Synthesis and Antitumor Activity of a GSH Inert Bisphosphonate Platinum (II) Complex
Abstract
Osteosarcoma is a highly aggressive neoplasm. Traditional platinum chemotherapeutic agents for osteosarcoma inevitably have acquired drug resistance and serious side effects, which have limited their utility. To slow down the reaction of platinum drugs with glutathione (GSH) is a strategy to overcome the resistance of platinum chemotherapeutic agents. Herein, the unique design of a GSH inert bisphosphonate platinum complex cis-{di(amino)platinum[tetraethyl 2,2-bis(2-pyridinylmethyl)methylidene-1,1-bisphosphonate]} (DBPP) is reported. MTT assay demonstrates that DBPP showed moderate inhibition towards human osteosarcoma cell line U2OS cells. The cytostatic action of DBPP is related to conformational conversion from B-DNA to A-DNA and the unwinding of pUC19 DNA. DBPP could also destroy the tertiary structure of human serum albumin (HSA). Notably, 31P NMR and 1H NMR indicate that DBPP can hardly chelate with GSH, which could overcome the GSH-induced side effects. We envision that this unique design of the platinum complex would open up new ways to overcome GSH-induced resistance.
1. Introduction
Osteosarcoma is a highly aggressive neoplasm that majorly occurs in patients younger than 25 years or older than 59 years [1, 2]. Annual incidence is estimated at 2–4 patients per million [1, 3]. Osteosarcoma arises from mesenchymal cells producing osteoid and/or bone, especially located in the long bones of the extremities [4–6]. The osteosarcoma cells show biological heterogeneity because of karyotypes that accompany patients for several months, and up to 30% of newly diagnosed patients will develop metastases [7, 8]. For those patients with metastatic or recurrent disease, therapeutic outcomes remain unsatisfactory, and the overall 5 years survival rate remains poorly at 20% [9, 10]. Therefore, it is urgent to develop more effective treatments for osteosarcoma.
Current clinical treatment of osteosarcoma is mainly based on neoadjuvant chemotherapy followed by surgery plus postoperative radiotherapy and chemotherapy [4]. The past decades have witnessed the effective improvement of osteosarcoma prognosis by using platinum chemotherapeutic agents such as cisplatin, which has improved 5-year survival rates for osteosarcoma to 60%–80% [11, 12]. The main mechanism of cisplatin is the inhibition of proliferation of rapidly dividing cells through DNA damage [13, 14]. After aquation inside cells, the platinum atom of cisplatin binds covalently to the N7 position of purines and DNA interstrand cross-linking occurs, which interferes with DNA synthesis, replication, and transcription, which is mainly responsible for the anticancer efficacy of platinum drugs [15]. However, the acquired drug resistance and severe side effects of cisplatin are inevitable and limit its utility [16].
A number of strategies, including structural modification in the platinum (II) coordination sphere, have been used to combat the resistance associated with platinum drugs [17]. For instance, the steric bulk ligand present around the platinum center enables the anticancer drug picoplatin to bypass platinum resistance by inhibiting cisplatin-resistant cell lines including human ovarian carcinoma xenograft ADJ/PC6cisR, L1210cisR, and CH1cisR [18]. The second example is N, N-chelate Pt (II) complexes, which possess micromolar activity in H460 cells. Another benefit of N, N-chelate Pt (II) complexes is that they prevent platinum from reacting with GSH [19, 20]. Therefore, well-designed platinum (II) complexes not only significantly advance our understanding of platinum complexes but also increase the likelihood that we will be able to overcome cellular resistance associated with platinum-based complexes.
The most widely known bone-targeting agents used in the formation of Pt complexes are bisphosphonates (BPs), which have been used to treat bone diseases such as osteoporosis, bone metastases, and multiple myeloma [21–24]. In view of these fascinating effects, Pt-BPs complexes with appealing characteristics have been explored. Particularly, the Pt-BPs complexes were discovered to have lower acute toxicity than the conventional drug cisplatin and preferentially inhibit osteosarcoma in in vitro studies [25, 26]. Some of the Pt-BPs complexes have been exploited as dual-targeting agents [26, 27].
Herein, we report the design, synthesis, and characterization of a bisphosphonate platinum complex, DBPP. In order to inherit the merits of reported Pt-BPs, we continue to use bisphosphonate as the nonleaving ligand. Considering that bis-or tridentate amine ligands could slow down the nucleophilic reactions by GSH [19], we adopted the strategy of employing bi-nitrogen pyridine ligands in DBPP. We evaluated and discussed the biological properties of DBPP through the investigation of their lipophilicity, accumulation, cytotoxicity, DNA, and HSA binding. We further demonstrate the stability of DBPP in the presence of GSH. This study provides a new strategy to overcome GSH-induced resistance.
