Development and Validation of a Novel High-Performance Liquid Chromatography (HPLC)/Refractive Index Detector (RID) Method to Determine Residual Polyvinyl Alcohol (PVA) in Microspheres
Abstract
Polyvinyl alcohol (PVA) is a commonly used emulsifier for the preparation of DL-lactide-co-glycolide (PLGA) and polylactic acid (PLA) microspheres with sustained-release profile. Although water-soluble PVA is normally considered to have low toxicity, monitoring residual level of PVAs is still necessary, especially for the chronic disease treatment medications containing PVA. In this study, a robust and validated method was developed for the determination of PVA using high-performance liquid chromatography (HPLC) and refractive index detector (RID). PVA in sustained-release microspheres containing PLGA, aripiprazole, mannitol, and PVA as active substances was analyzed by size exclusion chromatography (SEC) using acetonitrile/water/trifluoroacetic acid (TFA) (300 : 700 : 1; v/v/v) as mobile phase at a flow rate of 0.5 mL/min. The retention time was 11.2 min and PVA was well separated with other components with good linearity (r2 = 0.9997) in the range of 2.5∼75 μg/mL. The limit of detection (LOD) was 0.51 μg/mL and the limit of quantification (LOQ) was 2.53 μg/mL. The relative standard deviations of intra- and interday precision under different concentrations were not more than 2.1%, and the recoveries were all in the ranges of 95∼105%. The established method has been demonstrated to be accurate, precise, repeatable, specific, and robust and, therefore, suitable for routine analysis of PVA in not only pharmaceutical field but also food and textile industries.
1. Introduction
Polyvinyl alcohol (PVA) is a water-soluble linear hydrophilic polymer synthesized via partial or full hydrolysis of polyvinyl acetate, which has been widely used in many areas, including textiles, paper, adhesives, cement, and films [1–3]. Recently it has been applied extensively as an emulsion stabilizer, a carrier for controlled release of the drug molecules and other biopharmaceutical or medical applications [4–8], particularly for transdermal patches, nanoparticles, and microspheres. Although water-soluble PVAs are normally considered as low toxicity chemicals, monitoring the residual levels of PVAs is still necessary especially for the chronic disease treatment medications containing PVA. Moreover, according to the data of the International Agency for Research on Cancer (IARC), PVA is considered as the third carcinogen. It is recommended to monitor the residual PVA level in relevant pharmaceutical products; thus, developing and validating a sensitive, reliable, and easily available method in the pharmaceutical field is meaningful.
Since PVA has insufficient ultraviolet (UV) chromophores and fluorophores, HPLC with UV or fluorescence detector is not suitable unless preliminary treatments such as derivatization or complexation are employed. Due to the high molecular weight of the PVA of approximately 22 kDa, a SEC analysis procedure can be considered for quantification of PVA. SEC is a noninteractive technique which separates solutes according to their molecular size or chain length in solution. Resolution (R) in SEC is determined by the volume of pores with diameters between the inclusion and exclusion limits of the analytes. The elution volume is determined by the accessibility of the sample molecule to the pores. Maximum elution volume occurs if the sample can fully access the pores. While minimal elution volume occurs if the sample is larger than the pores, samples elute in order of size, with the highest molecular weight samples eluting first. If the analyte cannot enter the pores, it passes through the column in the channels between the particles. Analytes that can enter the pores, either partially or completely, elute later. Since molecules are eluted based on their size in solution, linear or rod-like molecules will elute before globular molecules of the same molecular weight.
Researches on quantitative analysis of PVA have been reported in the literature using ultraviolet spectrophotometric detection [9–12], infrared spectrophotometric detection [13], static laser scattering detection [14], gel filtration liquid chromatography with refractometry [15], adsorptive stripping voltammetry [16], SEC with evaporative light scattering detector (ELSD) [17] or RID [18], and reversed-phase high-performance liquid chromatography (RP-HPLC) with a chiral column [19]. Most of these methods are used in the textile or paper industry by detecting the absorbance of PVA-iodine-borate complex according to Beer-Lambert law. However, the reaction of PVA with boric acid and iodine can be easily affected not only by the reaction temperature, the amount of boric acid, and iodine added but also the PVA level in the samples to be tested, thus giving inaccurate quantitative results. In addition, other components in the sample matrix are prone to interfere with the detection, leading to low selectivity and specificity or even failure of detection, especially for complex sample matrix with low PVA levels. Almost all of these methods are not suitable for pharmaceutical field. Although there are sparse literature works [17–19] that aimed at developing a method for pharmaceutical use, these methods suffer from the need for pricey equipment, bad resolution power, band broadening, low sensitivity, and limited application fields. The SEC method in reference [17] has obvious advantages in terms of high sensitivity with LOD of 4∼20 μg/mL and excellent accuracy with recoveries of 96.03∼101.22%. However, expensive ELSD is necessary, resulting in low usability and universality. In literature [18], a SEC column was used for the analysis of PVA in injectable leuprorelin acetate microspheres according to molecular size or chain length. Although mannitol molecules are much smaller than those of PVA, mannitol in microspheres still cannot be completely separated from PVA with resolution of only 1.35, thereby interfering with the determination of PVA. Thus, an indirect quantitative method is adopted to evaluate the PVA level in injectable leuprorelin acetate microspheres by determining the PVA of microspheres which do not contain mannitol. The RP-HPLC method using a Chiral-AGP (150 mm × 4.0 mm, 5 μm) column in literature [19] is rapid and reliable and has low cost. Unfortunately, the sensitivity including LOD and LOQ has not been given exactly in the paper. In addition, no further research has been conducted to estimate the lowest detectable PVA in samples and the paper does not introduce the actual application of the developed method. Therefore, it is difficult to evaluate whether this method is suitable for other complex pharmaceutical preparations besides an ophthalmic solution. In order to overcome the above-mentioned drawbacks, this research aims to develop and validate a sensitive and reliable method, which can easily be applied in not only pharmaceutical preparations but also other industries, including food and textile. Compared with previous studies, the method established in this paper overcomes the shortcomings of low plate numbers, severe band tailing, band broadening, and poor separation power and is highly sensitive with LOQ of 2.53 μg/mL, which can be applied to a wide variety of samples in different fields. The developed method is especially suitable for complex samples with low PVA levels.
