[Retracted] Effect of miR-144-3p-Targeted Regulation of PTEN on Proliferation, Apoptosis, and Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells under Stretch
Abstract
Objective. To investigate the effects of miR-144-3p-targeted regulation of phosphatase and tensin homolog deleted on chromosome ten (PTEN) gene on proliferation, apoptosis, and osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) under retraction force. Methods. The BMSCs of rats were randomly divided into the tension MSC group with detrusor stimulation and the MSC group without detrusor stimulation, after which osteogenic differentiation of BMSCs was induced in both groups. Alkaline phosphatase (ALP) staining and alizarin red staining were used to detect the osteogenic differentiation ability of the two groups of cells. Real-time quantitative reverse transcription PCR (qRT-PCR) was used to detect the expression of miR-144-3p and PTEN in the two groups of cells after osteogenic differentiation. Bioinformatics website and dual luciferase reporter were used to detect the relationship between miR-144-3p and PTEN. The tension MSC group was used as a control group, and miR-144-3p mimics (miR-144-3p mimic group), mimic controls (mimic-NC group), PTEN interferers (si-PTEN group), and interference controls (si-NC group) were transfected into BMSCs. The BMSCs were then continuously stimulated for 24 h using a Flexercell in vitro cellular mechanics loading device, applying a draft force at a frequency of 1 Hz and a deformation rate of 18%. The cell proliferation was detected by Cell Counting Kit-8 (CCK-8) colorimetric assay; the expression levels of cyclin, cyclin-dependent kinases (CDK), BCL2-associated X (BAX), B-cell lymphoma-2 (BCL-2), and other cell cycle and apoptosis related proteins were detected by western blot (WB); and the osteogenic differentiation ability of MSC cells was detected by ALP staining and alizarin red staining. Results. Compared with the MSC group, the level of miR-144-3p was significantly lower and the level of PTEN was significantly higher in the tension MSC group. ALP staining showed normal activity in the MSC group and decreased ALP activity in the tension MSC group compared to the MSC group. Alizarin red staining in the MSC group showed scattered calcium nodule formation, and alizarin red staining showed red nodules with a more uniform color distribution. Compared to the MSC group, the tension MSC group showed fewer, smaller, and lighter staining mineralized nodules. Compared with the tension group and mimic-NC group (si-NC group), the proliferation rate of cells in the miR-144-3p mimic group (si-PTEN group) was significantly higher; the expression levels of PTEN and BAX were significantly lower; and the expression levels of cyclin, CDK, and BCL-2 protein were significantly higher. ALP staining results revealed that the miR-144-3p mimic group (si-PTEN group) showed significantly higher osteogenic differentiation ability and ALP activity of MSC than the tension group and mimic-NC group (si-NC group). Conclusion. miR-144-3p may inhibit apoptosis and promote proliferation and osteogenic differentiation of BMSCs under tension by targeting and regulating PTEN.
1. Preface
Osteoporosis (OP) is a complex group of systemic diseases of the skeleton, manifested microscopically by loss of bone mineral composition and bone matrix, reduced bone strength, and susceptibility to fracture [1, 2]. Fragility fractures are a serious complication of OP and are likely to occur in important functional areas such as the spine, hip, proximal humerus, and distal radius, resulting in a high rate of disability and even mortality, causing great harm and burden to families and society [3, 4]. Due to the limitations of current medical theory and technology, osteoporosis treatment is not ideal, treatment cycles are long, and there is no fundamental solution to this challenge. The causes of OP are complex and include declining oestrogen levels, natural aging and overuse of glucocorticoid drugs, all of which contribute to the development of osteoporosis [5–7]. Although the pathogenesis of osteoporosis varies, it is mainly characterized by a disruption in the balance of bone reconstruction with reduced bone formation and increased bone resorption. Therefore, promoting bone formation and inhibiting bone resorption to replace lost bone tissue are the key to treating osteoporosis. Bone marrow mesenchymal stem cells (BMSCs) are the main source of osteoblasts, and their reduced osteogenic differentiation and increased lipogenic differentiation are one of the major causes of osteoporosis [8, 9]. BMSCs are the only way to renew bone tissue in the body, so regulating the osteogenic differentiation of BMSCs will help in the treatment of OP.
