Therapeutic Effect of Cu(II) Complex with Photocatalytic Property on Gastric Cancer by Inhibiting the Proliferation of Tumor Cells
Abstract
In situ hydrolysis of 1,4,5,8-naphthalenetetracarboxylic dianhydride (L) and simultaneous self-assembly with Cu(II) ions afforded a new Cu(II) compound formulated as [Cu(L) (4,4′-bpy)0.5(H2O)]n (1), which is structurally determined by a series of characterization techniques, such as powder X-ray diffraction (PXRD), thermogravimetric Analysis (TGA), and elemental analysis. It is noteworthy that compound 1 can catalyze the degradation of methylene blue (MB) in aqueous solution under UV irradiation. This paper also investigated and evaluated its application value and research mechanism in gastric cancer. However, we also used real-time reverse transcription-polymerase chain reaction (RT-PCR) to detect the activation of vascular endothelial growth factor (VEGF) signaling pathway, and Cell Counting Kit-8 (CCK-8) method was used to calculate the inhibition activity of 1 on gastric cancer cell viability.
1. Introduction
One of the most common malignancies in the world is gastric cancer. Its pathogenic mechanism is complex and involves many factors [1]. Among them, part of the occurrence of gastric cancer is related to the VEGF signaling pathway, and its mechanism has unique molecular biological characteristics. Scholars at home and abroad have conducted continuous and in-depth research on this and put forward a number of theories [2].
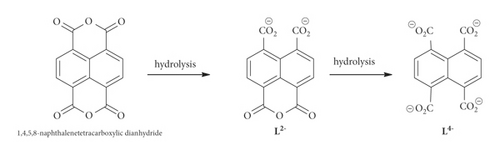
Metal-organic frameworks (MOFs) have many unique structures and functions and have been applied in many fields, such as high specific area, high porosity, tunable pore structure, and structural modifiability, that makes them show huge application potentials in gas storage/separation, heterogeneous catalysis, sensing and biomedicine [3–6]. The method of how to construct a MOF with the desired application property is of current research interest. For this purpose, we should subconsciously choose some metal ions and organic ligands with a suitable shape and high flexibility and symmetry [7–9]. Polycarboxylic acid ligands, showing diversified bonding modes to metal ions, have been demonstrated that they can serve as good organic building blocks for the construction of thermostable MOFs [10–13]. In this work, we selected 1,4,5,8-naphthalenetetracarboxylic dianhydride as the organic building block such that it can in situ hydrolyze into two different forms of L2− and L4− under appropriate conditions (Scheme 1). On the other hand, copper is an essential trace metal element in the human body. Modern medical research shows that copper complexes can produce antibacterial, antiviral, anti-inflammatory, antitumor, enzyme inhibitory, or chemical nuclease activities, which are increasingly attracting the attention of researchers [14–16]. Another drive for targeting copper(II) was due to its less toxic nature, which can be further decreased on complexation with ligands and thus proved to be promising in the development of copper complexes as anticancer agents. Copper can exist in two different oxidation states in cells. The anoxic character of cancer cells promotes the reduction of Cu(II) to Cu(I), which provides a therapeutic opportunity to target tumors [17]. Many Cu-based coordination polymers have been studied for their potential anticancer activity and promising achievements have been obtained. For instance, Yin and co-workers reported the inhibiting human gastric cancer cells activity of three Cu(II)-based coordination polymers [18]; Wu et al. found that the Cu(II)-based coordination polymer could significantly reduce the viability of MGC-803 cancer cells with a dose-dependent manner [19]; Xing reported that the nanosized Cu(II)-based coordination polymer could lead to the cell death of three human spinal tumor cells [20]. Via the in situ hydrolysis and simultaneous self-assembly with Cu(II) ions under hydrothermal conditions, a novel Cu(II) compound with the formula of [Cu(L2-) (4,4′-bpy)0.5(H2O)]n (1) has been resoundingly created. X-ray structural analysis revealed that it displays a 1D infinite chain structure. In addition, we studied the photocatalytic degradation of 1 to MB in aqueous solution under UV irradiation. In this study, two methods were used to study the anticancer activity of method compounds in gastric cancer cells.