Aripiprazole-loaded PLGA microspheres composed of PLGA, aripiprazole, mannitol, and PVA as active substances were selected as an example product. The microspheres are indicated for the treatment of schizophrenia, and its critical quality attributes include particle size, drug loading, initial burst, morphology, in vitro release features [20, 21], residual organic solvent content [22, 23], and residual PVA content. In this study, the residual PVA content in microspheres was successfully determined. The established HPLC-RID method was highly sensitive, selective, and precise for the quantification of PVA in pharmaceutical preparations, particularly biodegradable PLGA and PLA microspheres. This method was validated according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines.
2. Materials and Methods
2.1. Reagents and Chemicals
A pharmaceutical grade sample of PVA CRS (European Pharmacopoeia Reference Standard) was purchased from EP (European Pharmacopoeia) (assigned purity 100.0%) and PVA (MW≈22 kDa) raw material with purity of 99.6% was obtained from Nippon Synthetic Chemical Industry Co., Ltd. (Osaka, Japan). PLGA with MW of approximately 23 kDa was supplied from Evonik (Birmingham, AL, USA). Aripiprazole (purity 99.5%) was supplied by Neuland Laboratories Ltd. (Telangana, India). Mannitol and dichloromethane (DCM) were bought from Roquette (Guangdong, China) and Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), respectively. HPLC grade acetonitrile and TFA were purchased from Fisher Scientific (Guangdong, China). Milli-Q water was used for all studies.
2.2. Instrumentation
The HPLC (Shimadzu LC-2030C and Agilent 1260) equipped with a RID, quaternary pump, autoinjector, and column compartment with temperature control was used. Analyses were carried out on a BioBasic SEC-120 Å (300 mm × 7.8 mm, 5 μm) column.
2.3. Chromatographic Conditions
The quantification of PVA was detected using Agilent 1260 or Shimadzu LC-2030C HPLC system with a RID set at 40°C. The mobile phase was acetonitrile/water/TFA (300 : 700 : 1; v/v/v), and the flow rate was maintained at 0.5 mL/min. Injection volume of this method was 50 μL and the column temperature was maintained at 30°C. The detection lasted for 40 min with isocratic elution.
2.4. Preparation of the Aripiprazole-Loaded PLGA Microspheres and Placebo
Briefly, 166.7 g of PLGA was dissolved in DCM (16.67%, w/w) to obtain polymer solution, followed by adding the aripiprazole (500 g) solution in DCM (25%, w/w). The organic phase containing both the polymer and drug was then dispersed into a mixed solution of a 0.1% (w/v) PVA, and an oil-in-water (o/w) emulsion was prepared by emulsification (2000 rpm for 3 min) (IKA® Works, Inc.). The resultant o/w emulsion was stirred at about 300 rpm at 4°C to allow the microspheres to solidify. The organic solvent was then removed under a vacuum. The wet microspheres were collected by filtering the solidified solution and washed using injectable water. Mannitol solution was added to the wet microspheres followed by lyophilization to obtain aripiprazole-loaded PLGA microspheres. The placebo was prepared with the same preparation process as that of the aripiprazole-loaded PLGA microsphere except that no PVA was used.
2.5. Preparation of the Standard and Sample Solutions
A standard solution of PVA was prepared by dissolving an accurately weighed amount of 20 mg PVA in 20 mL volumetric flask using water by heat in a water bath at 80°C for 20 min. After the solution reached room temperature, volume was made up with water to obtain the stock standard solution of 1.0 mg/mL. The stock standard solution was diluted with water to obtain calibration standards of 2.5, 5, 10, 25, 50, 60, and 75 μg/mL. The aripiprazole-loaded PLGA microspheres (∼40 mg) were weighed and transferred into a 15 mL centrifuge tube, followed by adding 5 mL of acetonitrile and sonicating for 10 min. The mixture was centrifuged at 8000 rpm for 10 min and the supernatant was discarded carefully. The above extraction and centrifugation steps were repeated twice to completely remove aripiprazole and PLGA. The residue was dried with nitrogen, followed by adding 5 mL of water and heating in a water bath at 80°C for 20 min to completely dissolve the PVA and mannitol extracted from aripiprazole-loaded PLGA microspheres. The solution in the centrifuge tube was carefully transferred to a 20 mL volumetric flask. The centrifuge tube was washed with 3 mL of water 3 times. Then, the washing solutions were poured into the volumetric flask carefully. Finally, the volume was completed by adding water to obtain the sample solutions. The standards and sample extracts were centrifuged at 8000 rpm for 10 min or filtered through a 0.45 μm polytetrafluoroethylene (PTFE) filter (discard at least the first 5 drops during filtration), and then an aliquot was transferred to the injection vials for HPLC analysis.