Many studies [10, 11] have shown that mechanical stress plays a key role in regulating bone remodeling and bone homeostasis through mechanical signal transduction pathways. The stress-strain effect first causes changes in the cytoskeletal structure of the cell and subsequently activates a variety of cell biological processes within the osteoblast, such as cell metabolism, gene activation, and secretion of cytokines. The differentiation of BMSCs is under genetic regulation and is also influenced by environmental factors. A large number of scholars [12, 13] have conducted a variety of in vivo and in vitro mechanical stress stimulation experiments on mesenchymal stem cells and found that various biological characteristics of mesenchymal stem cells have changed under mechanical action. miRNAs are endogenous noncoding RNAs that regulate the expression of target genes mainly at the transcriptional or posttranscriptional level and play important roles in a variety of life events. Recent studies have shown that miRNAs are involved in the regulation of MSC proliferation, lipogenesis, and osteogenic differentiation or apoptosis and play an important role in the fate determination of MSCs [14, 15]. It has been reported [16] that miR-144-3p may be involved in the regulation of osteogenic differentiation and proliferation process of mouse MSCs. However, the mechanism of miR-144-3p action on osteogenic differentiation, especially in the molecular regulation of the effect of tension on osteogenic differentiation, is unclear and still needs further study. Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is a popular oncogene that has been studied in recent years and has a close relationship with tumorigenesis. Recent studies have found that it also plays an important regulatory role in the osteogenic differentiation of MSCs [17, 18]. This study focuses on the role of miR-144-3p in MSC proliferation, apoptosis, and osteogenic differentiation under tension and its possible mechanisms.
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Main Reagents
The following reagents were used: Modified Eagle Medium (MEM), whole medium (HyClone, USA); double antibodies mono-anti-penicillin mix, fetal bovine serum (FBS) (Gibco, USA), 0.25% trypsin–0.02% EDTA solution (Invitrogen, USA); bone induction medium, PBS buffer, alizarin red (Sigma, USA); miR-144-3p mimic and miR-144-3p mimic-NC, si-PTEN, and si-NC (Guangzhou RiboBio Co., Ltd. China); bicinchoninic acid (BCA) protein quantification kit, western blot primary antibody dilution, ALP staining kit, western and IP cell lysis solution (Shanghai Beyotime Company, China); sodium dodecyl sulfate, glycine (Solarbio); anti-GAPDH (Affinity Biotech); anti-PTEN, anti-cyclin, anti-CDK, anti-BAX, anti-BCL-2 (Santa Cruz), PVDF membrane, BSA solution, Trizol extraction kit (Takara, Dalian); and transfection kit (Invitrogen, USA).
2.1.2. Main Instruments
The following instruments were used: HERAcell CO2 thermostatic cell incubator (Heraeus, Germany); orthomorphic microscope, fluorescent inverted microscope (Nikon Corporation); real-time quantitative PCR amplification instrument (Roche, Switzerland); western blot electrophoresis kit (Beijing Liuyi Instrument Plant, China); desktop high speed freezing centrifuge (Thermo Inc.); and Flexercell in vitro cell mechanics loading device (Flexcell, Inc., USA).
2.1.3. Cell Lines
Rat BMSCs were purchased from the Shanghai Cell Bank, Chinese Academy of Sciences, China. After digestion in a constant temperature shaker at 37°C and 200 r/min for 1 h, the precipitate was filtered through a 70 μm cell strainer, collected after centrifugation, and resuspended using α-MEM containing 20% FBS and 1% double antibody. The suspensions were transferred to 60 mm cell culture dishes and incubated at 37°C in a 5% CO2 cell culture chamber. The 3rd to 6th generation passaged cells were taken for subsequent experiments.
2.2. Experimental Methods
2.2.1. Cell Grouping and Transfection
miR-144-3p mimics (miR-144-3p mimic group), negative controls (mimic-NC group), PTEN interferers (si-PTEN group), and interfering controls (si-NC group) were transfected with BMSCs, and a separate MSC group (without detrusor stimulation) and a detrusor MSC group (tension group) were set up. The miR-144-3p mimics, negative control, PTEN small interfering RNA, and interfering tension MSC were transfected into the BMSC lines according to the instructions of the transfection kit. The cells were transfected using liposomes when the cell fusion reached 70%–80%, and after 6 h, they were switched to osteogenic induction medium for further culture for subsequent assay experiments.