2. Experimental
2.1. Materials, Measurements, and Data Analysis
Cisplatin, (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), 2-(chloromethyl)pyridine hydrochloride, human serum albumin (HSA), and L-glutathione (GSH) were purchased from sigma. Supercoiled pUC19 plasmid DNA and 6X loading buffer (0.05% bromophenol blue, 0.035% xylene cyanol FF, 36% glycerol, and 20 mM EDTA) were purchased from Takara Biotechnology (Dalian) Co., Ltd. Calf thymus DNA (CT-DNA), Tris and ethidium bromide (EB) were purchased from Sunshine Bio. Co., Ltd (Nanjing, China). All chemicals were used as received without further purification unless otherwise stated. The human osteosarcoma cell lines U2OS and MG-63, the human breast carcinoma cell line MCF-7 and the human cervical cancer cell line HeLa were originated from the Cancer Institute & Hospital, Chinese Academy of Medical Sciences.
Anhydrous N, N-dimethylformamide (DMF), and dimethylsulfoxide (DMSO) were prepared by refluxing or stirring the commercial reagent in the presence of calcium hydride for 72 hours. 2-[2-pyridinylethylidene]bis[phosphonic acid] tetraethyl ester was synthesized according to the previous literature [26].
The 1H, 13C, 31P, and 195Pt NMR spectra were acquired on a Bruker DRX-500 spectrometer. Electrospray ionization mass spectrometry (ESI-MS) spectra were recorded using the LCQ fleet ESI-MS spectrometer (Thermo Scientific), and the isotopic distribution pattern for the complex was simulated using the ISOPRO 3.0 program. Elemental analyses were carried out with Elementar Vario ELIII. FT-IR spectra were recorded on a Tensor-27 FT-IR spectrometer (Bruker, Germany) with a resolution of 2 cm−1 and a spectral range of 4000–400 cm−1. The high-performance liquid chromatography (HPLC) sample was centrifuged, and the supernatant was subjected to analysis at 220 nm via an SPD-20AV Shimadzu HPLC instrument equipped with a C18 reverse-phase column (eluent: 50/50 H2O/CH3OH). The circular dichroism (CD) experiments were performed on a Jasco J-810 spectropolarimeter (Japan Spectroscopic, Japan) in a 1 cm path length cylindrical quartz cell at room temperature. The UV-vis spectra were determined on a Shimadzu UV 3600 (UV-vis-near-IR) spectrophotometer. The images of agarose gel electrophoresis were obtained by using a Bio-Rad Gel-Doc XR imaging system, and quantification analysis was performed with Quantity One software. The inductively coupled plasma mass spectrometry (ICP-MS) data were obtained on ELAN9000 ICP-MS (PerkinElmer Inc., U. S. A.). The optical density (OD) of formazan was tested on ThermoScientific Varioskan Flash.
All cell experiments were conducted in triplicate. Statistical analyses were performed with the SPSS software. Data were expressed as the mean ± standard deviation.
2.2. Synthesis of Tetraethyl 2,2-Bis(2-Pyridinylmethyl) Methylidene-1,1-Bisphosphonate (Ligand)
2-Chloromethyl pyridine hydrochloride (5.292 g, 32 mmol) was dissolved in water (7 mL), and the solution was made alkaline by adding sodium hydroxide (1.344 g, 32 mmol) to water (14 mL). The mixture was extracted with chloroform, the extract was dried over anhydrous MgSO4 and the solvent was removed in vacuo. The residue was purified by vacuum distillation to give 2-chloromethyl pyridine as a pale red oil. In a separate bottle, 2-[2-pyridinylethylidene]bis(phosphonic acid) tetraethyl ester (9.31 g, 24.56 mmol) in 20 mL dry DMSO was added to a mixture of 60% N (1.08 g, 29.47 mmol) in DMSO 20 mL at 0°C under nitrogen. The reaction mixture was stirred at 0°C for 30 min then at room temperature for 2 h. 2-Chloromethylpyridine (2.82 g, 20 mmol) was then added dropwise to the stirring reaction mixture. The reaction was allowed to stir for an additional 96 h at room temperature and then quenched by the addition of saturated aqueous ammonium chloride. The reaction mixture was extracted with methylene chloride, and the organic extracts were combined and concentrated under reduced pressure. The mixture was redissolved in excess toluene, washed with H2O and brine, dried over Na2SO4, and concentrated under a vacuum. The yellow product is purified by flash chromatography with isopropyl alcohol in methylene chloride in silica gel. 1H NMR (500 MHz, CDCl3): δ 8.60 (s, 2 H), 7.77 (d, J = 7.74 Hz, 2 H), 7.58 (t, J = 7.42 Hz, 2 H), 7.15 (s, 2 H), 4.02–3.97 (m, 8 H), 3.64 (dd, J = 12.15, 2.58 Hz, 4 H), and 1.12 (td, J = 7.05, 2.03 Hz, 12 H). 13C NMR (125 MHz, CDCl3): δ 158.14, 148.37, 135.39, 126.55, 121.43, 62.46, 50.55, 36.71, and 16.16. 31P NMR (202 MHz, CDCl3): δ 23.84. IR (KBr) cm−1: 3431, 2977, 2930, 2869, 1624, 1584, 1470, 1437, 1390, 1356, 1250, 1022, 968, 874, 807, 686, 613, and 513. ESI-MS (m/z, positive mode). Found (calcd): [Ma + H]+, 471.33 (471.44), [2M + Na]+, and 963.17 (963.88).