2.6. System Suitability
The blank solution, placebo, PVA standard solutions of LOQ (2.5 μg/mL), and 100% level (50 μg/mL) were injected to evaluate the system suitability, including injector carryover checking, sensitivity checking, and system performance verification. The theoretical plate number (N), the RSD% of peak area, and retention time for 100% level PVA standard solution were studied and recorded.
2.7. Method Validation
According to the recommendations of ICH guidelines, specificity, linearity, sensibility, precision, stability, accuracy, and robustness were conducted for validation requirements.
2.7.1. Specificity
Based on the different solubility of different components, mannitol and PVA were dissolved in water after being pretreated with acetonitrile. Thus, the blank solution, mannitol solution, PVA standard solution, mixing solution of PVA and mannitol, and sample solutions including the aripiprazole-loaded PLGA microsphere and placebo were injected to evaluate the method specificity.
2.7.2. Linearity
A calibration curve with different concentration levels of 2.5, 5, 10, 25, 50, 60, and 75 μg/mL was generated for the quantification of PVA.
2.7.3. Sensibility
Sensibility including the LOD and the LOQ was determined by the signal/noise (S/N) ratio of about 3 and 10, respectively, determined by injecting a series of diluted PVA standard solutions.
2.7.4. Accuracy, Precision, and Stability
To evaluate the accuracy and the intraday precision of the developed method, the percent recoveries of PVA in spiked samples prepared at different concentration levels of LOQ (2.5 μg/mL), 50% level (25 μg/mL), 100% level (50 μg/mL), and 150% level (75 μg/mL) were determined. Six individual sample solutions at each level were analyzed. The interday precision was assessed by injecting six individual sample solutions at each level prepared by another analyst for another day on a different brand of HPLC. Sample solution and 100% level of standard solution (50 μg/mL) were also injected at a predetermined time to evaluate the room temperature stability. The average percent recovery and the relative standard deviation (RSD) were calculated.
2.7.5. Robustness
The robustness of the developed method was evaluated by minor changes in certain analytical parameters including the flow rate (0.4 mL/min or 0.6 mL/min), the mobile phase ratio (acetonitrile:water, 28 : 72 or 32 : 68; v/v) containing 0.1% (v/v) TFA, the proportion of the TFA (0.05% or 0.15%; v/v), detector temperature (35°C or 45°C), and column temperature (25°C or 35°C). The average residual PVA content and RSD were used to evaluate the changes of these parameters.
3. Results and Discussion
3.1. Initial Method Development
Based on the high molecular weight (Mw) of approximately 22 kDa for PVA, SEC analysis was selected to determine the residual PVA in microspheres. However, the molecular weight for PLGA (approximately 23 kDa) in aripiprazole-loaded microspheres has no significant difference with that of PVA. We tried to separate PVA and other carbohydrates in aripiprazole-loaded microspheres on several SEC columns. Aripiprazole elutes later with retention time of 31.664 min (Figure S1), but PLGA cannot be separated from PVA due to the similar molecular size of the two analytes. Therefore, the interference of PLGA was eliminated by solvent extraction. PVA is hydrophilic, while PLGA and aripiprazole are hydrophobic. Solvents which can dissolve PLGA and aripiprazole but not PVA can be employed to remove PLGA and aripiprazole fully from the aripiprazole-loaded PLGA microspheres. PVA and mannitol in microspheres will be left and collected for separation on SEC column. An effort has firstly been made to develop a suitable HPLC-RID method using water as mobile phase and a column Agilent PL aquagel-OH 40 with 8 μm in particle size, 7.5 mm in internal diameter, and 300 mm in length to separate PVA and mannitol. Unfortunately, mannitol in microspheres cannot be completely separated from PVA with R of only 1.35. Although the Mw of mannitol is much smaller than that of PVA, according to the recommend Mw range of 10∼200 kDa for Agilent PL aquagel-OH 40 column (300 mm × 7.5 mm, 8 μm), both mannitol and PVA can penetrate through the pores and hence are all retained. Mannitol peaked adjacent to PVA and failed to resolve from PVA, which makes it impossible to directly quantify the PVA in microspheres, unless a method of indirect quantitative determination of PVA with microspheres which do not contain mannitol is adopted. Therefore, further attempts such as changing chromatographic columns or using different mobile phases have been made to optimize the chromatographic conditions. The analytes were then subjected to an analytical column Chiral-AGP (150 × 4.0 mm, 5 μm) using 10 mM potassium dihydrogen phosphate as mobile phase. However, severe band tailing, band broadening, and low plate number occurred. Moreover, the resolution between PVA and mannitol is only 0.89, which does not reach the baseline separation, thus the PVA cannot be reliably quantified. The separation ability of PVA and mannitol on chiral column seems to be