2.2.2. Application of Tensile Force to Cells
BMSCs from each group were digested with 0.25% trypsin solution and inoculated with 6 flexible wells of silica gel force plates at l × 105 cm−2. After the above operation, the cells were incubated for 48 h. When 80% to 90% of the cells fused, a tensile force was applied at a frequency of 1 Hz and a deformation rate of 18% for 24 h using a Flexercell in vitro cell mechanics loading device.
2.2.3. Alkaline Phosphatase (ALP) Staining
After each group of cells was inoculated in 12-well plates at 5 × 104/well, the osteogenic induction solution was continued for 3 d. (1) The medium was carefully aspirated with a gun tip; PBS was gently added to each well using a barrel, washed twice; and then PBS was carefully aspirated. (2) The cells were fixed with 4% paraformaldehyde and washed twice with PBS after 15 min. (3) 1.5 mL of prepared ALP staining solution was added to each well. (4) The cells were left for 30 min at room temperature and protected from light to allow sufficient staining. (5) After 30 min, the staining solution was removed, and the cells were washed twice with PBS (pH 4.2) and air-dried. (5) They were observed under the microscope and photographed.
2.2.4. Alizarin Red Staining
BMSCs were inoculated in 24-well plates at a density of about 30%, walled and treated with the corresponding factors, and incubated for 18 or 21 days before alizarin red staining. (1) The medium was carefully aspirated with a gun tip; PBS was gently added to each well using a barrel, washed twice; and then PBS was carefully aspirated. (2) 0.5% glutaraldehyde (0.2 ml/well) was added, and after 10 min, the staining solution was washed twice with PBS (pH 4.2). (3) 0.4% alizarin red staining solution (0.2 ml/well) was added, carefully aspirated after 20 min, put into water, and gently washed and dried. (4) Cells were scanned and photographed.
2.2.5. Real-Time Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
Total RNA was extracted by Trizol method and reverse-transcribed to complementary DNA (cDNA), and RT-PCR was performed on the machine according to the reagent instructions. The internal reference was glyceraldehyde phosphate dehydrogenase (GAPDH), every 3 replicate wells were used as one sample to derive the Ct value of each group, and the relative expression of mRNA in each group was calculated by the 2(-△△Ct) method. The primer sequences are shown in Table 1.
| Gene | Primer sequences | |
|---|---|---|
| miR-144-3p | Upstream | 5′-CTCTATCCAAAACAGGCCGC-3′ |
| Downstream | 5′-T T TACATCCCCAAGGCCCAT-3′ | |
| PTEN | Upstream | 5'--TCAGACTTTTGTAATTTGTGTATG-3′ |
| Downstream | 5′-ACAGGCTCCCAGACATGACA-3′ | |
| GAPDH | Upstream | 5′-CAGCGACACCCACTCCTC-3′ |
| Downstream | 5′-TGAGGATCCACCACCCTGT-3′ | |
2.2.6. Cell Counting Kit-8 (CCK-8) Colorimetric Method
After the end of the enrichment, incubation of each group of cells was continued for 48 h. (1) 10 μL CCK-8 solution was added, and the cells were incubated for 1 h. (2) After the end of the incubation, absorbance values were measured using an enzyme marker.
2.2.7. Western Blot (WB) Method
The transfected cells in each group were collected, and an appropriate amount of RIPA lysis buffer was used to extract the total protein in the cells. After quantification by BCA method, the sample volume was calculated, electrophoresed, transferred to a membrane, and then blocked in 5% nonfat milk powder solution for 2 h at room temperature. The membrane was washed with PBS-T, and the primary antibody (1 : 500) was added and stored in a refrigerator at 4°C overnight. After washing the membrane, secondary antibody (1 : 5000) was added, and cells were incubated at room temperature for 2 h. After adding developer solution and avoiding light development, the bands were analyzed by Image J software with GAPDH as internal reference after exposure.