2.3. Synthesis of DBPP
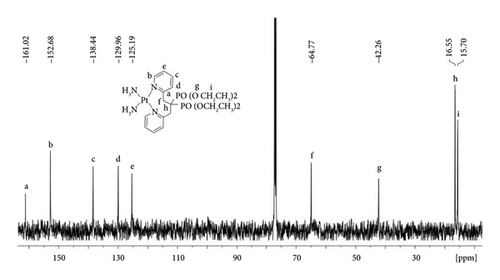
Cisplatin (75 mg, 0.25 mmol) and AgNO3 (42.5 mg, 0.25 mmol) were stirred in anhydrous DMF (4.0 mL) in the dark at 40°C for 24 h. The resulting AgCl precipitate was removed by filtration and Ligand (94.27 mg, 0.2 mmol) was added to the filtrate. After stirring for an additional 24 h at 40°C, AgNO3 (34.0 mg, 0.20 mmol) was added to the solution, and the solution was stirred for another 24 h at 40°C. The resulting AgCl precipitate was removed by filtration, and CH2Cl2 (100 mL) was added to the reaction mixture. The white precipitate was removed by filtration and the filtrate was evaporated under reduced pressure. The resulting oil was dissolved in CHCl3 (4 mL) and added to diethyl ether (100 mL). A yellow precipitate of DBPP was formed at a 58% yield. 1H NMR (500 MHz, CDCl3): δ 9.44 (s, 2 H), 7.68 (t, J = 7.85 Hz, 2 H), 7.58 (d, J = 7.75 Hz, 2 H), 7.45 (t, J = 5.04 Hz, 2 H), 5.05 (s, 4 H),4.42 (m, 6 H), 4.11 (t, J = 13.56 Hz, 2 H), 3.67 (s, 4 H), 1.87 (s, 6 H), and 1.50 (t, J = 7.01 Hz, 6 H); 13C NMR (125 MHz, CDCl3): δ 161.02, 152.68, 138.44, 129.96, 125.19, 64.77, 42.26, 16.55, and 15.70; 31P NMR (202 MHz, CDCl3): δ 22.88, 20.55; 195Pt NMR (107 MHz, CDCl3): δ−2287.651. IR (KBr) cm−1: 3433, 3225, 2990, 1611, 1484, 1443, 1376, 1356, 1223, 1049, 968, 780, 653, 607, and 533. ESI-MS (m/z, positive mode). [C21H38N4O6P2Pt]+ found (calcd.): 698.33 (699.19); [C21H38N4O6P2Pt]2+ found (calcd.): 349.92 (349.59). Calculated for C21H38N6O12P2Pt: C, 30.62; H, 4.65; N, 10.20. Found: C, 30.68; H, 4.42; N, 10.13%. The purity of DBPP was determined to be >98% by HPLC. The retention time was 5.07 minute.
2.4. Computational Modeling
Electronic properties of the chemical structures of the ligand and DBPP were calculated using density functional theory (DFT). In particular, the hybrid functional B3LYP, electron basis sets 6-311G (d, p), and an effective core potential LanL2DZ basis set as implemented in the Gaussian09 suite of programs were used for full geometry optimization. In all calculations, the integral equation form (IEFPCM) of the polarization continuum model was used to simulate the solvent (water) environment. The frequencies of all geometries are calculated at the same level. HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) were calculated for the optimized geometries.