inferior to that on SEC column. To improve the separation between PVA and mannitol, different SEC columns have been tried. BioBasic SEC columns employ highly base deactivated 5 μm silica, which is coated with a “Hydrolink” polymer to ensure separation occurs only on the basis of sample size. BioBasic SEC columns not only provide high efficiency separations for a wide range of samples, from 100 to 10000000 molecular weight, but also have advantages of superior column lifetimes and good tolerance of organic solvents. The columns are offered in a range of pore sizes of 60, 120, 300, and 1000 Å. The recommend Mw range is different depending on the pore sizes. When both analytes can penetrate to the pores or both analytes are excluded from the pores, the resolution is probably lower than that of only one analyte that can permeate through the pores. Hence, to improve the resolution, SEC column with suitable pore size should be selected to ensure that the larger PVA is excluded from the pores and the smaller mannitol is penetrated through the pores. Hence, analyses were then performed on a column BioBasic SEC-120 Å with 5 μm in particle size, 7.8 mm in internal diameter, and 300 mm in length using different proportions of acetonitrile and water containing 0.1% TFA (v/v) as the mobile phase in isometric elution. The peak shape, tailing factor, and the separation power were considered. For BioBasic SEC-120 Å column (300 mm × 7.8 mm, 5 μm), the recommend Mw range is 0.4∼10 kDa. PVA cannot enter the pores; it passes through the column in the channels between the particles, and rapid elution with retention time of approximately 11 min has been observed. The smaller mannitol molecules can penetrate the pores of the column and hence are retained with retention time of approximately 20 min. Aripiprazole has maximum elution volume with retention time of 31.664 min. Good resolution of 12.82 and 13.46 has been observed on BioBasic SEC-120 Å column (300 mm × 7.8 mm, 5 μm) when the proportion of acetonitrile in mobile phase is 20% and 30%, respectively. Moreover, the problems of band broadening, low plate numbers, and poor separation power have been significantly improved. The N of PVA increased significantly from 100 on chiral column to 5771 on SEC column. The R also has dramatically increased to 13.46. Finally, the parameters were determined, as shown in Section 2.3. The detailed chromatographic conditions and results for method development are summarized in Table 1S and Figure 2S.
3.2. Sample Pretreatment
Based on the different solubility of different components, acetonitrile was selected as an extraction solvent. In order to completely remove the PLGA and aripiprazole, the extraction times and the volume of acetonitrile were carefully studied. As shown in Table 1, the measured PVA concentration increased from 2.54 μg/mL to 7.60 μg/mL with the extraction times abruptly quadrupled when 5 mL acetonitrile was used. After three and four times of extractions, there was no obvious difference in the concentration of PVA. The number of extractions was fixed at four times, and the volume of acetonitrile was further increased to 10 mL, resulting in the PVA concentration of 7.70 μg/mL. In terms of effective sample determination, when 5 mL acetonitrile is used, the PVA content is significantly increased from 0.13% to 0.39% by increasing extraction times from one to four times. The above results indicated that PVA in aripiprazole-loaded PLGA microspheres can be completely extracted after extraction with 5 mL of acetonitrile for three times.
| Filtration parameters | Measured concentration ± SDa(μg/ml) | RSDb (%) | Measured PVA content ± SD (%) | RSD (%) | |
|---|---|---|---|---|---|
| Volume of acetonitrile (ml) | Extraction time | ||||
| 5 | 1 | 2.54 ± 0.22 | 8.7 | 0.13 | 8.1 |
| 5 | 2 | 5.62 ± 0.43 | 7.7 | 0.28 | 7.8 |
| 5 | 3 | 7.67 ± 0.34 | 4.3 | 0.40 | 3.4 |
| 5 | 4 | 7.60 ± 0.39 | 5.2 | 0.39 | 5.1 |
| 10 | 4 | 7.70 ± 0.08 | 1.0 | 0.39 | 2.3 |
- aSD: standard deviation. bRSD: relative standard deviation.
3.3. Filterable Membrane Adsorption
Polytetrafluoroethylene (PTFE) membranes and polyethersulfone (PES) membranes with different pore sizes were used to filter the sample solutions for adsorption research. The recovery rate (RR) was determined in triplicate according to Eq. RR (%) = A/B × 100, where A is the PVA concentration measured by PES membranes and PTFE membranes filtration with different pore diameters, and B is the PVA concentration measured by centrifugation. The results are listed in Table 2. As displayed in Table 2, when using 0.22 μm and 0.45 μm PES membranes and discarding the initial filtrate in 5 drops, the RRs were 86.69% and 85.43%, respectively, indicating that PVA of approximately 14% was absorbed. After discarding 5 drops of primary filtrate, still about 5% of PVA was lost when 0.22 μm PTFE membrane was used. When 0.45 μm PTFE membrane was employed, the RRs increased from 93.66% to 100.28% with droplets number increased from 0 to 10, which indicates that the adsorption can be completely avoided after discarding at least 5 drops of the initial filtrate.