2.2.8. Dual Luciferase Reporter Gene Assay
The 3′ UTR of PTEN containing miR-365-3p binding site was amplified by PCR technique, and the 3′ UTR wild-type luciferase vector of PTEN (WT-PTEN) and mutant luciferase vector (MUT-PTEN) were constructed. The log phase chondrocytes were inoculated in 6-well plates at 5.0 × 105 cells/well and incubated for 24 h. The medium was discarded and co-transfected with Lipofectamine™ 2000 kit with WT-PTEN and miR-144-3p mimic, WT-PTEN and mimic-NC, MUT-PTEN and miR-144-3p mimic, WT-PTEN and mimic-NC. After 12 h of transfection, the cells were replaced with fresh medium and incubated for another 24 h. The cells were collected and lysed, the lysate was centrifuged at 3,500 r/min for 5 min, and the supernatant was used to detect luciferase activity
3. Statistical Analysis
Three independent replicate experiments were performed for each group to obtain results. qRT-PCR results were plotted using GraphPad Prism 8 software, WB results were processed using ImageJ software, and statistical analysis was performed using SPSS22 software. The results were analyzed by independent sample t-test or nonparametric test according to the chi-square of the results, and the differences were statistically significant at p < 0.05
4. Result
4.1. Comparison of the Bone Differentiation Capacity of the MSC and Tension MSC Groups
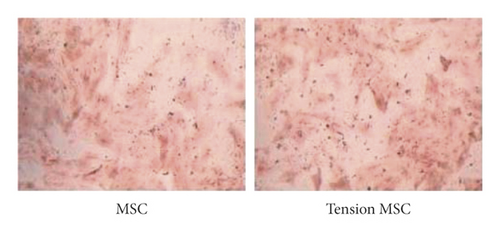
Osteogenic induction culture was followed by ALP staining with alizarin red staining. The ALP staining results (Figures 1(a)) showed significantly lower expression in the tension MSC group compared to the MSC group, suggesting that the loaded detrusor inhibited the ability of MSC cells to differentiate towards mature osteoblasts. The alizarin red staining results (Figures 1(b)) showed scattered calcium nodule formation in the MSC group, with alizarin red staining in the form of red nodules with a more uniform color distribution and fewer, smaller, and lighter staining mineralized nodules in the tension MSC group compared to the MSC group. This suggests that BMSCs have the potential for osteogenic differentiation and that pathological distraction inhibits their ability to do so (Figure 1).

4.2. Changes in miR-144-3p and PTEN Expression during Osteogenic Differentiation
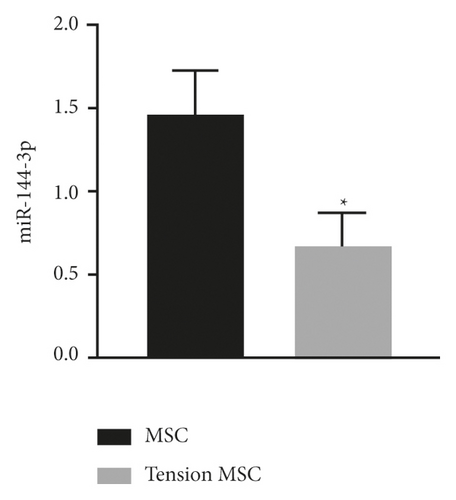
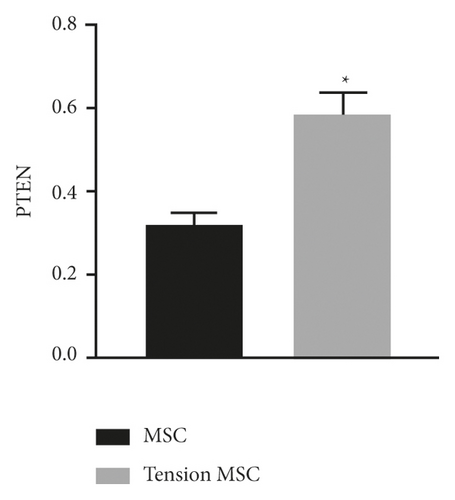
qRT-PCR was used to detect the expression levels of miR-144-3p (Figure 2(a)) and PTEN mRNA (Figure 2(b)) in the tension MSC group and the MSC group. The assay showed markedly lower expression of miR-144-3p and markedly higher mRNA expression of PTEN in cells of the tension MSC group than those in the MSC group (p < 0.05) (Figure 2).
