Stability Analysis and Optimal Control Strategies of Giving Up Relapse Smoking Model with Bilinear and Harmonic Mean Type of Incidence Rates
Abstract
In this manuscript, a giving-up smoking model is develop using bilinear incidence rate, harmonic mean type of incidence rate and keeping in view the relapse factor associated to smoking. It is shown that the model' solution is bounded and positive for the appropriate initial data. The equilibria of the model are obtained, and it is proved that the smoking-free equilibrium is both locally and globally asymptotically stable for R0 less than unity. It is shown that the model has a positive light smoker present equilibrium whenever R0 is greater than one, and it is locally asymptotically stable if we have 1 < R0 < 1 + 2β1(μ + d + δ)/Λβ2. Conditions for the global stability of light smoker present equilibrium are rigorously investigated. Also, it is proved that the model has a smoking present equilibrium when R0 satisfies some condition which is investigated both for local and global behavior. By considering a few control measures, optimal control strategies are achieved with the help of Pontryagin’s maximum principle. The analytical results are verified numerically, and effectiveness of the control program is presented.
1. Introduction
Among other diseases, infectious diseases are the most alarming threat to humanity from the last few centuries. The plague of Athens is considered to be the first ever epidemic which affects human life to a great extent [1, 2]. The life in Egypt and Roman was completely demolished by the smallpox in 165-180 C.E. in which millions of people died [3]. The epidemic Black Death in Europe is observed to be the first well-documented epidemic which killed more than 50 millions of people that region and Mediterranean. Various other epidemics affected human life throughout the history, and the current corona virus disease is the last epidemic whose disasters are in front of us. Thus, it is very crucial to understand the dynamics of such infectious disease (particularly the emerging epidemics) and to control its spread in early transmission phase. In the context of these extensive illnesses, the tobacco pandemic is one of the world’s most serious health threats. According to statistics, up to 50% of smokers die as a result of their habit, a tobacco-related mortality occurs every 8 seconds, and 10 percent of the adult population dies as a result of tobacco-related illnesses [4].
Smoking habit grows and spreads across a society in the same way as an epidemic illness does, and generally, it nearly follows the same process. To be specific, people are prone to smoking at first, then become active users, and eventually recover through certain control methods or self-abandonment of its usage. In the early twentieth century, William Hamer’s work on mathematical modeling of infectious illnesses achieved substantial progress. Perhaps, Hamer was the first who introduces mass action law in epidemic modeling. However, Sir Ronald Ross is considered the father of today’s mathematical biology due to his work on malaria. In 1911, he published a book regarding malaria in which he used mathematical models for describing the dynamics of the infection and calculated the threshold parameters. The formal mathematical biology started with the work of Kermack and McKendrick [5], and then, significant contributions were made in the subject, for instance, one can see [6–11].
To study the transmission of smoking habits and its control in the society, numerous researchers used the tools of mathematical modeling and optimal control theory. Castillo-Garrsow et al. [12] used a model of SIR type and well-presented the qualitative aspects of the model under discussion. Sharomi and Gumel [13] extended the work of Castillo-Garrsow et al. and introduced temporary quit class into the model. Zaman [14] incorporated the light smoker compartment and rigorously investigated the model from mathematical perspectives. Subsequently, considering various features and characteristics of smoking, sophisticated models have been developed and investigated, for example, [15–18].
Incidence rate plays a crucial role while investigating the dynamics of any epidemic disease. The bilinear and saturated incidence rates were widely used in case of epidemic diseases and smoking models, for instance, one can see [12, 19, 20]. The authors in [17, 21, 22] used the square root incidence rate and discussed the models from various mathematical aspects. In the sequel, Rahman et al. [4] used harmonic mean type of incidence rate in his giving-up smoking model, investigated the dynamics of the model, and set the controlling strategies for reducing the smoking habit. Similarly, Alzaid and Alkahtani used the same incidence rate and studied the effect of relapse [23]. This work as well as the work of Rahman et al. assumed that potential smokers will start smoking only if they make a contact with light smoker. However, this is usually not the case. A person (potential smoker) may start smoking when he/she comes into contact with a smoker. In this work, the authors intend to overcome this gap. We shall assume that smoking habit may spread in the population via two roots; (i) contacts of potential smoker and light smokers and (ii) contact between smoker and light smokers. For the former type of spread, we will use the usual harmonic mean type of incidence rate, and for the later, the standard bilinear incidence rate will be utilized. By doing so, the present model will cover models [4, 23] as a subcase.