| Extraction parameters | Measured concentration ± SD (μg/ml) | RR (%) | ||
|---|---|---|---|---|
| Material | Pore size (μm) | Discarded (drop number) | ||
| Centrifugation | / | N/A | 29.53 ± 0.37 | / |
| PES | 0.22 | 5 | 25.60 ± 0.65 | 86.69 |
| PES | 0.45 | 5 | 25.23 ± 0.63 | 85.43 |
| PTFE | 0.22 | 5 | 28.15 ± 0.37 | 95.33 |
| PTFE | 0.45 | N/A | 27.66 ± 0.30 | 93.66 |
| PTFE | 0.45 | 5 | 29.36 ± 0.35 | 99.41 |
| PTFE | 0.45 | 10 | 29.62 ± 0.32 | 100.28 |
3.4. System Suitability
The blank solution, placebo, PVA standard solutions of LOQ (2.5 μg/ml) and 100% level (50 μg/ml) were injected to evaluate the system suitability, including injector carryover checking, sensitivity inspection, and system performance verification. For the blank solution injected after 100% standard PVA solution, no peak was observed in the retention time of PVA, indicating that the syringe residue passed the inspection. The signal-to-noise ratio of LOQ of PVA standard solution (2.5 μg/ml) was about 9, which indicates that the system is sensitive enough to quantitatively measure PVA as low as 2.5 μg/ml. The RSD % of peak area and retention time for 100% level PVA standard solution (n = 5) were 1.4% and 0.1%, respectively, and the N was greater than 5000.
3.5. Validation of Analytical Method
ICH guidelines and technical guidelines for validation of analytical methods for quality control of chemical drugs were followed to validate the proposed analytical method with regard to specificity, LOD, LOQ, linearity, precision, stability, accuracy, and robustness.
3.5.1. Specificity
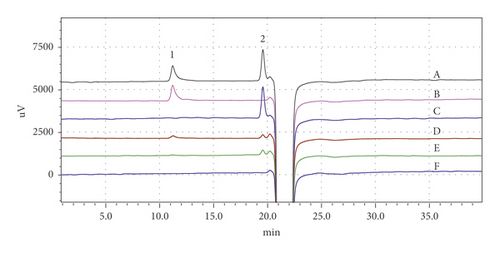
The specificity of this method was achieved by studying whether blank solvent, mannitol, and placebo interfere with the determination of PVA. As shown in Figure 1, other components in PLGA microspheres had no interference with PVA detection. In addition, PVA and mannitol were well separated with resolution of 13.46, far exceeding the limit parameter (R ≥ 1.5).
3.5.2. Linearity
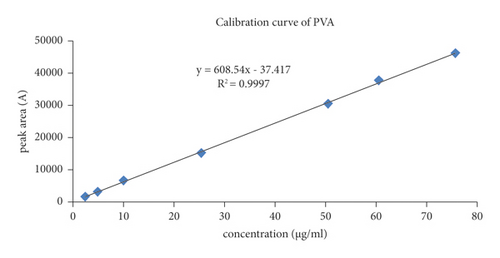
As illustrated in Table 2S, based on several concentrations in the range from 2.5 to 75 μg/ml, the linearity of the analytical method was evaluated and a calibration curve was constructed. As shown in Figure 2, the regression equation of peak area versus concentration data (Y = 608.54X − 37.417) was obtained with the correlation coefficient (R2) of 0.9997, which indicated that PVA has good linearity in the concentration ranges from 2.5 to 75 μg/ml.
3.5.3. Sensitivity
The lowest concentration of PVA that can be detected or quantified with acceptable precision was obtained by gradually diluting the standard solution to achieve S/N ratio of about 3 and 10, respectively. In this study, LOD was 0.51 μg/ml and LOQ was 2.53 μg/ml.
3.5.4. Precision
Six individual spiked sample solutions at different concentration levels including LOQ (2.5 μg/ml), 50% level (25 μg/ml), 100% level (50 μg/ml), and 150% level (75 μg/ml) were prepared by analyst A for intraday precision analysis. The interday precision was assessed by injecting another six spiked sample solutions at different concentration levels prepared by analyst B on another day (a total of 12 sample solutions for each concentration level). As shown in Table 3, both precisions for different concentration levels met the proposed acceptance criteria (RSD % ≤ 10%). The maximum RSD was 2.1%.
| Parameters | Levels | Analyst A | Analyst B | ||
|---|---|---|---|---|---|
| Ca ± SD (μg/ml) | RSD (%) | Ca ± SD (μg/ml) | RSD (%) | ||
| Intraday precision | LOQ | 2.59 ± 0.03 | 1.2 | 2.60 ± 0.06 | 2.1 |
| 50% | 25.18 ± 0.30 | 1.2 | 25.14 ± 0.28 | 1.2 | |
| 100% | 50.37 ± 0.86 | 1.8 | 50.33 ± 0.72 | 1.5 | |
| 150% | 75.49 ± 1.34 | 1.8 | 75.88 ± 1.01 | 1.4 | |
| Interday precision | RSD (n = 12) = 1.6(LOQ), 1.2(5%), 1.6(100%), 1.6(150%) | ||||
- aC: measured concentrations for spiked samples (n = 6) for each level of LOQ, 50%, 100%, and 150%.
