Thermophysical Studies of Chlorpheniramine with 1-Propanol at 303 K, 308 K, and 313 K in the Atmospheric Pressure
Abstract
A binary liquid system that consists of chlorpheniramine and 1-propanol is prepared at various concentrations by the mole fraction method. The density (ρ), viscosity (η), and ultrasonic velocity (U) of the system are observed at 303 K, 308 K, and 313 K. From the experimental observations, various thermophysical parameters such as adiabatic compressibility (β), free length (Lf), free volume (Vf), viscous relaxation time (τ), and Gibbs free energy (ΔG) are determined. The deviations of the excess parameters (βE, , , τE, and ΔGE) from their ideal values are calculated. The excess values are fitted with the Redlich–Kister polynomial function, and the corresponding coefficients are derived. Moreover, the standard deviations of the excess parameters are evaluated. The experimental and theoretical data confirmed the existence of hydrogen bonding interactions among the functional groups of the liquid mixture. The strength of intermolecular interactions decreased with increasing the temperature of the mixture. The strength of intermolecular interaction is noticed as 303 K > 308 K > 313 K.
1. Introduction
Intermolecular interactions between binary and ternary liquid mixtures are taking a vital role in the thrust area of theoretical and applied research [1–3]. The intermolecular interactions depend upon various intermolecular forces acting between the molecules present in the liquid system. Moreover, the change in temperature of the solutions leads to a significant change in the bonding and intermolecular forces among the dipoles [4, 5]. The measurement of ultrasonic velocity and related parameters is useful to analyse the strength of the intermolecular interactions between the liquid mixtures [6–8]. Furthermore, the interaction between the solute and solvent at various concentrations can be analysed by the experimental observations of the density (ρ), viscosity (η), and ultrasonic velocity (U) of the system. The adiabatic compressibility (β), free length (Lf), free volume (Vf), viscous relaxation time (τ), and Gibbs free energy (ΔG) can also be theoretically calculated by using the experimental data. The significant changes in those parameters are used to analyse the types of interactions between the functional groups present in the liquid system. The excess parameters that are deviated from their ideal values have also directed to the formation of bonding between the liquid mixtures. Manukonda et al. [9] reported changes in thermophysical changes of the N, N-dimethylaniline and 1-alkanols system. Preeti et al. [10] analysed the changes in the intermolecular interactions between acetonitrile with some alkyl methacrylates at various temperatures. Chlorpheniramine is one of the pharmaceutical valuable compounds. It takes part in the treatment of allergic rhinitis, cough, and the common cold [11]. In general, the interactions of alcohols respond to the temperature of the system. 1-Propanol has self-associative and polar nature. It can be used as a proton donor compound as well as a solvent [12]. The present work is an attempt to study the nature and strength of intermolecular interactions between chlorpheniramine and 1-propanol at 303 K, 308 K, and 313 K. Redlich–Kister polynomial function and standard deviations are also used to validate the experimental and theoretical observations.
2. Materials
The mole fraction method is adopted to prepare the various concentrations of the liquid mixtures. The airtight standard flasks are used to hold the solutions. Digital electronic balance (ACM-78094L, ACMAS Ltd., India) with the accuracy of ±1 mg was used to determine the mass of the liquid components. Specific gravity bottle and Ostwald’s viscometer are used to determine the density and viscosity of the solutions with the accuracy of ±0.01 kg/m3 and ±0.001 Ns/m2, respectively. A single frequency 2 MHz ultrasonic interferometer (Mittal Enterprises, India) with the accuracy of ±1 m/s is used to measure the ultrasonic velocity. The double distilled water is used to standardize the density, viscosity, and ultrasonic velocity measurements. AR grade chlorpheniramine and 1-propanol (purity mass fraction >0.99, S. D. Fine Chemicals, India) were used without further purification. The density, viscosity, and ultrasonic velocity of the pure liquids are compared with the available literature [13, 14] and given in Table 1. A constant temperature bath (INSREF model IRI-016C, India) with the accuracy of ±0.01 K is used to sustain the temperature of the solutions.