2. Experimental
2.1. Materials and Instrumentation
Unless otherwise stated, the materials used in this experiment have not been further purified and are all from commercial suppliers. Elemental analysis (C, H, and N) was performed with a Vario EL III elemental analyzer. The PXRD patterns of a Cu Kα X-ray source (λ = 1.54056 Å) were obtained by using a MiniFlex II powder diffractometer. TG analysis was performed using a NETSCHZ STA-449C thermoanalyzer in the temperature range from 30 to 800°C under a nitrogen atmosphere.
2.2. Synthesis of [Cu(L2-) (4,4′-bpy)0.5(H2O)]n (1)
Seal the mixture of 0.1 mmol 1,4,5,8-naphthalenetetracarboxylic dianhydride, 0.1 mmol NaHCO3, 0.100 mmol Cu(NO3)2·3H2O, 0.1 mmol 4,4′-bipy as well as 10.0 mL H2O in a 25 mL Teflon container and then it was heated at 150°C for 72 hours. When the temperature was reduced to indoor temperature with a rate of 5°C/min, the blue block crystal 1 was prepared with Cu(NO3)2•3H2O as a raw material, and the yield was 41%. Elemental analysis calcd. for C19H10NO8Cu (443.83): C, 51.37; H, 2.25; N, 3.15%. Found: C, 51.35; H, 2.27; N, 3.16%. IR(KBr pallet, cm−1): 3421 (m), 3058 (m), 2922 (m), 2798 (m), 1574 (s), 1577 (s), 1488 (s), 1454 (s), 1398 (m), 1361 (m), 1335 (s), 1311 (s), 1256 (s), 1161 (m), 1152 (m), 1101 (s), 1025 (m), 1002 (w), 922 (s), 837 (m), 774 (s), 752 (s), 709 (m), 682 (s), 614 (w), 582 (w), 531 (s).
2.3. X-ray Structural Determination
A suitable single crystal was selected under an optical microscope, glued to a thin glass fiber, and its structure was determined by a computer-controlled Rigaku Mercury CCD diffractometer at indoor temperature. The diffractometer uses graphite monochromatic Mo-Kα radiation. In addition, the structure of the compound was directly solved by SHELXTL crystal software package, and the full matrix least-squares optimization was carried out with F2 as reference [21]. All atoms except hydrogen are orientated from the differential Fourier map and further anisotropic refined. Hydrogen atoms are created according to theoretical models. Specific crystal parameters and related details of complex 1 are shown in Table 1. The bond lengths and bond angles of the central ion of Cu(II) are shown in Table S1.
| Formula | C19H10NO8Cu |
|---|---|
| Fw | 443.83 |
| Crystal system | Triclinic |
| Space group | P − 1 |
| a (Å) | 8.2135(16) |
| b (Å) | 10.254(2) |
| c (Å) | 10.807(2) |
| α(°) | 70.19(3) |
| β(°) | 70.92(3) |
| γ(°) | 68.28(3) |
| Volume (Å3) | 774.1(4) |
| Z | 2 |
| Density (calculated) | 1.904 |
| Abs. Coeff. (mm−1) | 1.469 |
| Total reflections | 5952 |
| Unique reflections | 3495 |
| Goodness of fit on F2 | 1.091 |
| Final R indices [I > 2sigma(I2)] | R = 0.0457, wR2 = 0.1133 |
| R (all data) | R = 0.0577, wR2 = 0.1226 |
| CCDC | 2118730 |
2.4. CCK-8 Assay
The inhibitory activity of novel compounds on the activity of gastric cancer cells was detected by CCK-8. This experiment was carried out after a slight modification on the basis of protocol specification. Because compound 1 could not be dissolved in the organic solvents and water, we used its stock solution in the following bioactivity tests according to the literature methods [22,23]. With this in mind, about 50 mg of the as-prepared crystalline samples of 1 was taken in a mortar. It was then ground manually for 30 min by using a pestle. The produced fine powders were soaked in 20 mL DMSO and subjected to the ultrasonic treatments for 2 hours to obtain the well dispersed solution. The obtained stock solutions were diluted by cell culture medium to various working concentrations. In simple terms, gastric cancer cells collected from logarithmic growth phages were inoculated into 96-well plates with 5000 cells per well and incubated at 37°C in a 5% CO2 incubator. When the degree of cell fusion reached 75%, the compound was added, treated with continuous dilution, and incubated for 48 hours. In the end, the absorbance of each hole was measured and the survival curve of cancer cells was drawn according to the results.