3.5.5. Accuracy
The precision results were also used for accuracy assessment. The percent recoveries (PR) of PVA from the spiked samples were analyzed according to Eq. PR (%) = (A − B)/C × 100%, where A and B are the measured PVA (μg) in the spiked samples and the initial samples, respectively. C is the added PVA (μg) in the spiked samples. The PR at different concentration levels and its RSD were described in Table 4. The average recovery rate of analyst A (n = 6) was 98.0% (RSD % = 2.1, LOQ level), 99.2% (RSD % = 1.3, 50% level), 99.7% (RSD % = 1.9, 100% level), and 99.7% (RSD % = 1.9, 150% level). The average recovery rate for analyst B (n = 6) was 98.2% (RSD % = 3.7, LOQ level), 99.1% (RSD % = 1.4, 50% level), 99.6% (RSD % = 1.6, 100% level), and 100.3% (RSD % = 1.5, 150% level). The average recovery rates for both analysts A and B (n = 12) were 98.1% (RSD % = 2.8%), 99.2% (RSD % = 1.3%), 99.7% (RSD % = 1.6%), and 100.0% (RSD % = 1.6%), respectively. The results indicated that the developed method was of good accuracy and reliability.
| Level | Analyst A | Analyst B | Average recovery ± SD (%) (n = 12) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B b (μg) | C c (µg) | A a (μg) | Recovery | Average recovery ± SD (%) | B b (μg) | C c (µg) | A a (μg) | Recovery | Average recovery ± SD (%) | ||
| LOQ | 27.12 | 25.29 | 52.74 | 101.32 | 98.01 ± 1.97 | 27.50 | 25.29 | 52.44 | 98.65 | 98.19 ± 3.55 | 98.10 ± 2.74 |
| 27.13 | 25.29 | 51.48 | 96.28 | 26.78 | 25.29 | 50.87 | 95.26 | ||||
| 26.90 | 25.29 | 51.20 | 96.09 | 27.79 | 25.29 | 51.41 | 93.40 | ||||
| 26.80 | 25.29 | 51.36 | 97.15 | 26.38 | 25.29 | 52.27 | 102.37 | ||||
| 27.00 | 25.29 | 52.05 | 99.06 | 27.91 | 25.29 | 53.67 | 101.87 | ||||
| 26.75 | 25.29 | 51.57 | 98.14 | 26.36 | 25.29 | 51.04 | 97.59 | ||||
| 50% | 53.40 | 452.93 | 500.13 | 98.63 | 99.23 ± 1.27 | 54.32 | 452.93 | 501.01 | 98.62 | 99.12 ± 1.31 | 99.17 ± 1.23 |
| 54.01 | 452.93 | 511.25 | 100.95 | 53.73 | 452.93 | 512.13 | 101.21 | ||||
| 54.39 | 452.93 | 495.67 | 97.43 | 54.82 | 452.93 | 495.75 | 97.35 | ||||
| 54.37 | 452.93 | 505.44 | 99.59 | 53.91 | 452.93 | 503.11 | 99.18 | ||||
| 55.10 | 452.93 | 509.03 | 100.22 | 53.68 | 452.93 | 505.72 | 99.80 | ||||
| 53.77 | 452.93 | 500.20 | 98.57 | 53.29 | 452.93 | 499.61 | 98.54 | ||||
| 100% | 53.67 | 956.18 | 1009.41 | 99.95 | 99.70 ± 1.82 | 54.06 | 956.18 | 1007.31 | 99.69 | 99.61 ± 1.50 | 99.66 ± 1.59 |
| 54.08 | 956.18 | 1035.23 | 102.61 | 53.79 | 956.18 | 1021.05 | 101.16 | ||||
| 53.98 | 956.18 | 1013.68 | 100.37 | 54.17 | 956.18 | 1024.43 | 101.47 | ||||
| 54.20 | 956.18 | 1008.03 | 99.75 | 54.65 | 956.18 | 1005.20 | 99.41 | ||||
| 54.88 | 956.18 | 987.21 | 97.51 | 54.82 | 956.18 | 991.61 | 97.97 | ||||
| 53.68 | 956.18 | 991.03 | 98.03 | 52.97 | 956.18 | 989.79 | 97.98 | ||||
| 150% | 53.83 | 1459.43 | 1497.50 | 98.92 | 99.74 ± 1.85 | 54.18 | 1459.43 | 1498.04 | 98.93 | 100.30 ± 1.41 | 100.02 ± 1.60 |
| 54.04 | 1459.43 | 1538.47 | 101.71 | 53.39 | 1459.43 | 1537.65 | 101.70 | ||||
| 53.97 | 1459.43 | 1535.49 | 101.51 | 54.22 | 1459.43 | 1535.03 | 101.46 | ||||
| 54.32 | 1459.43 | 1474.37 | 97.30 | 54.61 | 1459.43 | 1491.73 | 98.47 | ||||
| 54.44 | 1459.43 | 1487.61 | 98.20 | 53.25 | 1459.43 | 1533.17 | 101.40 | ||||
| 53.68 | 1459.43 | 1525.02 | 100.82 | 53.54 | 1459.43 | 1510.40 | 99.82 | ||||
- a A: amount found for the spiked samples (n = 6) for each level of LOQ, 50%, 100%, and 150%. bB: amount measured for the initial samples (n = 6); the weight percentage content of PVA for initial sample was 0.13%. cC: amount added for spiked samples (n = 6) for each level of LOQ, 50%, 100%, and 150%.