The rest of the work is organized as follows. The model formulation and essential biological features to the model are discussed in Section 2. The smoking generation number and equilibria of the model are presented in Section 3. We investigated the local stability of each equilibrium point in Section 4, whereas the criteria for global dynamics of smoking-free, light smoker present, and smoking-present equilibria are derived in Section 5. The local sensitivity will be performed in Section 6. Based on sensitivity analysis, we take into account some control variables and formulated a control problem for further analysis in Section 7. The desired goals were obtained, and the results are verified through simulations in Section 8. Finally, we presented the conclusion of the work.
2. Model Formulation and Well-Posedness
| Parameter | Interpretation |
|---|---|
| Λ | Constant recruitment into the potential smokers |
| β1 | The contact rate of occasional and potential smokers |
| β2 | The contact rate between smokers and light smokers |
| μ | The natural mortality rate |
| d0 | Death rate due to smoking |
| δ | Temporarily quitting rate of smokers |
Theorem 1. For the nonnegative initial data, if a solution to model (1) exists, it must be positive.
Proof. Consider the second equation in model (1), and its solution is given by
By using this relation in the third equation of system (1), we have S(t) ≥ 0 which assures the positivity of Q(t). Finally, using these facts in the first equation, we have P(t) ≥ 0.
Theorem 2. For nonnegative initial conditions that are not identically zero on any interval, all possible solutions of system (1) are bounded in the region
Letting t⟶∞, we get T(t) ≤ Λ/μ. Thus, all solutions of the model are bounded by 0 and Λ/μ, and hence, the desired feasible set is (4).
Theorem 3. For system (1), the nonnegative space is positive invariant.
Proof. Consider the vector Ψ = (P, L, S, Q); then, in matrix form, we can write model (1) in the form
Clearly, the nondiagonal entries of matrix are nonnegative which guarantees that is Metzler matrix [24]. Further, , and thus, system (1) is positively invariant in the desired space. In the next section, we intended to find the smoking generation number (an important parameter describing the behavior of a system) as well as the possible equilibria of the proposed system.
3. Equilibria of the Model and Smoker Generation Number
This fixed point is known as smoking-free equilibrium of the model.
By using the number R0, we can obtain other possible equilibria of system (1) using equation (3).
Theorem 4.
- (1)
If R0 > 1, then model (1) has a positive light smoker present equilibrium given by
Proof. By solving systems (10)–(13) and keeping in view that there are no smokers (i.e., S(t) = 0) in the community, we obtained the light smoker present equilibrium (21). However, for R0 < 1, the light smoker becomes negative and hence, the model will have just the smoking-free equilibrium (14). In the similar way, if we assume R0 = 1, then the fixed point (21) becomes the smoking-free equilibrium as we have Λ/2β1 = Λ/(μ + d)R0.
Theorem 5. There exists a positive smoking present equilibrium of model (1) blue if R0 ≥ 1 and β2S∗ < μ + d.
4. Local Stability Analysis of Equilibria
Theorem 6. The SFE (14) of system (1) is locally asymptotically stable for R0 < 1.
From relation (25), the Jacobian of system (1) at E0 is given by
Matrix (26) has eigenvalues t1 = −(μ + d), t2 = −(μ + d + δ), t3 = 2β1 − (μ + d), and t4 = −(μ + d + γ). As the parameters of the model assume positive values, thus the eigenvalues t1, t2, and t4 are negative and t3 = (μ + d)(2β1/μ + d − 1) = (μ + d)(R0 − 1) is negative only if R0 < 1. Hence, for R0 < 1, all of the eigenvalues of the Jacobian matrix at E0 are negative and so E0 is locally asymptotically stable. Further, if R0 = 1, then t3 = 0. However, if we assume R0 > 1, then E0 becomes unstable as one of the eigenvalues is positive in such case.
Theorem 7. The local smoking present equilibrium (LSPE) (21) of system (1) is locally asymptotically stable for 1 < R0 < 1 + 2β1(μ + d + δ)/Λβ2.