2.5. Real Time RT-PCR
We used the real-time RT-PCR to evaluate how the opening degree of the VEGF signaling pathway in gastric cancer cells would be affected after compound treatment. This experiment was carried out after a slight modification on the basis of protocol specification. In summary, normal cells gastric cancer cells collected from logarithmic growth stage phages were inoculated into 6-well plates whose destiny is 105 cells/well and incubated at 37°C at 5% CO2. When the convergence degree of cells reached 75%, compounds with different concentration gradients (10 ng/mL, 20 ng/mL, 50 ng/mL) were added for treatment. After treatment, cells were collected, the TRIZOL reagent can be used to extract cell total RNA. After the RNA concentration is determined, it is converted into cDNA using an RNA reverse transcription detection kit. The relative expression of vegf and erk were measured with GAPDH as an internal reference. The results of three repeated preformation were calculated by a 2−ΔΔCt method.
3. Results and Discussion
3.1. Structural Characterization
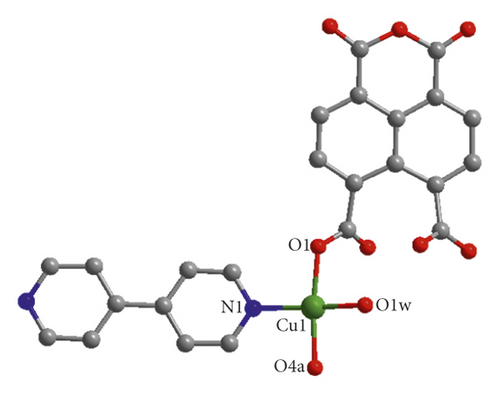
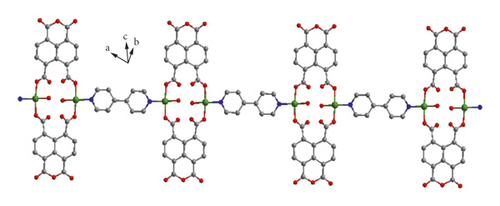
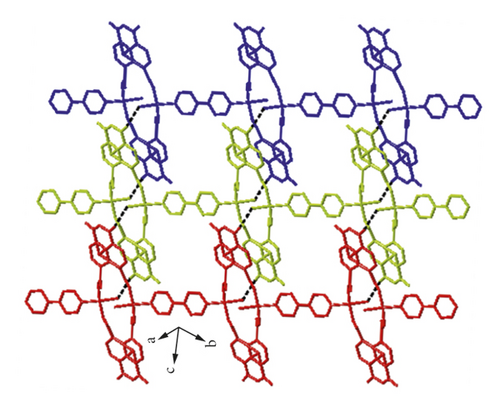
X-ray crystal analysis showed that compound 1 mainly crystallized in the P-1 space group, and its structure was mainly a one-dimensional chain, and each basic building unit was composed of a Cu(II) ion, one L2- ligand, a half 4,4′-bipy ligand as well as one terminal water ligand. As shown in Figure 1(a), the central Cu(II) ion is located in the center of the quadrilateral and can be divided into four parts: two carboxylic oxygen atoms from different L2−ligands, a terminal water ligand, and a nitrogen atom from a 4,4 ′-bipyridine ligand. The bond distance of Cu–O varies from 1.938(2)-1.947(2) Å, while that of Cu–N varies from 1.990(3) Å. Two neighboring Cu(II) ions are bridged by two L2- ligands in uniform bis-monodentate mode, leading to the formation of a dinuclear [Cu2(L)2] subunit with the Cu…Cu distance of 4.98 Å, and such a coordination pattern has also been observed in other reported literature [24, 25]. The adjacent binuclear [Cu2(L)2] subunit can be linked into a one-dimensional chain structure by the action of the 4,4 ′-diligand (Figure 1(b)). Intermolecular hydrogen bond interactions formed between the terminal water ligands and anhydride groups of L2- ligand from the adjacent chain (O1w-H…O = 2.6203–3.2462 Å, ∠O1wHO = 101–141°) combined these 1D chains together, affording a hydrogen bond-bridged 2D supramolecular layer (Figure 1(c)). Finally, these hydrogen bond-bridged 2D layers are interlaced with each other, stacking into a 3D supramolecular framework under the direction of weak Van der Waals interactions (Figure 1(d)).