3.5.6. Stability
The room temperature stability was determined by injecting each level of the sample solutions for precision assessment at predetermined time. The PR of each level relative to 0 h was all in 90%∼110% (92%∼101%) and the RSD of each level was 3.3%, 3.1%, 2.8%, and 3.0%, respectively, which indicated that the PVA solution was stable for at least 40 h at room temperature. The stability of standard solution was also investigated by injecting the standard solution of 100% level (50 μg/ml) at predetermined time and then the RSD of peak areas was calculated. The RSD of standard solution was 1.1%, indicating that the standard PVA solution was stable at room temperature for at least 24 h.
3.5.7. Robustness
The developed method was of good robustness when it remained unchanged even in small variations in the analytical parameters including the flow rate, mobile phase ratio, column temperature, and detector temperature. The retention ability, peak shape, separation power from mannitol, PVA content, and RSD were investigated. As shown in Table 5, the retention of target analyte decreased as the flow rate increased from 0.4 ml/min to 0.6 ml/min. The higher the flow rate, the faster the PVA will pass through the SEC column. The retention time of PVA advances from 13.978 min to 9.326 min. The influence of column temperature on retention depends on the physicochemical properties of analyte. At different temperatures, polymers may have different chain lengths and angles, thus exhibiting different molecular sizes. When the column temperature rises from 25°C to 30°C and 35°C, the retention time of PVA is 11.236, 11.249, and 11.262 min, respectively. This proves that the column temperature has no obvious effect on the retention of PVA. Other parameters including mobile phase ratio and detector temperature have little influence on PVA retention. The measured PVA content was between 0.129% and 0.136%, with the maximum RSD of 4.6%. The retention time was 9.326 min∼13.978 min. The symmetry factors were all between 2.16 and 2.37, and the lowest resolution was 9.03, which indicates that this method is of good robustness when considering these changes.
| Different parameters | Retention time (min) | Symmetry factor | Resolution | Measured PVA content ± SD (%) (n = 6) | RSD (%) | |
|---|---|---|---|---|---|---|
| Flow rate | 0.4 ml/min | 13.978 | 2.37 | 11.64 | 0.129 ± 0.0056 | 4.4 |
| 0.5 ml/min | 11.249 | 2.23 | 11.35 | |||
| 0.6 ml/min | 9.326 | 2.17 | 11.38 | |||
| Acetonitrile proportion | 28% | 11.167 | 2.35 | 9.03 | 0.136 ± 0.0060 | 4.4 |
| 30% | 11.249 | 2.23 | 11.35 | |||
| 32% | 11.248 | 2.21 | 11.67 | |||
| TFA proportion | 0.05% | 11.252 | 2.26 | 11.19 | 0.135 ± 0.0062 | 4.6 |
| 0.10% | 11.249 | 2.23 | 11.35 | |||
| 0.15% | 11.170 | 2.22 | 12.18 | |||
| Column temperature | 25°C | 11.236 | 2.25 | 10.75 | 0.129 ± 0.0044 | 3.4 |
| 30°C | 11.249 | 2.23 | 11.35 | |||
| 35°C | 11.262 | 2.19 | 11.86 | |||
| RID temperature | 35°C | 11.161 | 2.24 | 12.06 | 0.134 ± 0.0058 | 4.4 |
| 40°C | 11.249 | 2.23 | 11.35 | |||
| 45°C | 11.191 | 2.16 | 10.64 | |||
3.6. The Acceptable Level of PVA in Aripiprazole-Loaded PLGA Microspheres
PVA has been widely used in various fields for many years, which is generally considered to be less toxic. In addition, PVA eye drops with a nominal content of 1.4% have been used clinically for many years to relieve discomfort associated with xerophthalmia, which further confirms the safety of PVA to the human body. Up to now, neither the daily minimum acceptable amount of PVA in any pharmaceutical preparations containing PVA has been accurately given, nor the daily allowable intake amount of PVA in pharmaceutical preparations has been precisely given according to ICH guidelines. As food additive, the maximum allowable dosage of PVA is 18.0 g/Kg (1.8%, v/v). According to literature [24], the lethal dose 50% (LD 50) of PVA for rabbit after hepatic artery administration was 1.94 mg/kg, which was converted to obtain the LD 50 of about 19.4% (calculated by 1.94 mg/kg × 60 kg/600 mg) for human with average body weight of 60 kg and the actual loading amount of 600 mg for aripiprazole-loaded PLGA microspheres. Another document [25] proves that the daily dose of 0.28 mg PVA is safe enough for mice after 160 days of continuous administration. The sustained-release period for aripiprazole-loaded PLGA microsphere is 28 days. Taking 0.28 mg/day as the safe dose, the acceptable safe dose of aripiprazole-loaded PLGA microspheres for human is 1.3% (calculated by 0.28 mg/day × 28 day/600 mg). Based on the above research, the advised acceptable level for PVA in aripiprazole-loaded PLGA microspheres was strictly limited to 1.0%. Further safety data will be provided in the future to support the established limit for PVA.