Proof. From relation (25), the Jacobian of system (1) at El is given by
The eigenvalues of matrix (30) are t1 = −(μ + d), t2 = −(μ + d)(R0 − 1), t3 = −(μ + d + γ), and t4 = (R0 − 1)Λβ2/2β1 − (μ + d + δ). Now, for positive parameters of the model and for R0 > 1, the eigenvalues t1, t2, and t3 are negative. However, t4 is negative only for R0 < 1 + 2β1(μ + d + δ)/Λβ2. Hence, if 1 < R0 < 1 + 2β1(μ + d + δ)/Λβ2, all of the eigenvalues of the Jacobian matrix at El are negative and so El is locally asymptotically stable. Moreover, for R0 = 1, we have t2 = 0. In the case of R0 < 1, t2 becomes positive and thus, the equilibrium El will be unstable.
Theorem 8. The smoking present equilibrium (SPE) is locally asymptotically stable of if 1 < R0 < 1 + β2S∗/μ + d.
Proof. The Jacobian at SPE is given by
The characteristic equation of matrix (28) is given by
Clearly, a11, a12, a21, a32, and a44 are positive and
only if β2S∗ > (d + μ)(R0 − 1). Thus, all of the coefficients of the characteristic equation (35) are positive, and hence, by Descartes’ rule of signs, this equation has no positive real solution. By using −τ in place of τ in equation (35) and utilizing Descartes’ rule of signs, we get that all of the eigenvalues of this equation are negative or complex conjugates with dominant negative real part. Therefore, the SPE is locally asymptotically stable whenever R0 < 1 + β2S∗/μ + d. Further, for the existence of SPE, we must assume that R0 > 1. Moreover, the case R0 = 1 does not affect the stability of the equilibrium point; however, this condition will challenge the existence of smoking-present equilibrium.
5. Global Stability Analysis of the Equilibria
Theorem 9. Let R0 ≤ 1; then, the smoking-free equilibrium (SFE) E0 of model (1) is globally asymptotically stable (GAS).
Proof. For proving the required result, the Lyapunov function of the following form is assumed:
By considering the derivative of (37) with respect to time t and using model (1), we have
Hence,
Theorem 10. If 1 < R0 < β2Λ + 2β1(d + μ)/β2Λ, then the local smoking present equilibrium (LSPE) El of model (1) is globally asymptotically stable (GAS).
Proof. To prove the main result, we will take into consideration the Lyapunov function of the form
By considering the derivative of (37) with respect to time t and using model (1), we have
Theorem 11. If R0 > 1, β2S∗ < μ + d and , then (1) is GAS at SPE E∗.
Proof. By considering the Jacobean (25) of the system at the SPE, we have
The third additive compound matrix of J∗ is given by
where
Consider ρ(χ) = diag{Q, S, L, P} such that ρ(χ−1) = diag{1/Q, 1/S, 1/L, 1/P} and time derivative is Therefore,
By defining B = ρfρ−1+∣2∣ρ−1 so that
Consequently,
Now we have
By combining the preceding four inequalities, we have the following inequality.
6. Local Sensitivity Analysis
The term R0 is generally influenced by the inconsistencies in data gathering and estimated values. Sensitivity analysis is used to assess the relative impact of epidemic factors for disease propagation and control.
Definition 12. For the basic reproduction number, the normalized sensitivity index of a parameter ψ (which depends on the partial derivative of R0 w.r.t ψ) is of the following form:
These indices enable us to determine the relevance of numerous variables involved in disease transmission, as well as the relative change in the number of reproductions as a function of parameter changes. Using such indices, we may identify the characteristics that have a significant impact on R0 and are critical for disease prevention.