| S. no. | Liquid | Temperature (K) | (ρ) kg/m3 | (η)/10−3 N s/m2 | (U) m/s | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Expt | Lit | Expt | Lit | Expt | Lit | ||||
| 1 | Chlorpheniramine | 303 | 1260 | 1264.7 | 2.791 | 2.055 | 1820 | 1819 | [13] |
| 308 | 1257 | 1257.9 | 2.201 | 2.1949 | 1786 | 1786 | |||
| 313 | 1250 | 1250.1 | 1.980 | 2.3750 | 1766 | 1765 | |||
| 2 | 1-Propanol | 303 | 752 | 752.2 | 1.095 | 1.0948 | 1194 | 1194 | [14] |
| 308 | 751 | 751.3 | 0.920 | 0.9187 | 1192 | 1192 | |||
| 313 | 750 | 750.1 | 0.744 | 0.7426 | 1190 | 1191 | |||
3. Methods
4. Results and Discussion
The density (ρ), viscosity (η), and ultrasonic velocity (U) of chlorpheniramine and 1-propanol are measured at 303 K, 308 K, and 313 K. Furthermore, adiabatic compressibility (β), free length (Lf), free volume (Vf), viscous relaxation time (τ), and Gibbs free energy (ΔG) are evaluated at various concentrations.
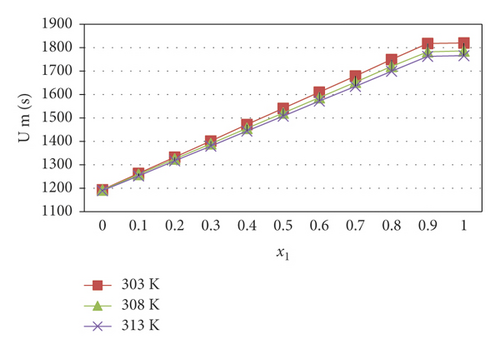
The observed significant changes in these parameters at various temperatures with the mole fraction of chlorpheniramine in the 1-propanol medium are given in Table 2. It is noted that the density of the liquid mixture increased with increasing chlorpheniramine concentration. It leads to the structure maker of the solute to the chosen solvent. This trend may also suggest the increasing solute-solvent interactions with chlorpheniramine concentrations. The shrinkage of chlorpheniramine molecules makes the solution in higher density comparisons than that of 1-propanol. The viscosity reflects the same trend as in the density effect of the solution. While increasing the temperature from 303 K to 313 K, both these parameters decreased. It may suggest the dissociation of the molecular clusters in the mixture. In general, the ultrasonic velocity increases with the active interaction of the solute-solvent system [19]. In the present study, the ultrasonic velocity increases with the chlorpheniramine concentrations, as shown in Figure 1. At the entire concentration, the ultrasonic velocities linearly vary with the mixing of 1-propanol. However, while increasing the temperature, the resonant wavelength of the ultrasonic wave in the solution decreased at the constant ultrasonic frequency. The corresponding changes in ultrasonic velocity with the chlorpheniramine concentrations reveal the existence of intermolecular interactions between the functional groups present in the liquid system. In general, the adiabatic compressibility is inversely proportional to the ultrasonic velocity and density. In this work, both the density and ultrasonic velocity increase with the chlorpheniramine concentration. The adiabatic compressibility decreases with increasing chlorpheniramine concentration. The decreasing trend of adiabatic compressibility signifies an increase in electrostrictive compression. After mixing chlorpheniramine with 1-propanol, the free length (Lf) decreased. In this study, the values of free length are inversely proportional to the ultrasonic velocity data. This result follows the Kincaid model [20].