4.3. There Is a Targeting Relationship between miR-144-3p and PTEN
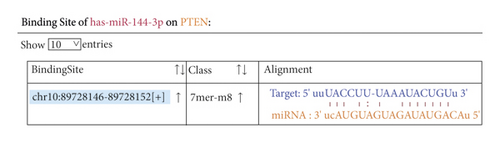
Bioinformatics sites were used for predicting target genes for mature miR-144-3p sequences, and the results suggested the presence of binding sites for miR-144-3p to PTEN (Figure 3(a)). To further confirm the direct binding action of miR-144-3p to PTEN, PTEN wild-type and mutant fluorescent reporter vectors were constructed, and MSC cells were transfected. Dual luciferase reporter gene assays further confirmed PTEN as a direct target of miR-144-3p (Figure 3(b)). In addition, WB analysis showed that the mixture of miR-144-3p overexpression group had clearly lower concentration of PTEN protein compared to the other two groups (Figure 3(c)) (Figure 3).
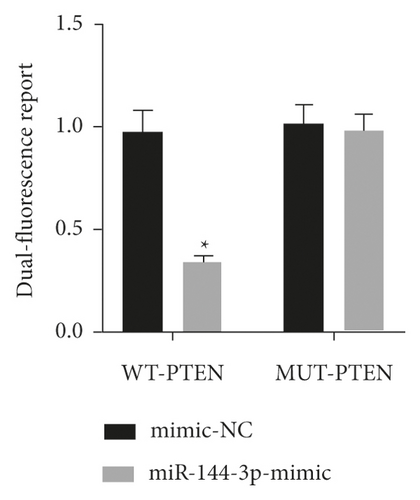
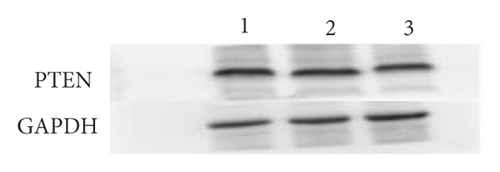
4.4. Expression of miR-144-3p and PTEN in BMSCs under Posttransfection Drafting Force
The expression of miR-144-3p, PTEN mRNA, and protein in each group of BMSCs under posttransfection traction was detected by qRT-PCR assay and WB method. Analysis showed that miR-144-3p overexpression group had substantially higher miR-144-3p content compared to the other two groups, while PTEN mRNA and protein content were substantially lower (p < 0.05) (Figures 4(a)–4(c)). Compared with the tension MSC and si-NC group, the si-PTEN group had substantially greater concentrations of miR-144-3p and substantially less mRNA and protein content of PTEN (p < 0.05) (Figures 4(c) and 4(d)) (Figure 4).