3.7. Applications
This method has been successfully applied to the determination of PVA in different samples (A, B, C, D, and E) including eye drops (polyvinyl alcohol eye drops), PLA microspheres (injectable leuprorelin acetate microspheres), and PLGA risperidone microspheres with different molecular weights, food industry (glutinous rice paper), and textile industry (printing thickeners). Utilizing the dissimilarity of solubility, PLA and PLGA with different molecular sizes can be easily removed from PVA, which makes it possible to quantify PVA for other microspheres besides the ones mentioned above. In addition, the analysis results of this method are compared with those of the published ultraviolet spectrophotometric method and HPLC method, which verifies the effectiveness of this method. As displayed in Table 6, the developed method can be successfully applied to not only the pharmaceutical industry but also the food and textile industries, which further confirms the good selectivity and the universality of the established method. As low as 0.36%, PVA in sample matrix can be accurately detected with maximum RSD of 3.3%. Unfortunately, when using ultraviolet spectrophotometry to detect PVA in sample B, brick-red precipitate appeared due to the interaction between water-soluble leuprorelin acetate and iodine, which leads to the failure of PVA detection. The PVA level in sample C is too low to detect using the published ultraviolet spectrophotometric method and the published HPLC method, while the actual PVA content is 0.86% ± 0.02% by the developed method. When the published HPLC method was used for sample E, an unknown peak in the matrix appeared, which interfered with the PVA detection. Undoubtedly, the developed method was less likely to be interfered by other components and was highly sensitive, especially for complex matrix samples. There was no significant difference between the quantitative results for sample A and sample D when the developed method and both of the published methods were used, which further confirms the effectiveness of the developed HPLC method.
| Different samples | The developed method | The published ultraviolet spectrophotometric method | The published HPLC method | |||
|---|---|---|---|---|---|---|
| Measured PVA content ± SD (%) (n = 6) | RSD (%) | Measured PVA content ± SD (%) (n = 6) | RSD (%) | Measured PVA content ± SD (%) (n = 6) | RSD (%) | |
| A a | 1.39 ± 0.04 | 2.9 | 1.36 ± 0.11 | 8.1 | 1.38 ± 0.07 | 5.1 |
| B b | 0.36 ± 0.01 | 2.6 | N/A | N/A | N/A | N/A |
| C c | 0.86 ± 0.02 | 2.4 | N/A | N/A | N/A | N/A |
| D d | 3.81 ± 0.12 | 3.2 | 3.74 ± 0.16 | 4.3 | 3.71 ± 0.14 | 3.9 |
| E e | 1.59 ± 0.05 | 3.3 | 1.64 ± 0.08 | 4.9 | N/A | N/A |
- a A: polyvinyl alcohol eye drops with marked content of 1.4% (1.4 g/100 ml). bB: injectable leuprorelin acetate microspheres. cC: risperidone microspheres. dD: glutinous rice paper. eE: Printing thickeners.
4. Conclusion
This work provides an easily available and reliable HPLC method with high sensitivity using a RID to determine the PVA through SEC in many fields, including not only the pharmaceutical field but also the food and textile industries. Firstly, solvent extraction is employed to eliminate the interference of other polymeric materials that might be present in microspheres such as PLGA or PLA based on the different solubility, thus making assay of PVA possible for other microspheres besides the ones mentioned in this study. Secondly, a SEC column with appropriate pore size was selected to successfully separate PVA and mannitol, thus, eliminating the interference of mannitol and accurately determining the residual PVA in microspheres. Compared with the traditional ultraviolet spectrophotometric method, the established HPLC method was easily available with no requirement of expensive equipment and was less likely to be disturbed by other components, thus having good selectivity and sensitivity. Most importantly, PVA in the samples can be directly determined with no need for derivatization or complexation. Compared with the HPLC method in literature, this method has higher sensitivity (LOQ is 2.53 μg/mL with this method vs. LOD is 4∼20 μg/mL with the published method) at lower injection volume (50 μl of this method vs. 100 μl or 200 μl of the published method). It is particularly suitable for detecting complex matrix samples with low PVA levels. Thirdly, the defects including severe band tailing, band broadening, low plate numbers, and bad separation power in the published HPLC analysis have greatly been improved. In addition, this method is simple and reliable and meets the requirements of specificity, sensitivity, linearity, precision, stability, and accuracy. The established method is also of good robustness when minor changes are considered. When different methods are used, the quantitative results are statistically the same, which strongly proves that the proposed method is of good effectiveness. Finally, the developed method can be applied to a wide variety of samples. It also allows samples to be analyzed, which is not compatible with currently published methods that require complexation or interference elimination. It has remarkable advantages in simple preparation of mobile phase, wider application fields, convenient operation, no need of expensive equipment, high sensitivity, high selectivity, and low cost. The established method not only provides an alternative solution for PVA detection in the pharmaceutical field but also offers alternatives of PVA quantification for food and textile industries.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publishing of this work.
Acknowledgments
The authors gratefully acknowledge the financial support for this study by the National Major New Drugs Innovation and Development (project no. 2017ZX09201004-018) and the Pearl River Talent Plan of Guangdong Province (project no. 2016ZT06Y229). The authors also gratefully acknowledge Lizhu Pharmaceutical Group Co., Ltd. for providing infrastructure facilities and financial support for carrying out this work.
Open Research
Data Availability
Data used to support the findings of this study are included within the article and the supplementary information. All other information not included could, however, be obtained on request from the corresponding author.