2.5. Anticancer Activity Assays
The in vitro MTT assay was carried out according to a previously reported procedure [28]. Briefly, the U2OS (osteosarcoma), MG-63 (osteosarcoma), MCF-7 (breast carcinoma), and HeLa (cervical cancer) cells were inoculated in 96-well plates and incubated in different media. U2OS was maintained in DMEM with 10% fetal bovine serum. MG-63, MCF-7, and HeLa cells were maintained in RPMI 1640 medium with 10% fetal bovine serum. These cells were cultured at 37°C in an atmosphere of 5% CO2 and 95% air and 100% relative humidity overnight. After plating, different concentrations of DBPP in PBS were added to the above culture medium. After the cells were incubated for another 48 h, MTT (20 μL, 5 mg mL−1 in PBS) was added to each well. After another 4 h of incubation, the culture plates were centrifuged, and the medium was removed. Then DMSO (200 μL) was added to each well, and the absorbance of dissolved formazan was measured at 570 nm by an ELISA plate reader. The inhibition rate (%) or IC50 was the mean of three independent results.
2.6. Lipophilicity Determination
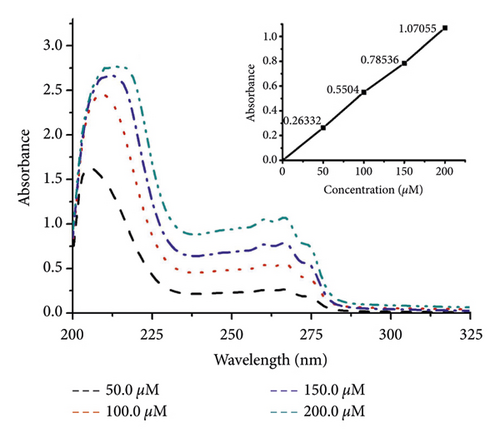
Lipophilicity determination was carried out by using the 1-octanol/buffer system of the shake-flask method [29]. Briefly, solutions of DBPP (50, 100, 150, and 200 μM) were prepared in the phosphate buffer (10 mM, pH 7.4) which was presaturated with 1-octanol. 2.0 mL of the solution and equal volumes of 1-octanol were mixed and placed in a thermostatic (25.0 ± 0.1°C) air-bath orbital shaker at 200 rpm for 4 h. After centrifugation at 2500 rpm for 15 min, the samples were separated into two phases. According to the law of mass conservation, the concentration of the solute in the aqueous phase was determined by spectrophotometry (λmax = 267 nm). The drug concentration of the corresponding 1-octanol phase and the lipo-hydro partition coefficient Po/w(Co/Cw = Ao/Aw), where A stands for absorbance) were calculated.
2.7. Platinum Cellular Uptake
U2OS cells (15 × 104) were seeded in 6-well plates, which were allowed to grow for 18 h, then treated with DBPP (50.0 μM and 100.0 μM) for 24 h, 48 h, and 72 h, respectively. After incubation, the medium was removed and all of the wells were washed with phosphate-buffered saline (PBS) three times. The cells were trypsinized, and live cells were counted by the trypan blue method. After that, cells were suspended in 500 μL PBS and digested with 0.5 mL of hot (≈95°C) concentrated nitric acid for 2 h, followed by the addition of H2O2 (50 μL) and HCl (100 μL). The obtained homogenized solution was diluted by water and analyzed by ICP-MS to determine the total platinum content per well. The amount of platinum was calculated by subtracting the average amount of platinum found in the blank wells from the average amount of platinum found in the cell-containing wells and normalizing it to the average number of cells per well.
2.8. Studies on DNA Interaction
The DNA binding experiments were performed in Tris-HCl/NaCl buffer (5 mM Tris-HCl, 50 mM NaCl, pH 7.4) using different concentrations of DBPP. After CT-DNA was incubated with different ratios of DBPP at 37°C for 24 h in the dark, CD spectra were recorded in the wavelength range of 220–320 nm. Each CD spectrum was recorded after averaging over at least 3 accumulations, and the buffer background was subtracted.
The impact on the secondary structure of plasmid DNA was determined by agarose gel electrophoresis. pUC19 plasmid DNA (200 ng) was treated with different concentrations of DPP at 37°C for 24 h. All the samples were finally loaded onto a 1% agarose gel containing EB (0.5 μg·mL−1). Electrophoresis was carried out for 2 h at 100 V in a TAE buffer (50 mM Tris-acetate and 1 mM ethylenediaminetetraacetic acid). The resulting bands were visualized under UV light. The resulting photographs were obtained by using a Bio-Rad Gel-Doc XR imaging system.
