The CON−H…+NH2 Blue-Shifting H-Bond Stabilizing Effect on Z Secondary Amides and Cyclic System Conformational Rearrangement through an Alkylamine-Chain Migration Pathway
Abstract
The paper is focusing on the amide linkage exceptional properties and usage of chemistry (conformational rearrangement, geometrical stereoisomers, spectroscopic blue shift phenomenon, protonation and deprotonation reactions, synthetic scope, and mechanistic implications). Hydrogen-bond-stabilized acylation reactions of a diamine with thioamides or nitriles reveal how substituents influence both the outcome of stereoselectivity and interactions. Inferring the chemical mechanism from the structures of reactants is dissimilar to the appropriate E isomers, the Z form becomes more favored in the secondary amides obtained. One conclusion from the estimation of Z structures, based on the 1H-15N 2D NMR spectra in comparison with the references, is the existence of the intramolecular, blue shifting CON−H…+NH2CH3 hydrogen bonds. The rearrangement of a methylamino residue provided the free base stabilized in the CH3N−H…O=CNH after deprotonation. An essential part of the publication describes systems in a highly stereoselective fashion, so the stereochemical outcome of the product is predictable now.
1. Introduction
Reversibility in biology is associated with well-ordered structures formed from flexible molecules. Ionic hydrogen bonds are an important subclass of hydrogen bonds and have recently come into focus [1–6]. They appear in many biologically important aggregates [7–9]. Proton-transfer reactions take place at the active sites of enzymes and lead to a large increase in the reactivity of neutral organic molecules. The formation of functional positively charged molecules is prevalent in many systems at physiological pH values. These agents can hydrogen-bond with the major groove and thereby recognize base pairs. Compounds whose structures contain cationic groups usually exhibit useful activities, [10, 11] and such groups are present in many synthetic drugs. Orthopramides and substituted benzamides are capable of acting as selective dopamine D2-receptor antagonists, antiemetics, antispasmodics, antidepressants, antipsychotics, and digestive aids (e.g., clebopride, metoclopramide, and sulpiride) [10]. Hence, studies concerning the conformational aspects of ammonium units in complexes are a very actual research field in gaining a better understanding of the mechanism of action of drugs.
Several new protonation reactions of (thio) amides have been discovered (Scheme 1). From the chemical point of view, the synthesis and study of such compounds, carrying the +NH3…N or N…H+…N bridges, become of interest because they participate in preparation and stabilization of monoacylated diaminoalkanes [2, 3]. The hitherto reported data leave no doubt that the strength of these bonds is of fundamental importance for the explanation of the reactivity differences (intramolecular “noncovalent” catalysis). Multifurcated hydrogen bonds can alter both the stability and the reactivity of thioamides towards zinc ammoniates [4]. These results are in touch with the previous hydrolysis and cyclization studies [2]. It has been shown that the strong interactions prevent the subsequent, theoretically possible, protonation reaction of the neighboring α-amino groups in dispirocyclic benzenes [5]. The existence of NH…Cl−…HN hydrogen bonds in the centrosymmetric dimer of (Z)-N-[2-(dimethyl amino)ethyl] thioacetamide hydrochloride was found [6].
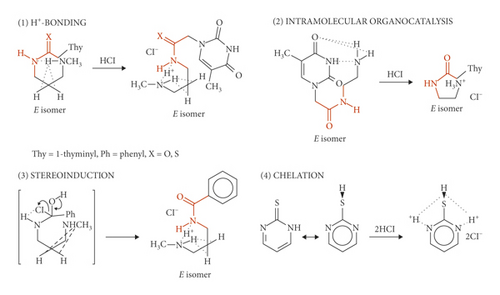
The spectroscopic properties of N-substituted secondary thioamides and amides, in particular, in connection with the occurrence of intramolecular N…H+…N hydrogen bonding (covalent nature of the extremely strong, homonuclear HBs) have been discussed. Published findings on the E configurations are included in Scheme 1. However, not all secondary amides interact according to equations (1)–(3). This paper highlights a new stereoselective strategy to prepare the isomeric Z secondary amides. Mechanistic pictures on structurally related benzamides have already provided some conformational insight [10]. The present article concerns the secondary benzamide ammonium salts. Most approaches to this area have not focused on the geometrical isomerism about carbon-nitrogen partial double bond [11]. No stereochemical studies have been carried out with derivatives in such systems. Furthermore, the more general implications are here provided.