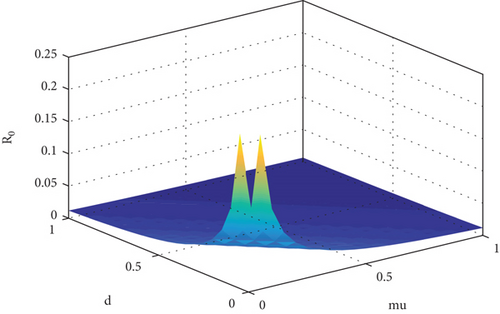
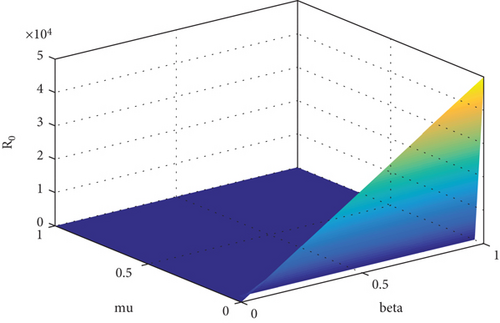
Table 2 suggests that there is a positive relation between R0 and β1 while negative influence of μ and d on R0. The same argument is supported by Figure 1. This means that if you raise or reduce the value of parameter β1 by 10%, it will rise or decrease R0 by 10%. Similarly, if we reduce or increase μ and d by 1 percent, its relative inverse impact on R0 will be 99.9% and 0.04%, respectively. To prevent smoking from the population, we must consider those parameters which have high sensitivity indices. Based on the sensitivity indices, we concluded that β1 will directly affect R0 (100%) whereas μ affects R0 inversely about 99%. Thus, to reduce smoking habit, we must focus on the contacts between potential smokers and light smokers. It is simple to develop a control mechanism program for eradication of smoking habit based on this study.
| Parameters | Sensitivity index | Value |
|---|---|---|
| β1 |
|
1 |
| μ | Sμ | −0.999500 |
| d | Sd | −0.00049975 |
7. Formulation and Analysis of Optimal Control Problem
In this part, we simulate the eradication of smoking habit from the population by using the tools of control theory [9, 28]. We focus on reducing transmission, which has a sensitivity index of 1, to develop a control plan based on local sensitivity analysis. Further, to make the control program more effective, we take into consideration the following four control measures.
7.1. Existence of Solution to the Control Problem
Theorem 13. There exists a control vector which minimizes the objective functional.
Proof. In order to show that actually such control variables exist, we need to follow [28], as
- (a)
Both the state and control functions are nonnegative
- (b)
Convexity and closedness properties hold by the set of admissible controls
- (c)
The boundedness of the control model assures the compactness
- (d)
The function inside the integral in the objective functional (57) is convex in the control measures
Therefore, there exist the control variables ui(t) for i = 1, 2, 3, 4 which minimize the objective functional.
7.2. Optimality Conditions
Theorem 14. Let P⋆, L⋆, S⋆, and Q⋆ be optimal states associated with the optimal controls for problem (55). Then, there exist adjoint variables λ1(t), λ2(t), λ3(t), and λ4(t) satisfying
Proof. The adjoint system (72) is obtained by using the adjoint equation (69c) in the maximum principles. The terminal conditions of (76) were derived as a direct consequence of equation (71). We took the partial derivatives of with respect to the control measure ui for i = 1, ⋯, 4 in turns and used (69b) for the derivation of (77)–(80).
8. Simulation Results
For solving the problems (both with and without controls), we used the standard RK method of fourth order. To verify numerically the key theorems on dynamical analysis, we utilized parameter values from Tables 3 and 4. Specifically, values of parameters from Table 3 were used to show the stability of smoking-free equilibrium, and sequentially, parameter values from Table 4 guarantee the stability of light smoker present equilibrium. For presenting the effect of control measures, we assumed values from Table 5. For simulating the control problem, firstly, we solved model (55) forward in time and then used backward RK4 method for solving the adjoint system (72) with the help of terminal conditions and characterization of the control variables (77)–(80). The simulation clearly illustrates the results on dynamical analysis (Figures 2 and 3) as well as on control theory (Figures 4 and 5).