3.2. PXRD and TGA of 1
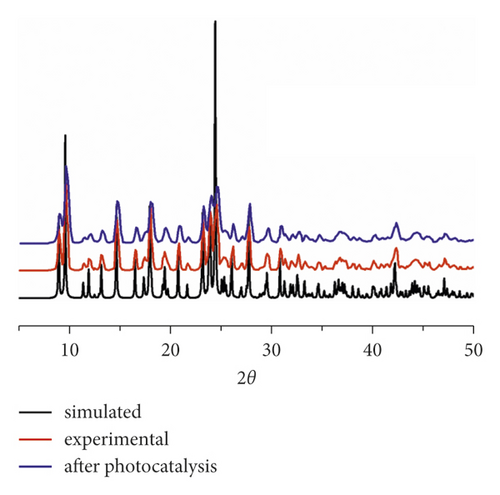
In order to verify whether the synthesized polycrystalline solids belong to the same phase, a PXRD experiment was performed at indoor temperature. In Figure 2(a), we show that the experimental spectrum based on polycrystalline solids highly matched with the spectrum simulated from the single crystal diffraction data, clearly suggesting that the synthesized bulk polycrystals have high purity.
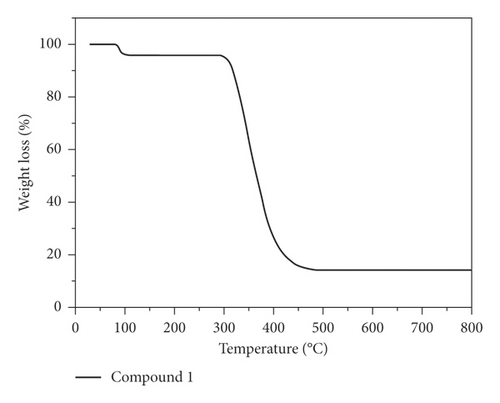
The structural stability of 1 was also evaluated through the TGA experiment under a nitrogen atmosphere. In Figure 2(b), we show the TGA curve of 1, and it can be seen that there are two main weight loss steps: one occurred from 78 to 112°C that was attributed to the loss of terminal water ligand (obsd: 4.11%, calcd: 4.06%), and the other occurred from 292 to 486°C that was caused by the combustion of the organic ligand. The final products after decomposition may be metal copper with an observed weight of 14.27%, corresponding well to the theoretical value of 14.31%.