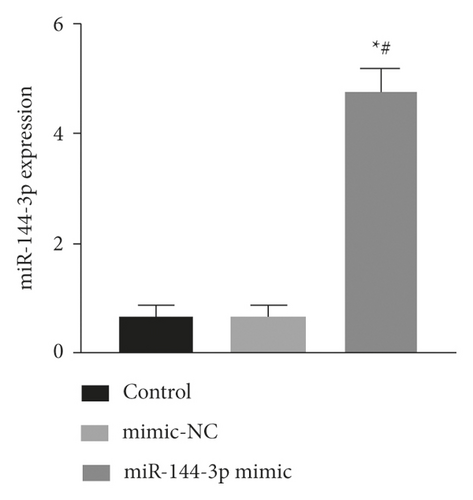
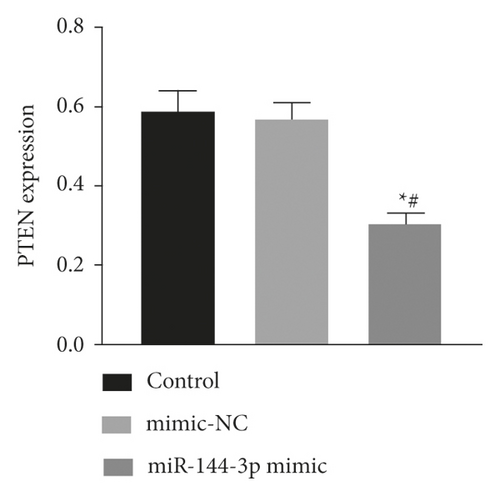
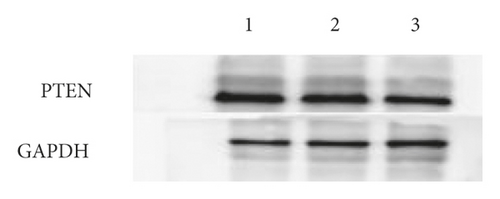
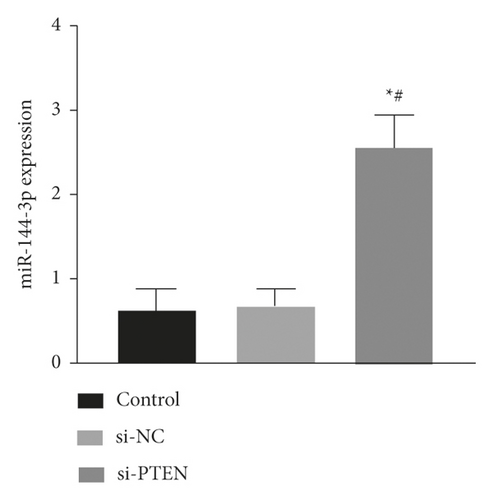
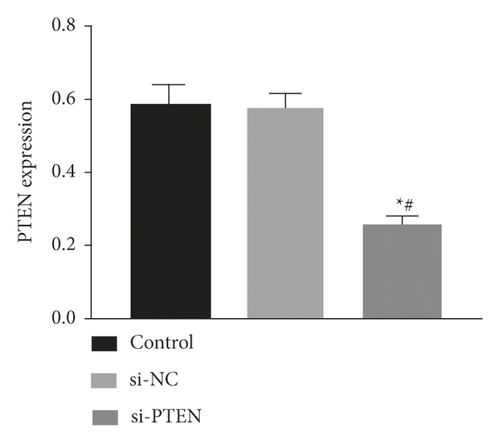
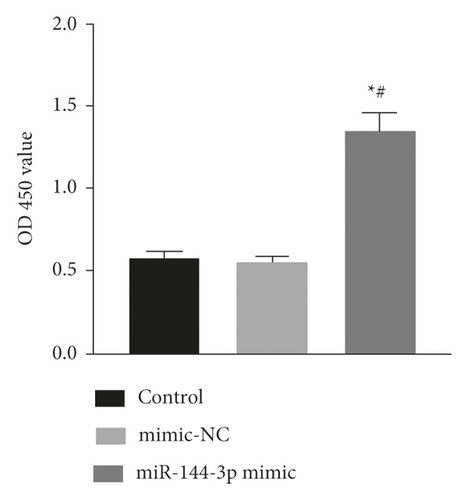
4.5. Effect of Upregulation of miR-144-3p on BMSCs Proliferation, Cycling, Apoptosis, and Osteogenic Differentiation under Tension
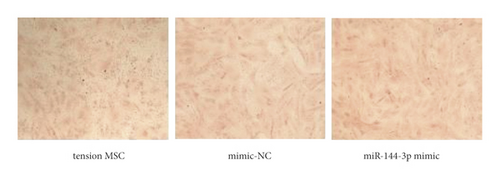
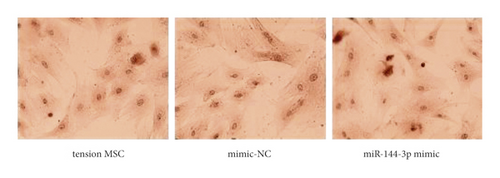
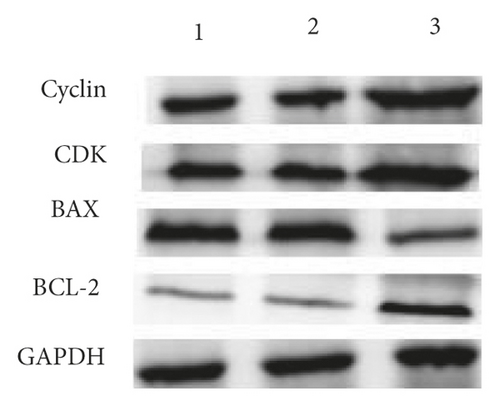
The exposure of cyclin, CDK, BCL-2, BAX, and other cycle and apoptosis related proteins in BMSCs under traction after overexpression of miR-144-3p and the value-added rate of BMSCs was measured by WB and CCK-8 assays, respectively. The analysis showed that the level of cyclin, CDK, and BCL-2 protein production was much higher in the miR-144-3p mimic group compared to the other two groups, while the level of BAX protein production was slightly lower (p < 0.05). Osteogenic induction culture was followed by ALP staining with alizarin red staining, and the results showed that the ALP activity was obviously more in the miR-144-3p mimic group compared to the other two groups. In addition, the alizarin staining showed scattered calcium nodule formation in the form of red nodules, with darker cells and more uniform color distribution (Figures 5(a)a–d5(a), 5(d)).