| Parameters | Values | Parameters | Values |
|---|---|---|---|
| Λ | 10.25 | μ | 0.08 |
| γ | 0.006 | β1 | 0.008 |
| β2 | 0.00038 | d | 0.0019 |
| δ | 0.000274 |
| Parameters | Values | Parameters | Values |
|---|---|---|---|
| Λ | 10.25 | μ | 0.0111 |
| γ | 0.006 | β1 | 0.038 |
| β2 | 0.00038 | d | 0.0019 |
| δ | 0.041 |
| Parameters | Values | Parameters | Values |
|---|---|---|---|
| Λ | 10.25 | μ | 0.0111 |
| γ | 0.006 | β1 | 0.9 |
| β2 | 0.00038 | d | 0.0019 |
| δ | 0.000274 | q | 0.2 |
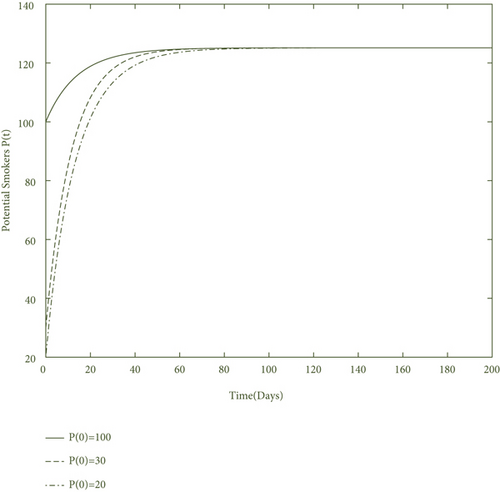
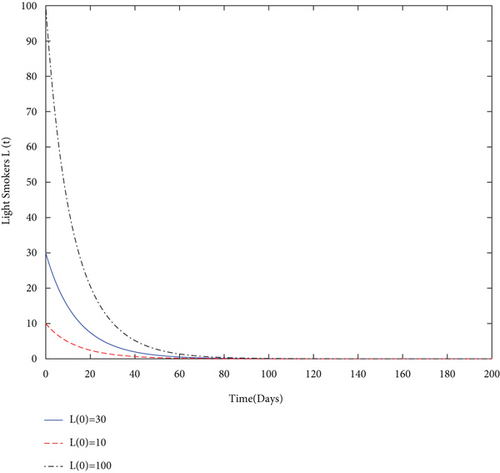
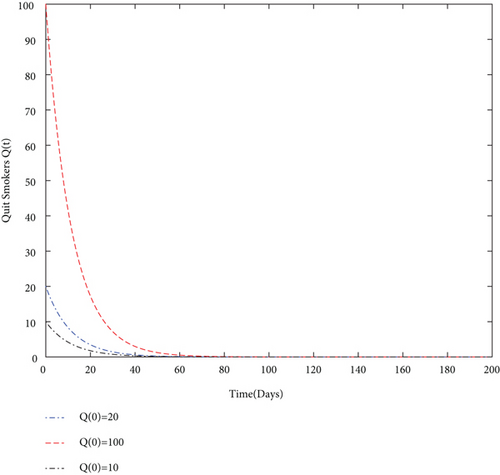
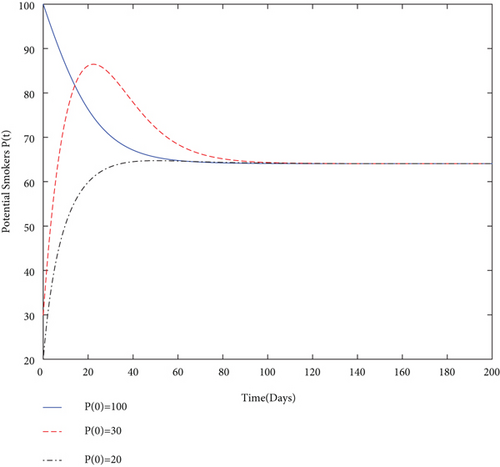
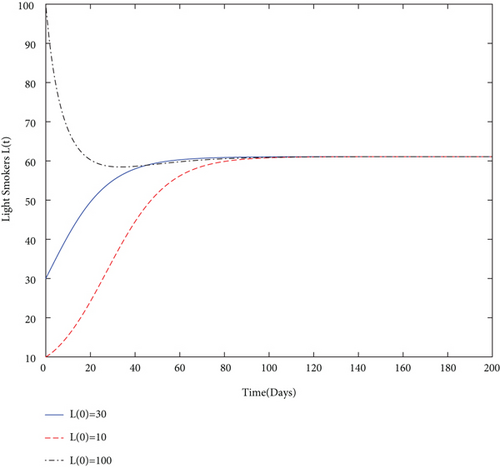
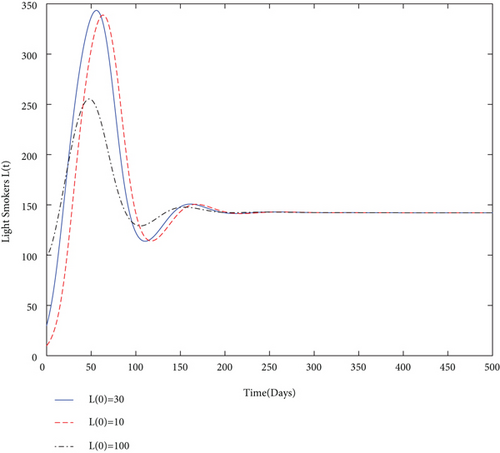
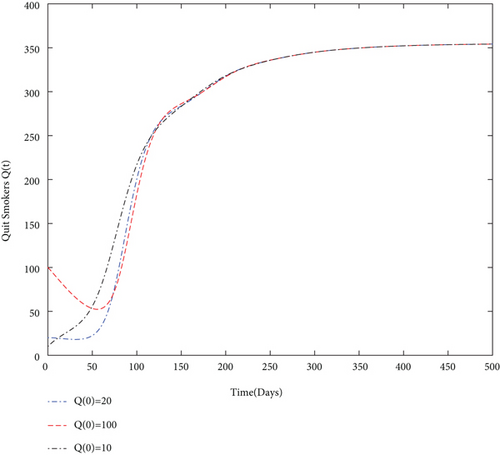