2. Experimental Section
2.1. Materials and Measurements
Melting points are uncorrected and were determined by using a Boetius melting-point apparatus. Thin-layer chromatography was performed with Merck silica gel 60F254 glass plates (0.25 mm thickness) in the appropriate solvent systems: 44 : 8:1, 11 : 4:1, 7 : 5:2 CHCl3-CH3OH-NH4OH. N-Methylaminopropane, N-methyl-1,3-diaminopropane, thiobenzamide, and ammonium sulfide, 20–22% solution in water were commercially available from Aldrich and purified by standard methods, if necessary. The syntheses of compounds 3, [12] 7, [13, 14] and 8, [15] were accomplished according to the cited literature. The identity of benzamide 8, prepared from benzoyl chloride and 1-aminopropane under the Schotten–Baumann reaction conditions, was confirmed by 1H- and 13C-NMR spectra [16–19]. Final samples were dried first in an oven at 120°C and then stored in a vacuum desiccator over P2O5.
NMR spectra were recorded on a Varian 300 Gemini spectrometer in DMSO-d6 with TMS as a standard and IR spectra on a Bruker 113v FT-IR spectrometer (νmax, KBr discs). The GHSQC (gradient heteronuclear single quantum coherence) and GHMBC (gradient heteronuclear multiple bond correlation) spectra were measured under standard conditions [2–4] on a Bruker Avance DRX 600 spectrometer operating at 600.3 MHz for 1H and 60.8 MHz for 15N (accuracy of data, 0.05 ppm). Sweep width and data points in both dimensions for GHSQC spectra: 2, 7123 Hz, 1024 × 256; 4, 7645 Hz, 1024 × 256 and 7645 Hz, 1024 × 249 (in turn); 7, 7441 Hz, 1024 × 256; 8, 7441 Hz, 1024 × 256; and for GHMBC spectra: 2, 7123 Hz, 1024 × 256; 4, 7645 Hz, 1024 × 249; 8, 7441 Hz, 1024 × 256. The pulse parameters are as follows: acquisition time, 0.067–0.072 s; π/2 pulse lengths, 19.0 µs; and relaxation delay, 1 s. The 15N resonances were referenced externally to the signal of liquid CH3NO2 (381.7 ppm). All spectra were obtained with the natural abundance of the nitrogen-15 isotope. The sample was 40 mg of the compound dissolved in DMSO-d6 (0.55 ml) in 5 mm TBI 1H/31 tubes. The temperature range: 296.1 to 303.1 K. High-resolution mass spectra were obtained using an AMD 402 or 604 mass spectrometer in the electron ionization (EI) or fast atom bombardment (FAB) mode, respectively.
2.2. Synthesis and Characterizations of the Z Isomers (2 and 4–6)
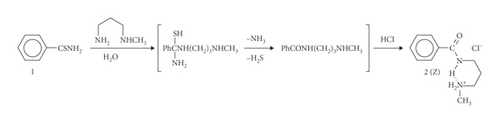
The following secondary amides 2 and 4 were prepared under comparable conditions to the own procedures [2]. Two equivalents of 1N HCl were used to obtain salt 4. The 1N potassium hydroxide addition induces a conformational rearrangement of hydrochloride salt 2 to free base 5. A change of the hydrogen bonding interactions by the deprotonation reaction is observed.
(Z)-N-[3-(methylamino)propyl]benzamide hydrochloride (2): 92%, stereochemically pure. M.p. 162–163°C. IR (KBr): 3317, 2960, 2854, 2794, 2737, 2505, 2446, 2420, 2404, 1640, 1634, 1602, 1579, 1531, 1484, 1464, 1446, 1436, 1425, 1317, 1297, 1279, 1165, 1026, 930, 921, 803, 719, 694, 670, 665. 1H NMR (300.1 MHz, DMSO-d6): 1.90 (quintet, 2H, J = 7.1 Hz, CH2CH2CH2), 2.52 (s, 3H, CH3), 2.91 (t, 2 H, J = 7.3 Hz, CH2N), 3.35 (dt, 2H, J = 6.0, 6.5 Hz, CONHCH2), 7.34–7.60 (m, 3H), 7.92 (dd, 2H, J = 6.9, 1.6 Hz), 8.82 (t, 1H, J = 5.7 Hz, CONH), 9.09 (s, 2H, +NH2CH3). 13C NMR (75.5 MHz, DMSO-d6): 25.8, 32.3, 36.2, 46.0, 127.2, 128.3, 131.2, 134.2, 166.3. LRMS (EI): 192 (17), 177 (7), 148 (41), 105 (87), 44 (100). HRMS (EI): 192.1269 (M+⋅, C11H16N2O+⋅; calc. 192.1263) and 177.1038 ([M-CH3]+, C10H13N2O+; calc. 177.1028).