3.3. Photocatalytic Property of Compound 1
In order to elucidate the photoresponse region, the solid state UV-vis diffuse reflection (DRS) spectra of 1 and the free organic ligands were measured at room temperature (Figure S1 left). The maximum UV absorption bands for the L ligand were at ca. 254 and 296 nm, which may be attributed to the π⟶π∗ transition. In Figure S1 left, two absorption components in both the UV and visible regions for 1 were observed. The UV absorption band (ca. 275 nm) can be attributed to the ligand-to-metal charge transfer (LMCT), while the visible absorption band (ca. 548 nm) may be due to the d–d transition of the Cu2+ ions. In previous investigations, the band gap energy (Eg) was one of the key factors evaluating the degradation efficiency of the photocatalysts. Thus, the Kubelka–Munk (K–M) equation, F = (1 − R)2/2R (where R is the reflectance of an infinitely thick layer at a given wavelength) was used to calculate the band gap energy (Eg). The Eg value of 1 was estimated to be 3.14 eV (Figure S1 right), suggesting that 1 may be responsive to UV light and has potential for photocatalytic reactions [26–28].
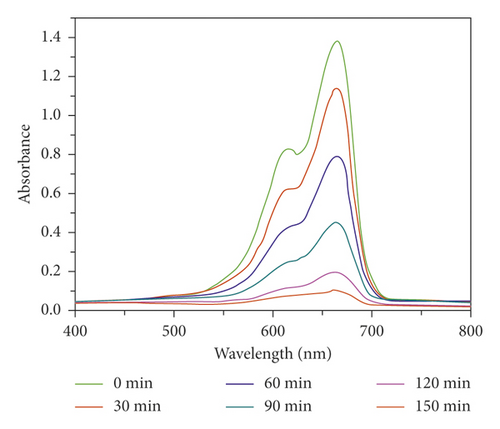
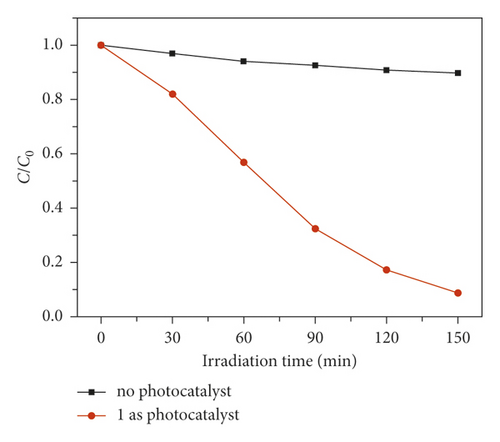
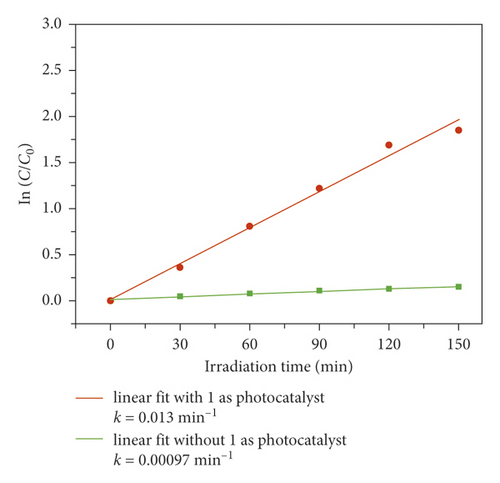
In order to evaluate its photocatalytic performance, a model dye of methyl blue (MB) was chosen in this work. The photocatalytic experiment was performed in a typical process as follows: The adsorption-desorption equilibrium was established by adding 40 mg compound 1 powder samples into a reactor containing 100 mL MB aqueous solution (10 mg/L) and conducting an ultrasonic treatment under dark conditions for 60 min. After that, the reactor was irradiated by the UV light under magnetic stirring. Every 30 minutes, 3 mL of the mixture was removed and it was centrifuged. The solution was analyzed by a UV-vis spectrometer, and the photocatalytic reaction process was monitored at 664 nm using the characteristic peak of MB as a reference. As can be seen from Figure 3(a), in the presence of 1, the characteristic peak of MB decreased sequentially as the reaction time increases. After 150 min, the degradation efficiency of MB can attain 91.3% (Figure 3(b)). However, the degradation efficiency of MB is only 10.3% after 150 min in the absence of any photocatalysts (Figure 3(b)). At the same time, we also analyze it from the point of view of reaction kinetics, assuming that it follows the pseudo first-order kinetic equation of ln(C0/Ct) = kt, where K is the rate constant, then we can calculate the lowest K value of 1 as 0.013 min−1 with 1 as photocatalyst, 0.00097 min−1 for the blank experiment (Figure 3(c)). It acts as a photocatalyst with a minimum of 0.00097 for a blank experiment (Figure 3(c)). Obviously, the addition of compound 1 increases the value of K, indicating it could behave as a catalyst to accelerate the degradation rate of MB. These results also indicate that 1 is favorable for catalytic degradation of MB in aqueous solution under UV irradiation. The structural stability is one of the important indictors to determine whether one catalyst can be used for industrialization. The PXRD pattern after photocatalytic reaction exhibits a high similarity to the simulated pattern (Figure 2(a)), indicating a good structural integrity during the photocatalytic process.