| x1 | Ρ (kg/m3) | η/10−3 (Ns/m2) | U (m/s) | Β/10−10 (m2/N) | Lf/10−10 (m) | Vf/10−11 (m3/mol) | τ/10−12 (s) | ΔG (kJ/mol) | |
|---|---|---|---|---|---|---|---|---|---|
| T = 303 K | |||||||||
| 0.0000 | 752 | 1.095 | 1194 | 9.327 | 0.634 | 0.598 | 1.358 | 5.406 | |
| 0.1203 | 808 | 1.283 | 1264 | 7.746 | 0.578 | 0.877 | 1.322 | 5.340 | |
| 0.2065 | 865 | 1.472 | 1333 | 6.506 | 0.529 | 1.035 | 1.274 | 5.249 | |
| 0.3165 | 920 | 1.659 | 1402 | 5.529 | 0.488 | 1.267 | 1.220 | 5.135 | |
| 0.4077 | 977 | 1.847 | 1472 | 4.723 | 0.451 | 1.436 | 1.160 | 5.015 | |
| 0.5489 | 1032 | 2.034 | 1541 | 4.080 | 0.419 | 1.761 | 1.104 | 4.888 | |
| 0.6382 | 1088 | 2.223 | 1610 | 3.545 | 0.391 | 1.918 | 1.048 | 4.756 | |
| 0.7291 | 1145 | 2.398 | 1679 | 3.098 | 0.365 | 2.101 | 0.988 | 4.605 | |
| 0.8216 | 1201 | 2.599 | 1750 | 2.718 | 0.342 | 2.260 | 0.940 | 4.479 | |
| 0.9326 | 1256 | 2.787 | 1818 | 2.408 | 0.322 | 2.488 | 0.893 | 4.352 | |
| 1.0000 | 1260 | 2.791 | 1820 | 2.396 | 0.321 | 2.697 | 0.889 | 4.340 | |
| T = 308 K | |||||||||
| 0.0000 | 751 | 0.92 | 1192 | 9.371 | 0.641 | 0.774 | 1.147 | 5.105 | |
| 0.1203 | 807 | 1.062 | 1259 | 7.817 | 0.585 | 1.157 | 1.104 | 5.009 | |
| 0.2065 | 863 | 1.198 | 1325 | 6.600 | 0.538 | 1.397 | 1.052 | 4.882 | |
| 0.3165 | 920 | 1.346 | 1390 | 5.625 | 0.497 | 1.711 | 1.007 | 4.774 | |
| 0.4077 | 975 | 1.501 | 1456 | 4.838 | 0.461 | 1.928 | 0.966 | 4.666 | |
| 0.5489 | 1032 | 1.632 | 1521 | 4.188 | 0.428 | 2.402 | 0.909 | 4.509 | |
| 0.6382 | 1087 | 1.772 | 1587 | 3.652 | 0.400 | 2.638 | 0.861 | 4.371 | |
| 0.7291 | 1142 | 1.915 | 1653 | 3.204 | 0.375 | 2.876 | 0.816 | 4.238 | |
| 0.8216 | 1199 | 2.056 | 1720 | 2.819 | 0.352 | 3.129 | 0.771 | 4.088 | |
| 0.9326 | 1254 | 2.198 | 1782 | 2.511 | 0.332 | 3.448 | 0.734 | 3.967 | |
| 1.0000 | 1257 | 2.201 | 1786 | 2.494 | 0.331 | 3.744 | 0.730 | 3.949 | |
| T = 313 K | |||||||||
| 0.0000 | 750 | 0.744 | 1190 | 9.415 | 0.648 | 1.062 | 0.932 | 4.690 | |
| 0.1203 | 805 | 0.882 | 1253 | 7.912 | 0.594 | 1.518 | 0.928 | 4.684 | |
| 0.2065 | 861 | 1.019 | 1317 | 6.696 | 0.547 | 1.765 | 0.907 | 4.623 | |
| 0.3165 | 915 | 1.158 | 1380 | 5.738 | 0.506 | 2.121 | 0.884 | 4.551 | |
| 0.4077 | 972 | 1.297 | 1444 | 4.934 | 0.469 | 2.371 | 0.851 | 4.455 | |
| 0.5489 | 1025 | 1.436 | 1508 | 4.290 | 0.438 | 2.873 | 0.819 | 4.358 | |
| 0.6382 | 1081 | 1.573 | 1572 | 3.743 | 0.409 | 3.110 | 0.783 | 4.238 | |
| 0.7291 | 1135 | 1.712 | 1635 | 3.295 | 0.383 | 3.347 | 0.750 | 4.130 | |
| 0.8216 | 1191 | 1.849 | 1699 | 2.908 | 0.360 | 3.602 | 0.715 | 4.003 | |
| 0.9326 | 1246 | 1.987 | 1763 | 2.582 | 0.339 | 3.947 | 0.682 | 3.883 | |
| 1.0000 | 1250 | 1.980 | 1766 | 2.565 | 0.338 | 4.315 | 0.675 | 3.853 | |
It is observed that the free volume (Vf) of the system increased with increasing temperature as well as the concentration of chlorpheniramine. It may be due to the dispersive forces acting on the molecules and signify the dissociation of the bonds in the mixture. Furthermore, increasing the temperature of the solution weakened the intermolecular interactions between the proton donor and acceptor functional groups present in the liquid system. The viscous relaxation time decreased with the increasing mole fraction of chlorpheniramine at the entire range of temperatures. It suggested the rupture in the molecular cluster of the liquid system. The significant changes in the Gibbs free energy with the chlorpheniramine concentration are shown in Figure 2. The plot signifies the decreasing Gibbs free energy with increasing temperature and chlorpheniramine. It confirmed that solute-solvent interaction occurs and reveals the corresponding changes in the equilibrium states and the existence of specific weak hydrogen bonding interaction (N-H…O-H) between the molecules in the liquid system.