2.9. Interaction with HSA
Reactions of DBPP with HSA were carried out in PBS (10 mM, pH 7.4). Reactions were monitored by UV-Vis and absorption spectra were obtained after HSA (3.0 μM) with different concentrations of DBPP in PBS at 37°C for 24 h in the dark.
2.10. Solubility Test
Solubility testing was carried out using distilled water and chloroform as test media. Each test was repeated 3 times. One milliliter of each test medium was put in a glass tube and stirred with a constant speed of 80 rpm for 10 min after the start of the test. Then, the samples were incubated at 37°C for 30 min and centrifuged for 10 min at 10,000 rpm. The solvent of each sample was removed, and the dry sample was redissolved in 1.0 mL of distilled water. The platinum concentration of the samples was determined by ICP-MS. The dissolution values were calculated and expressed in the form of Mol/L (M).
2.11. Stability of DBPP
DBPP (ca. 0.0056 mmol) was dissolved in a solution of deuterated aqueous and placed into an NMR tube. GSH (2.45 mg, ca. 0.0132 mmol) was added to the same NMR tube. Then, the tube was kept at 37°C, and the obtained solution was monitored by 1H NMR and 31P NMR at different time intervals.
3. Results and Discussion
3.1. Characterization
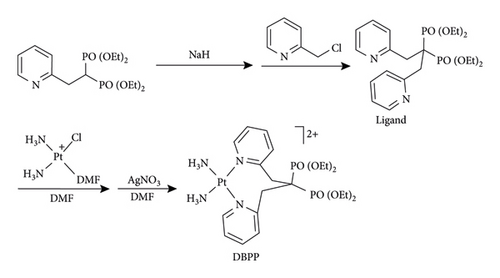
Preparation of DBPP was conducted according to the procedure shown in Scheme 1. Briefly, the ligand was straightforwardly obtained from nucleophilic substitution reaction of 2-(chloromethyl)pyridine hydrochloride and 2-[2-pyridinylethylidene]bis(phosphonic acid) tetraethyl ester. Then, the ligand was reacted with cationic cis-[Pt(NH3)2Cl(DMF)]+, after which AgNO3 was added to afford DBPP in high yield. Since there are two pyridyl rings in DBPP, it is assumed that DBPP is more difficult to react with GSH. In addition, the coordination of the ligand with platinum (II) affords an eight-membered ring. To the best of our knowledge, it is the first example reported till now.
The ligand and the resulting platinum complex (DBPP) have been characterized by using NMR, ESI-MS, and IR spectroscopy. Compared to the free ligand, the aromatic proton signals in the 1H NMR data of DBPP significantly shift to a lower field, indicating that the pyridine nitrogen has been coordinated to the PtII center. Additionally, 31P NMR data of the free ligand provided a singlet at 23.84 ppm, whereas two new signals at 22.88 and 20.55 ppm were observed for the DBPP indicating a change in the P atoms’ signals as a result of coordination with platinum. Moreover, the 195Pt NMR signal from DBPP falling at −2287.651 ppm further confirmed that the PtII complex was successfully developed. The IR spectrum of the ligand and DBPP complex showed a POO-stretching band in the 1000–1250 region [30]. The ESI-MS spectrum of DBPP showed two main peaks at m/z 349.92 and 698.33, which could be ascribed to the positively charged species [C21H38N4O6P2Pt]2+ and [C21H38N4O6P2Pt]+, respectively.
3.2. DFT Calculation
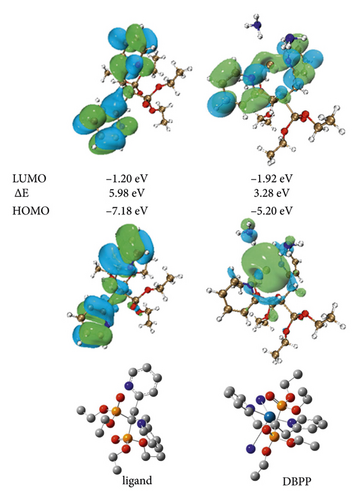
We carried out the DFT calculation using a collection of Gaussian 09 programs in order to theoretically comprehend the coordination of DBPP [31–33]. Figure 1 demonstrates how the central Pt2+ coordinates with two N atoms from NH3 and two N atoms from the pyridine units, the latter of which almost stand in a straight line in opposite directions. Figure 1 also displays the spatial distributions of the HOMO and LUMO of the ligand and DBPP. The ligand’s electron density, whether HOMO or LUMO, is evenly distributed, primarily on the pyridine unit. In contrast, the majority of the electrons in the HOMO orbital are distributed on the N-Pt binding site in the DBPP upon binding with platinum ions. Additionally, DBPP’s energy gap (E) between HOMO and LUMO is 3.28 eV, lower than the ligand’s (5.98 eV), indicating that DBPP is more stable than the free ligand.