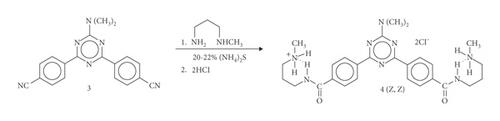
(Z, Z)-4,6-bis{4-{N-[3-(methylamino)propyl]carbamoyl}phenyl}-2-dimethylamino-1,3,5-triazine dihydrochloride (4): 72%, stereochemically pure. M.p. 240–242°C. IR (KBr): 3290, 3235, 3044, 3010, 2941, 2777, 2726, 2492, 2442, 1635, 1628, 1589, 1571, 1542, 1512, 1502, 1472, 1465, 1436, 1404, 1388, 1368, 1326, 1318, 1298, 1259, 1237, 1210, 1180, 1167, 1167, 1144, 1104, 1062, 1031, 1016, 1001, 932, 922, 908, 885, 874, 849, 821, 790, 775, 759, 726, 704, 677, 668, 658, 626. 1H NMR (300.1 MHz, DMSO-d6): 1.92 (quintet, 4H, J = 6.9 Hz, CH2CH2CH2), 2.55 (s, 6H, +NH2CH3), 2.95 (m, 4H, CH2N), 3.35 (s, 6H, CH3NCH3), 3.40 (m, 4H, CONHCH2), 8.07 (d, 4H, J = 8.8 Hz), 8.60 (d, 4H, J = 8.2 Hz), 8.91 (s, 4H, +NH2CH3), 8.98 (t, 2H, J = 5.8 Hz, CONH). 13C NMR (75.5 MHz, DMSO-d6): 25.5, 32.2, 35.9, 36.2, 46.0, 127.1, 127.7, 137.0, 138.3, 164.6, 165.6, 169.0. HRMS (FAB, NBA): 505.3002 (MH+, C27H37N8O2+; calc. 505.3040).
(Z)-N-[3-(methylamino)propyl]benzamide (5-6): 97%, mixture of isomers. M.p. oil after chloroform extraction (155–158°C [11]), the mole ratio of 2 to KOH was 1 : 1. IR (KBr): 3289, 3059, 3029, 2932, 2868, 2801, 1640, 1603, 1578, 1539, 1489, 1447, 1383, 1345, 1307, 1262, 1186, 1158, 1076, 1027, 1001, 928, 806, 774, 708, 670, 666, 617. 5 (25%), 1H NMR (300.1 MHz, DMSO-d6): 1.94 (quintet, 2H, J = 5.8 Hz, CH2CH2CH2), 2.81 (s, 3H, CH3), 3.18–3.25 (m, 2H, CH2NHCH3), 3.35–3.39 (m, 2H, CONHCH2), 3.41 (m, 1H, NH), 7.35–7.60 (m, 3H), 7.83–7.88 (m, 2H), 8.53 (m, 1H, CONH). 13C NMR (75.5 MHz, DMSO-d6): 20.0, 40.3, 41.0, 47.8, 128.0, 127.5, 130.3, 133.1, 159.5. 6 (75%), 1H NMR (300.1 MHz, DMSO-d6): 1.66 (quintet, 2H, J = 6.8 Hz, CH2CH2CH2), 2.27 (s, 3H, CH3), 2.51 (t, 2H, J = 5.6 Hz, CH2NHCH3), 3.31 (dt, 2H, J = 6.8, 5.9 Hz, CONHCH2), 3.43 (m, 1H, CH2NH), 7.35–7.60 (m, 3H), 7.85 (d, 2H, J = 6.7 Hz), 8.62 (br t, 1H, J = 5.1 Hz, CONH). 13C NMR (75.5 MHz): 29.0, 36.1, 37.6, 49.2, 127.1, 128.2, 131.0, 134.7, 166.1. LRMS (EI): 192 (13), 173 (48), 148 (36), 134 (45), 105 (100). HRMS (EI): 192.1253 (M+⋅, C11H16N2O+⋅; calc. 192.1263).