3.4. Compound 1 Can Significantly Decrease the Viability of Gastric Cancer Cells
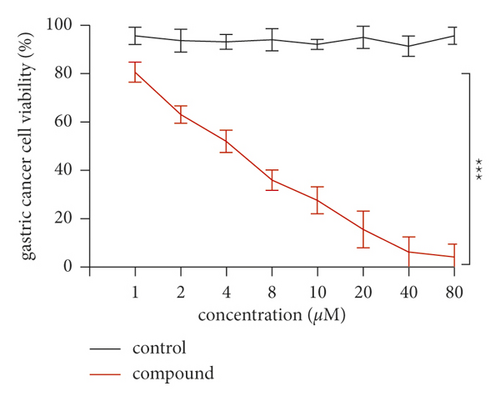
The inhibitory activity of the new compounds on gastric cancer cell viability was evaluated by the CCK-8 method (Figure 4). Specifically, gastric cancer cells were inoculated into 96-well plates and treated with the new compound for 2 days. The results showed that it could reduce the survival rate of gastric cancer cells compared with control cells. This result indicated that the compound has an excellent anti-gastric cancer activity through reducing the viability of the gastric cancer cells.
3.5. Activation Level of VEGF Signaling Pathway in Gastric Cancer Cells Decreased due to the Effect of Compound 1
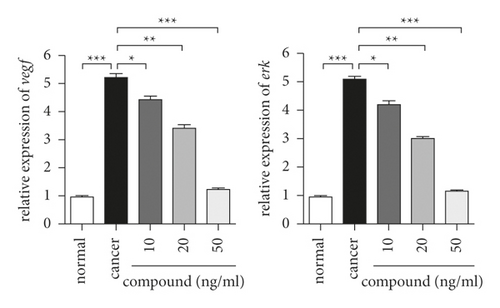
In these experiments, we drew a conclusion that the complex is one of the candidates for gastric cancer drugs. For gastric cancer cells, the role of the VEGF signaling pathway is mainly to regulate their survival ability. Later, we also used real-time RT-PCR to study the opening of this pathway in gastric cancer cells after compound treatment. The results in Figure 5 showed that the experimental group has the highest level of pathway activation in the two groups. The new compound significantly reduced the activation level of the VEGF signaling pathway in gastric cancer cells, and the degree of reduction was dose-dependent.
4. Conclusion
All in all, we have synthesized a new Cu(II) compound, which features a 1D infinite chain structure via the in situ hydrolysis and self-assembly reaction. Interchain hydrogen bond interactions result in a 2D supramolecular layer; furthermore, the three-dimensional supramolecular framework is formed by stacking layer to layer in staggered mode. In the photocatalytic experiment, under UV irradiation, the compounds can catalyze the degradation of MB in aqueous solution, and CCK-8 experiment results also proved that it could reduce the survival rate of gastric cancer cells. In addition, the compound also inhibits the activation level of this pathway in different ways.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.