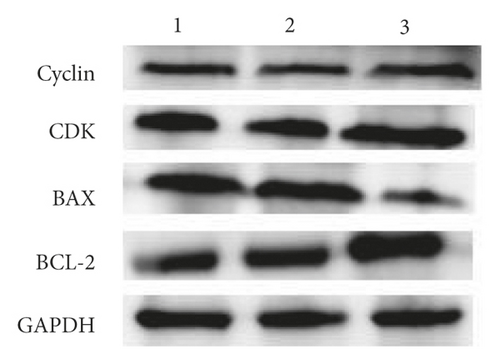
4. 6. Effect of PTEN Inhibition on BMSCs Proliferation, Cycling, Apoptosis, and Osteogenic Differentiation.
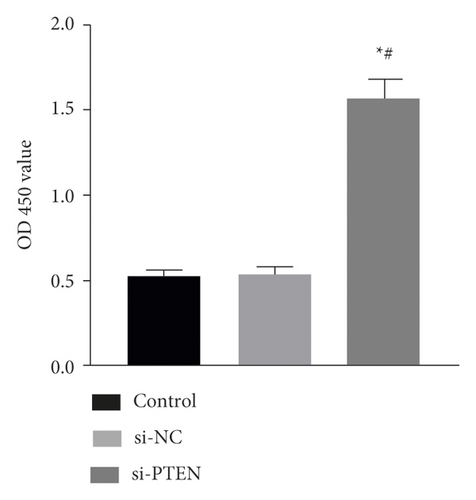
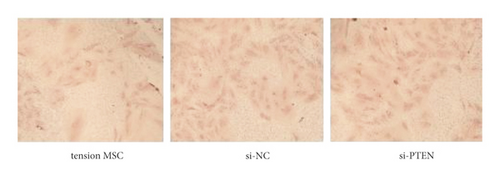
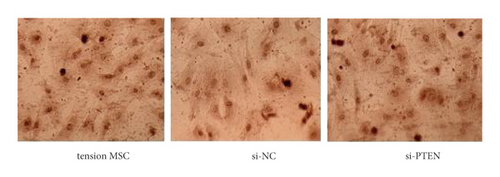
The exposure of cyclin, CDK, BCL-2, BAX, and other cycle and apoptosis related proteins in BMSCs under traction after inhibition of PTEN and the value-added rate of BMSCs was measured by WB and CCK-8 assays, respectively. The analysis showed that the level of cyclin, CDK, and BCL-2 protein production was much higher in the si-PTEN group compared to the other two groups, while the level of BAX protein production was slightly lower (p < 0.05). Osteogenic induction culture was followed by ALP staining with alizarin red staining, and the results showed that the ALP activity was obviously more in the si-PTEN group compared to the other two groups. In addition, the alizarin red staining showed scattered calcium nodule formation in the form of red nodules, with darker cells and more uniform color distribution (Figures 6(a)∼6(d)).