3.3. Antiproliferative Activity
The cytotoxic potency of DBPP was evaluated. As shown in Table 1, DBPP did not show significant activity against MG-63, MCF-7, and HeLa cell lines at a concentration of 50 and 100 μM after 24 h incubation. However, DBPP exhibits a significant inhibitory effect on U2OS cells’ ability to proliferate, indicating a higher selectivity than cisplatin. The high systemic toxicity of cisplatin is thought to be associated with nonselectivity [34]. By redirecting drugs at the specific site of injury or disease is the most promising strategy to compel the associated drug resistance and may be helpful in lowering systematic toxicities [35]. The difference in selectivity between DBPP and cisplatin may be due to the presence of a bisphosphonate moiety in DBPP as opposed to cisplatin.
| Cell | Relative cellular viability at 50.0 μM concentration | Relative cellular viability at 100.0 μM concentration |
|---|---|---|
| MG-63 | 100.0% ± 2.5% | 85.9% ± 1.8% |
| U2OS | 85.8% ± 1.6% | 55.7% ± 3.0% |
| HeLa | 100.0% ± 1.1% | 100.0% ± 1.6% |
| MCF-7 | 100.0% ± 0.9% | 100.0% ± 3.8% |
- aRelative cellular viability of cisplatin toward MG-63, U2OS, HeLa, and MCF-7 at 10.0 μM concentration for 24 h is 59.0% ± 1.0%, 52.1% ± 2.2%, 48.5% ± 1.0%, and 51.2% ± 1.2%, respectively.
At the concentration of 50 and 100 μM, the relative cellular viability is 85.8% ± 1.6% and 55.7% ± 3.0%, respectively (Table 1). With the extension of incubation time from 24 h to 48 h, the relative cellular viability reduced to 65.6% ± 3.8% and 48.2% ± 0.9%, respectively. When the incubation time was extended to 72 h, the relative cellular viability reduced to 58.3% ± 4.8% and 30.7% ± 1.6%, respectively (Table 2). The 50% inhibition appeared at concentrations up to about 60 μM. In contrast, cisplatin was more cytotoxic. For example, the relative cellular viability of cisplatin toward MG-63 at 10.0 μM concentration for 24 h is 59.0% ± 1.0%.
| Concentration (μM) | Relative cellular viability | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 50.0 | 85.8% ± 1.6% | 65.6% ± 3.8% | 58.3% ± 4.8% |
| 100.0 | 55.7% ± 3.0% | 48.2% ± 0.9% | 30.7% ± 1.6% |
Analysis of the structure-activity relationship revealed that the leaving group has an important influence on the cytotoxicity. The result of cytotoxicity indicated that DBPP which has no leaving group in the structure, shows moderate cytotoxicity against U2OS cells but is quite less potent than cisplatin and BPP, the latter of which have at least one leaving the group in the structure [26].
3.4. Lipophilicity and Cellular Uptake
The lipophilicity parameter (log P) is a crucial factor in membrane transport [36, 37]. Here, the log P value of DBPP was determined as water-octanol partition coefficients using the shake-flask method. The log P is calculated to be −1.1 ± 0.1 (Figure 2), showing that DBPP is more lipophilic than BPP (log P = −1.7) [26] and cisplatin (log P = −2.3) [38].
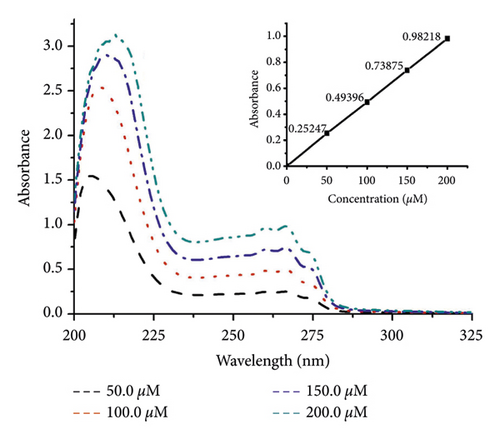
The U2OS cellular uptake of DBPP was determined by ICP-MS. When U2OS cells were treated with 10.0 μM cisplatin for 24 h, the concentration of cellular was 2.6 ± 0.2 μg/106 cells. However, treating U2OS cells with 50.0 μM of DBPP for 24 h only resulted in 0.6 ± 0.5 μg/106 cells concentration of cellular platinum (Table 3), which was quite lower than that of cisplatin. Prolonging the incubation time to 48 h or 72 h could not bring about a significant increase in cell uptake. After exposure to 100.0 μM of DBPP for 48 and 72 h, the concentration of cellular platinum rose to 3.7 ± 0.5 and 5.0 ± 0.3 μg/106 cells, respectively. In addition, the results indicate that the Pt uptake is correlated with the cytotoxicity.