By using values of parameter from Table 3, calculate the smoking generation number R0 = 0.1954 and SFE E0 = (125.1526,0, 0, 0). It is clear from Figure 2 that each solution curve in the subplots tends to its SFE irrespective of the initial size of the population whenever R0 < 1.
To show numerically the global stability of light smoker present equilibrium El (21), we assumed the same values of Table 3 except β1 = 0.08 which gives R0 = 1.9536 and as a result, El = (Pl, Ll, Sl, Ql) = (64.0625,61.0901,0, 0). Thus, Figures 6(a)–6(d) shows a clear interpretation of Theorems 7 and 10, that is, if R0 > 1, then (P, L, S, Q)⟶(Pl, Ll, Sl, Ql) as t⟶∞.
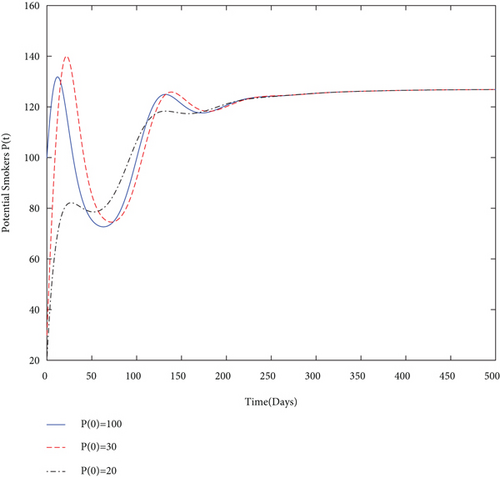
Similarly, to show the long-term behavior of each class whenever R0 > 1 and the side condition of Theorem 8 holds, we take into consideration values from Table 4. From these values of parameters, we calculated R0 = 12.3077 > 1 and (P∗, L∗, S∗, Q∗) = (127,142.1,164.5,354.9). Figure 3 proves the statement of Theorem 8 as well as the global stability of SPE numerically.