5. Discussion
The microstructure of bone consists of mineralized extracellular matrix and bone remodeling units, including osteocytes, osteoblasts, and osteoclasts [19]. The osteoblasts differentiate to form new bone, and the osteoclasts digest and resorb old bone, keeping the normal adult bone metabolism in a dynamic equilibrium, which, once disrupted, is prone to insufficient bone formation or excessive bone resorption, resulting in reduced bone mass and osteoporosis [20,21]. In the present experiment, we found that PTEN expression levels were increased in the tension MSC group by applying pathological traction stimulation to BMSCs and that PTEN overexpression significantly impaired the mineralization capacity and ALP activity of BMSCs. Software prediction and dual luciferase reporter gene detection revealed that miR-144-3p was capable of target-binding PTEN gene sequences. In addition, this study found that the expression level of miR-144-3p was significantly decreased and PTEN expression level was significantly increased in the cells of the MSC group under tension. Overexpression of miR-144-3p or inhibition of PTEN expression could inhibit apoptosis, promote BMSC proliferation, and enhance BMSC mineralization capacity and ALP activity, which could reverse the inhibitory effect of PTEN on osteogenic differentiation of BMSCs under tension.
MiRNA is a small noncoding RNA of approximately 19–25 nucleotides in length and is highly conserved. It is involved in the early development and growth, proliferation, differentiation, division, and apoptosis of cells in various organisms; is involved in the regulation of important genes, humoral regulation, tissue reconstruction, endocrine regulation; and has important implications for the onset, development, and regression of disease [22–24]. MiRNAs have been extensively studied in osteoblast and osteoclast growth and differentiation. For example, Zeng et al. [25] found that during osteogenic differentiation of MC3T3 cells, miR-29b could promote osteogenesis by acting on the target genes HDAC4, TGF-β3, and ACVR2A, which inhibit osteogenesis, and blocking their expression through degradation. He et al. [26] found that miR-20b promoted osteogenic differentiation by binding to its target gene PPARγ and reducing PPARγ expression, thereby increasing the transcription of the core osteogenic transcription factor Runx2. In this study, we found that the expression of miR-144-3p and its osteogenic differentiation ability were significantly decreased in cells from the tension MSC group compared to the MSC group. To clarify the effect of miR-144-3p on the proliferation, apoptosis, and osteogenic differentiation ability of rat BMSCs under distraction force, we transfected miR-144-3p mimics into cells to alter the expression level of miR-144-3p in the cells and then applied distraction force to each group of cells. After upregulating the expression of miR-144-3p and loading with pathological distraction force, ALP activity, calcification ability, cyclin, CDK, and BCL-2 protein expression levels were enhanced in the miR-144-3p mimic group, and BAX protein expression level was decreased. This suggests that miR-144-3p plays a regulatory role in the proliferation, apoptosis, and osteogenic differentiation of rat BMSCs under distraction force.
PTEN is an oncogene with phosphatase activity that has been identified and is also closely related to the differentiation of MSC, especially in terms of osteogenesis, providing new ideas for the treatment of OP, fracture healing, osteosclerosis, and other bone-related diseases [27–29]. It was shown that miR-19b could enhance the osteogenic differentiation of BMSCs by regulating PTEN-mediated AKT/GSK3β pathway expression; miR-140-3p could regulate the growth and differentiation of osteoblasts and osteoclasts by targeting PTEN and activating the PTEN/PI3K/AKT signaling pathway [30,31]. This study confirmed PTEN as a direct target of miR-144-3p by bioinformatics prediction display and dual luciferase reporter gene assay. Inhibition of PTEN expression increased ALP activity and cyclin, CDK, and BCL-2 protein expression levels in BMSCs; decreased BAX protein expression levels; and enhanced cellular calcification. However, the overexpression of miR-144-3p reduced the mRNA and protein expression levels of PTEN and also reversed the inhibitory effect of PTEN on osteogenic differentiation. It is evident that PTEN is also essential in the proliferation, apoptosis, and osteogenic differentiation of BMSCs. miR-144-3p may regulate osteogenic differentiation of BMSCs under tension by targeting PTEN.
In summary, overexpression of miR-144-3p inhibited the apoptosis of BMSCs under pathological tension and promoted their proliferation and osteogenic differentiation, and its mechanism of action may be related to the targeted inhibition of PTEN. miR-144-3p/PTEN may provide a potential target for the prevention and treatment of osteoporosis.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Open Research
Data Availability
The data can be obtained from the corresponding author upon reasonable request.




