0043n2 + 60.0881n − 3.6279 | 0.03015n2 + 127.04n − 15.7054 |
| (5, n) | 0.0059n2 + 75.2533n − 4.5214 | 0.06667n2 + 160.8653n − 19.433 |
| (6, n) | 0.0079n2 + 90.4161n − 5.4113 | 1.6043nn2 + 196.5316n − 24.9634 |
| (7, n) | 0.0103n2 + 105.5765n − 6.2976 | 4.0707n2 + 234.1325n − 34.0192 |
| (8, n) | 0.0131n2 + 120.7345n − 7.1803 | 9.6511n2 + 273.6896n − 45.7622 |
| (9, n) | 0.0163n2 + 135.8901n − 8.0594 | 20.5835n2 + 316.0045n − 59.6014 |
| (10, n) | 0.0199n2 + 151.0433n − 8.9349 | 39.7109n2 + 363.9428n − 81.673 |
| n | Energy (E(N)) |
|---|---|
| n2 | 2 × 10−4m2 − 2 × 10−4m + 1.9 × 10−3 |
| n | −1.2 × 10−3m2 + 15.176m − 5.967 × 10−1 |
| 1 | 1.8 × 10−3m2 − 9.097 × 10−1m − 1.79 × 10−2 |
| n | Estrada index EE(N) |
|---|---|
| n2 | −0.0001m6 + 0.0041m5 − 0.0423m4 + 0.1805m3 − 0.2864m2 + 0.0798m + 0.0529 |
| n | 0.0006m6 − 0.0163m5 + 0.159m4 − 0.5569m3 + 0.1969m2 + 34.682m − 5.6672 |
| 1 | −0.0051m6 + 0.1755m5 − 2.3788m4 + 15.91 m3 − 54.385m2 + 83.077m − 45.943 |
Also, the error between the Eexact(N), Eestimated(N), and EEexact(N), EEestimated(N) are shown in Tables 9 and 10.
| (m, n) | Eexact(N) | Eestimated(N) | Error |
|---|---|---|---|
| (2, 1) | 27.9798 | 27.9227 | 0.0571 |
| (2, 2) | 57.6801 | 57.6322 | 0.0479 |
| (2, 3) | 87.4421 | 87.3202 | 0.1219 |
| (2, 4) | 117.2087 | 117.12 | 0.0887 |
| (2, 5) | 146.9799 | 146.9514 | 0.0285 |
| (2, 6) | 176.7557 | 176.739 | 0.0167 |
| (2, 7) | 206.5361 | 206.248 | 0.0345 |
| (2, 8) | 236.3211 | 236.248 | 0.0731 |
| (2, 9) | 266.1107 | 266.0206 | 0.0901 |
| (2, 10) | 295.9049 | 295.8252 | 0.0797 |
| (m, n) | EEexact(N) | EEestimated(N) | Error |
|---|---|---|---|
| (3, 1) | 83.11256 | 91.6297 | –8.51714 |
| (3, 2) | 177.7729 | 186.3548 | –8.5819 |
| (3, 3) | 272.012 | 281.2499 | –9.2379 |
| (3, 4) | 366.2511 | 376.315 | –10.0639 |
| (3, 5) | 460.4902 | 471.5501 | –11.0599 |
| (3, 6) | 554.7293 | 566.9552 | –12.2259 |
| (3, 7) | 648.9684 | 662.5303 | –13.5619 |
| (3, 8) | 743.2075 | 758.2754 | –15.0679 |
| (3, 9) | 837.4466 | 854.2309 | –16.7843 |
| (3, 10) | 932.1173 | 950.2756 | –18.1583 |
5. Conclusion
- (1).
Eexact(N) > Eestimated(N)
- (2)
Eexact(T) > Eestimated(T)
This concludes that the error among the abovementioned structures is always positive.
- (1).
EEexact(N) < EEestimated(N)
- (2)
EEexact(T) < EEestimated(T)
This concludes that the error among the abovementioned structures is always negative.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Acknowledgments
This work was supported by the Researchers Supporting Project Number (RSP-2021/401), King Saud University, Riyadh, Saudi Arabia. (RSP-2021/401).
Open Research
Data Availability
No data were used to support this study.