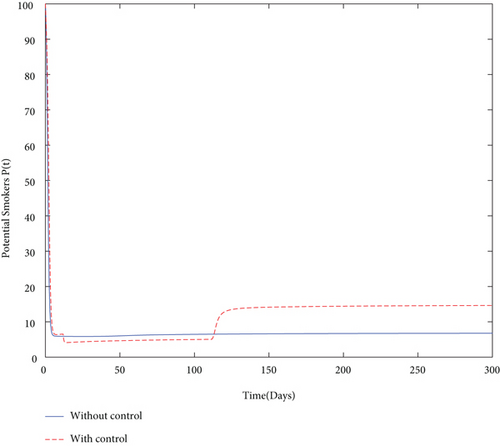
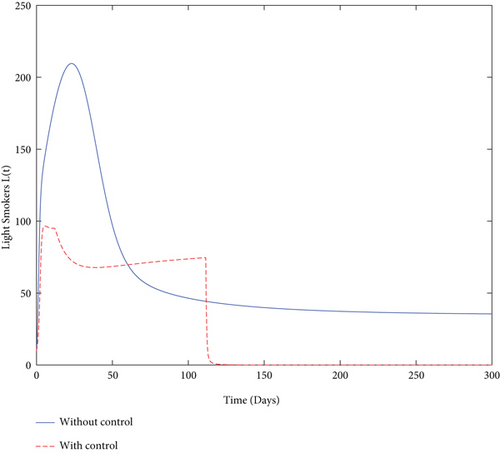
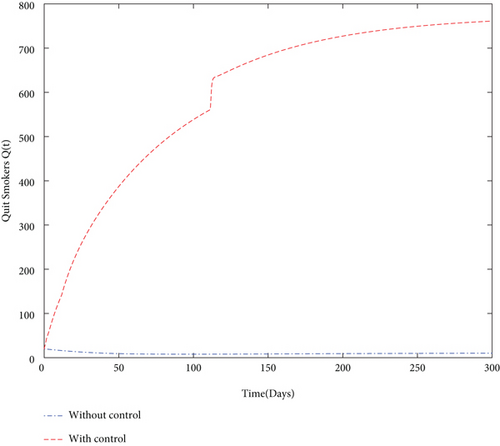
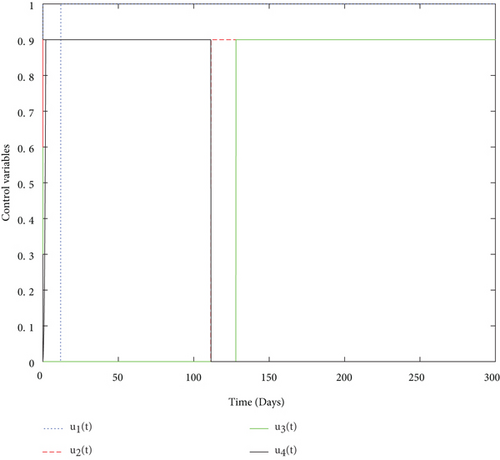
Finally, we used values from Table 5 and simulated both with and without control problems and presented the effect of the control variables. The simulation (in Figure 4) depicts in a clear way the effect of the control variables: to decrease the size of smoker population and to increase the number of quit smokers.
9. Concluding Remarks
In this work, we formulated and analyzed a giving-up smoking model utilizing three main factors related to smoking habit: the bilinear incidence rate showing the spread of smoking habit within the population from the contact of light smokers and smokers, the harmonic mean type of incidence rate (for incorporating contacts between potential smokers and occasional smokers), and the relapse factor associated to smoking. After model formulation, we investigated the model for bounded and positive solution subject to appropriate initial conditions. The model was checked for possible fixed points, and three equilibria were derived under certain conditions, namely, the smoking-free equilibrium, light smoker present equilibrium, and smoking present equilibrium. It was shown that the smoking-free fixed point always exists and is both locally and globally asymptotically stable when R0 < 1. The positive light smoker present equilibrium exists if we assume R0 > 1, and the same fixed point is locally as well as globally asymptotically stable if we impose additional conditions on the basic reproduction number. Further, we proved that the model has a smoking present equilibrium whenever additional conditions are satisfied by R0. Also, a criteria for the local analysis has been derived, and the global stability of smoking present equilibrium was presented both analytically and numerically. Keeping in mind a few control measures, we set a control problem and optimal control strategies were achieved with the help of Pontryagin’s maximum principle. The obtained analytical results were verified through simulations and effectiveness of the control program is presented by comparing with and without control curves.
This work is indeed a very good contribution to the existing literature on smoking epidemics. However, this social evil has a dramatic impact on every society, and thus, one cannot ignore the memory effect while studying such and related problems. Keeping in view the importance of generalized fractional derivatives in this regard [10–30], the authors have a keen interest to formulate and analyze the fractional models for smoking epidemics in near future.
Conflicts of Interest
The author declares no conflicts of interest.
Acknowledgments
The author extends appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. RG-1437-017.
Open Research
Data Availability
Data availability is not applicable to this research.