The sign and deviation of the excess parameters from their ideal values are useful to analyse the nature and strength of the intermolecular interactions. Fort et al. [21] reported that the negative excess parameters raised due to the existence of hydrogen bonding in the intermolecular interactions and positive values lead to weak interactions between the unlike functional group constituents in the system.
The variations of excess adiabatic compressibility (βE), excess free length (), excess free volume (), excess viscous relaxation time (τE), excess Gibbs free energy (ΔGE), and excess molar volume are given in Table 3.
| x1 | βE/10−10 (m2/N) | /10−11 (m) | /10−8 (m3/mol−1) | τE/10−12 (s) | ΔGE (kJ/mol) | /10−6 (m3/mol) |
|---|---|---|---|---|---|---|
| T = 303 K | ||||||
| 0.0000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 0.1203 | −0.261 | −0.048 | −0.081 | −0.018 | −0.247 | 0.977 |
| 0.2065 | −0.538 | −0.123 | −0.191 | −0.063 | −0.523 | 1.223 |
| 0.3165 | −0.795 | −0.196 | −0.300 | −0.106 | −0.781 | 1.547 |
| 0.4077 | −1.022 | −0.263 | −0.406 | −0.144 | −1.007 | 1.479 |
| 0.5489 | −1.199 | −0.318 | −0.501 | −0.176 | −1.185 | 1.660 |
| 0.6382 | −1.306 | −0.357 | −0.576 | −0.197 | −1.292 | 1.302 |
| 0.7291 | −1.308 | −0.367 | −0.611 | −0.201 | −1.294 | 0.850 |
| 0.8216 | −1.151 | −0.331 | −0.575 | −0.178 | −1.137 | 0.345 |
| 0.9326 | −0.752 | −0.216 | −0.404 | −0.111 | −0.738 | 0.151 |
| 1.0000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| T = 308 K | ||||||
| 0.0000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 0.1203 | −0.271 | −0.051 | −0.103 | −0.013 | −0.257 | 0.974 |
| 0.2065 | −0.557 | −0.128 | −0.236 | −0.053 | −0.542 | 1.232 |
| 0.3165 | −0.824 | −0.203 | −0.369 | −0.092 | −0.809 | 1.524 |
| 0.4077 | −1.057 | −0.271 | −0.497 | −0.126 | −1.043 | 1.483 |
| 0.5489 | −1.242 | −0.328 | −0.612 | −0.155 | −1.227 | 1.627 |
| 0.6382 | −1.353 | −0.368 | −0.702 | −0.174 | −1.339 | 1.282 |
| 0.7291 | −1.355 | −0.378 | −0.746 | −0.177 | −1.340 | 0.859 |
| 0.8216 | −1.192 | −0.340 | −0.703 | −0.156 | −1.178 | 0.333 |
| 0.9326 | −0.779 | −0.223 | −0.496 | −0.097 | −0.765 | 0.167 |
| 1.0000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| T = 313 K | ||||||
| 0.0000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 0.1203 | −0.281 | −0.053 | −0.129 | −0.009 | −0.267 | 0.976 |
| 0.2065 | −0.577 | −0.133 | −0.289 | −0.046 | −0.563 | 1.226 |
| 0.3165 | −0.852 | −0.210 | −0.448 | −0.080 | −0.838 | 1.554 |
| 0.4077 | −1.094 | −0.280 | −0.601 | −0.112 | −1.079 | 1.473 |
| 0.5489 | −1.284 | −0.340 | −0.740 | −0.137 | −1.269 | 1.672 |
| 0.6382 | −1.398 | −0.380 | −0.848 | −0.154 | −1.383 | 1.300 |
| 0.7291 | −1.398 | −0.390 | −0.901 | −0.157 | −1.384 | 0.884 |
| 0.8216 | −1.231 | −0.351 | −0.849 | −0.139 | −1.216 | 0.363 |
| 0.9326 | −0.805 | −0.231 | −0.601 | −0.084 | −0.790 | 0.149 |
| 1.0000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
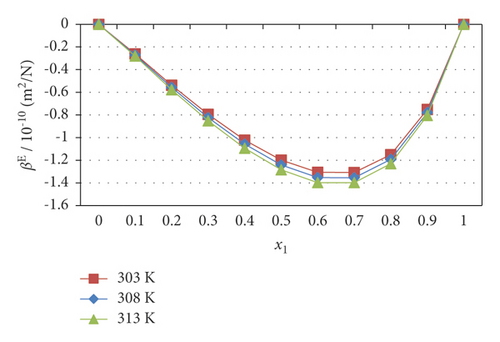
In this study, the above parameters show negative signs. The negative excess adiabatic compressibility (βE) values are progressively increased and decayed with the increasing concentration of chlorpheniramine, as shown in Figure 3. The negative values slightly decrease with an increase in the entire range of temperature. Moreover, the negative excess compressibility (βE) increased up to a critical concentration (x1, 0.5), decreasing in the 1-propanol medium. This trend suggested that the dissociation of dipoles increased in 1-propanol rich concentrations. Furthermore, the nonlinear variations in the excess free volume () also suggested that the strength of intermolecular interaction increased with the increasing mole fraction of chlorpheniramine. The excess viscous relaxation time (τE) and excess Gibbs energy (ΔGE) also supported the experimental observations. The excess free molar volume increase with 1-propanol concentrations and decrease with increasing chlorpheniramine molecules. Furthermore, the positive signifies dispersive force acting on the mixture. It leads to dissociation in order to increase 1-propanol concentrations. The changes in excess parameters reveal the polynomial curve. Therefore, the Redlich–Kister polynomial coefficients and the standard deviation of the theoretical data are also calculated and given in Table 4. The standard deviation values validated the theoretical calculation of these excess parameters.