| Concentration (μM) | Pt content (μg/106 cells) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 50.0 | 0.6 ± 0.5 | 0.8 ± 0.9 | 1.3 ± 0.9 |
| 100.0 | 1.4 ± 0.5 | 3.7 ± 0.5 | 5.0 ± 0.3 |
- aTreating U2OS cells with cisplatin (10.0 μM) for 24 h resulted in 2.6 ± 0.2 μg/106 cells concentration of cellular platinum.
3.5. Interaction with DNA
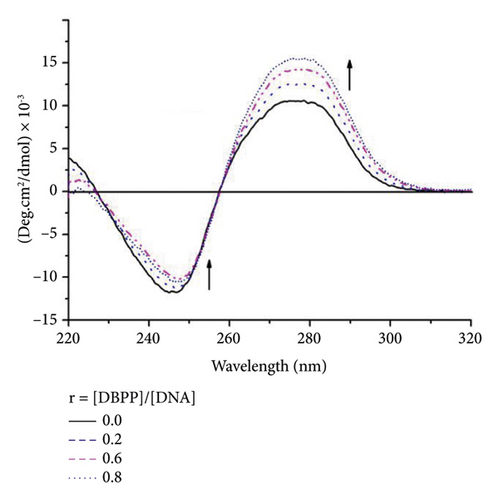
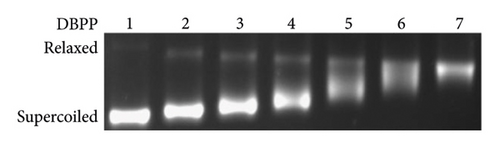
Since DNA is the primary target of Pt (II)-based antitumor complexes, to get more insight into the antitumor activity of DBPP, the ability of DBPP to bind calf thymus DNA (CT-DNA) and unwind supercoiled pUC19 DNA was investigated. Figure 3(a) displays the circular dichroism CD spectra of CT-DNA in the absence or presence of varying amounts of DBPP, where the negative band at 245 nm and the positive one at 275 nm represent the characteristics of B-DNA. With the rising of the [DBPP]/[DNA] ratio from 0 to 0.8, the intensity of the negative bands decreases and the positive bands increases. Moreover, the maximum wavelength of the negative bands has a tendency to redshift. Such a changing tendency indicates a conformational conversion from B-DNA to A-DNA. The changes of the negative and positive bands are quite similar to that of DNA modified by cisplatin and this suggests that DBPP can induce a similar formation of intrastrand cross-links with cis-[PtCl2(Py)2] [39]. Figure 3(b) shows the DNA unwinding property of DBPP by native agarose gel electrophoresis using pUC19 DNA. Upon incubation with DBPP, retardation of supercoiled DNA was observed, indicating untwisting by DNA binding. Additionally, the separation between supercoiled and relaxed DNA decreases as the molar ratio (ri) increases. The coalescence point ri (c) for complete removal of supercoiled DNA is 1.8, which is higher than that of cisplatin (ri (c) = 0.076) [40]. The results suggest that DBPP cannot perturb the tertiary structure of supercoiled DNA as efficiently as cisplatin, which is consistent with their cytotoxicity.
3.6. Interaction with HSA
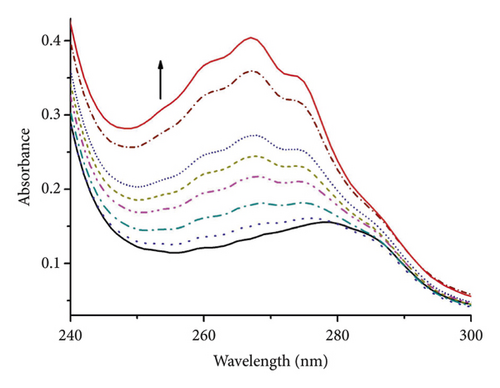
HSA plays a crucial role in the transport of drugs because of its remarkable binding properties and the highest abundance in blood plasma [41]. UV-Vis spectrophotometry was used as a simple method to explore the interaction of DBPP with HSA. The effect of DBPP on the UV-Vis absorption of HSA is shown in Figure 4. With the addition of DBPP, the absorption intensity of HSA was enhanced with an obvious blue shift of about 12 nm (from 278 to 266 nm) indicating that the microenvironment of tryptophan (Trp), tyrosine (Tyr), and phenylalanine (Phe) residues were altered and they were extended into the aqueous milieu [42]. These observations confirm that DBPP can destroy the tertiary structure of HSA and form a novel complex with HSA.