| Parameters | Temperature (K) | a0 | a1 | a2 | σ |
|---|---|---|---|---|---|
| βE/10−10 (m2/N) | 303 | −0.454 | 0.328 | −0.214 | 0.049 |
| 308 | −0.324 | −0.312 | −0.096 | 0.045 | |
| 313 | −0.324 | 0.402 | −0.103 | 0.046 | |
| /10−11 (m) | 303 | −1.010 | 0.481 | 0.180 | 0.062 |
| 308 | −0.772 | 0.177 | 0.202 | 0.049 | |
| 313 | −1.170 | 0.520 | 0.308 | 0.063 | |
| /10−8 (m3/mol) | 303 | −0.255 | −0.625 | 0.542 | 0.051 |
| 308 | −0.309 | −1.005 | 0.973 | 0.057 | |
| 313 | −0.341 | −1.463 | 1.397 | 0.065 | |
| τE/10−12 (s) | 303 | −0.093 | −0.111 | −0.133 | 0.048 |
| 308 | −0.027 | −0.089 | −0.220 | 0.052 | |
| 313 | 0.027 | −0.034 | −0.329 | 0.047 | |
| ΔGE (kJ/mol) | 303 | −2.034 | 1.120 | 0.570 | 0.055 |
| 308 | 0.082 | 0.438 | −0.856 | 0.048 | |
| 313 | 0.332 | −2.146 | −2.424 | 0.062 | |
| /10−6 (m3/mol) | 303 | 0.134 | 2.825 | 1.836 | 0.134 |
| 308 | 1.974 | 0.815 | 1.373 | 0.683 | |
| 313 | 0.137 | 0.2068 | 1.434 | 0.8545 |
5. Conclusion
The density (ρ), viscosity (η), and ultrasonic velocity (U) of chlorpheniramine with 1-propanol were measured at 303 K, 308 K, and 313 K in the atmospheric pressure. Using the experimental data, adiabatic compressibility (β), free length (Lf), free volume (Vf), viscous relaxation time (τ), and Gibbs free energy (ΔG) are derived. These parameters significantly vary with the temperature and concentrations of the solute-solvent in the liquid system. The deviations of these parameters from the ideal values are also calculated. The systematic observation reveals that the presence of hydrogen bonding interaction between the amine group and hydroxyl group existed in chlorpheniramine and 1-propanol, respectively. Redlich–Kister coefficients and standard deviations are also supporting the legitimacy of the observations. The experimental and theoretical calculations confirmed the existence of intermolecular interaction between chlorpheniramine and 1-propanol. The strength of the interaction is found greater in the higher chlorpheniramine concentrations. The dissociation is observed in 1-propanol concentrations. Furthermore, while increasing the temperature, the strength of the intermolecular interaction decreased and was found in the order of 303 K > 308 K > 313 K.
Conflicts of Interest
The author declares that there are no conflicts of interest.
Acknowledgments
The author is thankful to the Department of Physics, Research and Technology Transfer Centre, Wollega University, Nekemte, Ethiopia (WU/S1/108), for their support by providing necessary facilities to complete this work.
Open Research
Data Availability
The data used to support the findings of this study are included within the article.