3.7. Stability of DBPP
Having the U2OS selectively inhibited platinum complex in hand, we next turned our attention to investigating the reactivity between DBPP and Glutathione (GSH). GSH is a soluble tripeptide whose intracellular concentrations are between 0.5 to 10 mM. As a nucleophilic molecule, GSH could chelate with platinum (II) to form a Pt (GS)2 conjugate, which is then exported out of the cells. It would decrease the platinum (II)-mediated DNA damage and weaken the therapeutic efficacy. The interactions between GSH and Pt (II) complexes are therefore considered to play a critical role in mechanisms that are relevant to the inactivation of platinum complexes, their resistance, and side effects [43–45].
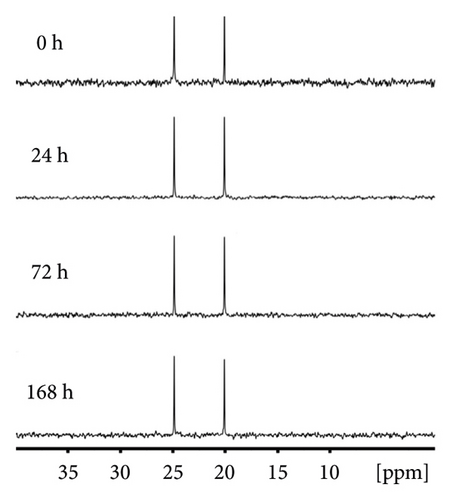
Figure 5 demonstrated that DBPP has good solubility in chloroform. Since solubility affects the bioavailability and individual variability of drugs [46], we carried out the solubility test to explore the details of the solubility of DBPP. The solubility values of DBPP in distilled water and chloroform were 0.13 and 0.084 M, respectively. Therefore, the reaction of DBPP with GSH in D2O was studied at millimolar concentrations at 37°C by 31P NMR and 1H NMR.
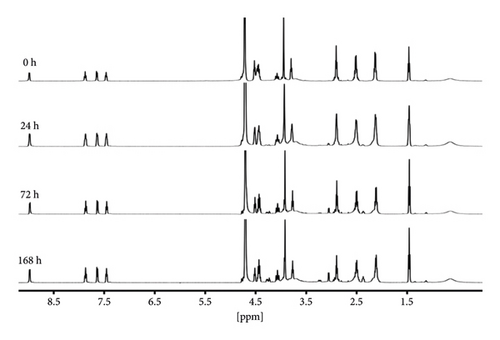
As shown in Figure 6(a), the peaks of DBPP are located at 24.88 and 20.09 ppm. After even 168 hours incubation, no obvious changes were observed, indicating that DBPP is quite inert to GSH. Similar results were observed in Figure 6(b). Overall, these results demonstrate that DBPP can hardly chelate with GSH. This is a promising result since GSH-Pt conjugates cannot form, suggesting that DBPP could avoid the GSH-induced side effects. It is worth mentioning that due to the high inertness of GSH, the cytotoxicity deficiency of DBPP may be compensated by raising the therapeutic dose.
4. Conclusions
In conclusion, a bisphosphonate platinum complex DBPP was synthesized and characterized. DBPP showed moderate inhibition toward OS cells. The cytostatic action of DBPP is related to the conformational conversion from B-DNA to A-DNA and the unwinding of pUC19 DNA. DBPP could also destroy the tertiary structure of HSA. The objective of this study is to slow down the GSH substitution of platinum complexes. By means of using bulky nitrogen-bisphosphonate and changing the structural motif of complexes, this goal has been basically achieved.
Regardless of the cytotoxicity, these results will open up new avenues to overcome GSH-induced resistance. Our future plans include further activity optimization of BPs-Pt compounds with similar structures, so that BPs-Pt may be used for therapeutic applications in platinum drug resistance.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work has been supported by the Innovation and Entrepreneurship Program of College Students in Jiangsu (for Zhichao Zhou) and Guizhou Provincial Department of Education Youth Science and Technology Talent Growth Project (no. KY-2018-330).
Open Research
Data Availability
All data related to this work are presented in the results section along with references.