Characteristic spectral data, brief comments, and description for two reference compounds are prepared by trivial methods. No hydrogen bond assisted activation (no stereoinduction essential to the E isomers) results in the Z isomer formation. N-methylaminopropane hydrochloride (7): the stretching vibrations νNH+ for the ammonium species [2]. 1H NMR (300.1 MHz, DMSO-d6): 2.48 (s, 3H, NCH3), 9.03 (br s, 2H, +NH2CH3). Comparative data for (Z)-N-propylbenzamide (8) recrystallized from a mixture of hexane and ethyl ether. M.p. 82–82.5°C (81–82°C [15]). IR (KBr): 3303, 3083, 3064, 3057, 3030, 2966, 2955, 2934, 2872, 1634, 1602, 1578, 1550, 1493, 1465, 1448, 1433, 1373, 1327, 1316, 1291, 1244, 1189, 1162, 1150, 1108, 1075, 1029, 932, 914, 885, 827, 804, 742, 699, 677, 666, 617, 543. 1H NMR (300.1 MHz, DMSO-d6): 8.47 (t, 1H, CONHCH2). 13C NMR (75.5 MHz, DMSO-d6): 166.1 (CONH). 1H NMR (300.1 MHz, CDCl3): 6.43 (br s, 1H, CONHCH2). 13C NMR (75.5 MHz, CDCl3): 167.6 (CONH). This may reflect a structural dependence of solvation revealed by other NMR data.
3. Results and Discussion
3.1. Stereochemistry of the Acylation Reaction Leading to the Z Isomers
The present work shows how substituents of varying steric and electronic requirements influence the intramolecular H-bonding of secondary amides. The transamination reaction of primary thioamide 1 with N-methyl-1,3-diaminopropane and subsequent hydrolysis of the unstable four-centered intermediate, upon exposure to atmospheric moisture, yield the secondary amide of the type shown in Scheme 2. Also, the sterically demanding diaryltriazine substituent in 4 forces this diamide in the Z form after the addition of acid (Scheme 3). The discrepancy between the configurational stability of 2 or 4, and that of (E)-N-[3-(methylamino)propyl](1-thyminyl)acetamide hydrochloride (Scheme 1, Equation (1)) or (E)-N-[3-(methylamino)propyl]benzamide hydrochloride (Scheme 1, Equation (3)) can be attributed to the occurrence of strong intramolecular hydrogen bond activations in the latter two different reactions. Their intermediate species generate different stereoinduction systems of the reactions. In this way, the stereochemical course of the reaction depends on the potential for hydrogen bonding.
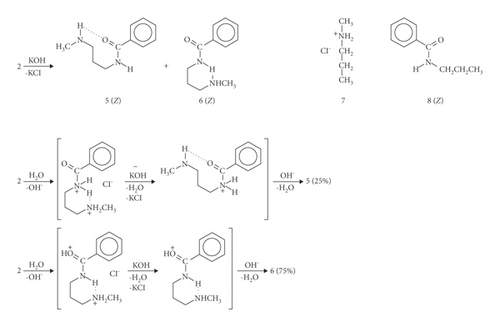
A study of the conversion of salt 2 into free bases 5-6 has brought about an excellent spectroscopic evidence for various geometrical arrangements of atoms (Scheme 4). The background to structures 5-6 has been already discussed. The literature studies yield only limited information on the conformational behavior of related compounds investigated [10]. The compounds 5-6 have strong structural resemblance but differ in the position of the N-methylamino group. As determined by NMR (chemical shifts, multiplicity, and intensity of individual signals), a mixture of the conformational isomers consisting of 5 and 6 at the ratio 1 : 3 is formed, in which relative stability is chiefly dependent upon intramolecular hydrogen bonding interactions. The governing factor in destabilizing 5 (bent HB, less in percentage terms) is the +NH…+NH2 interaction.
3.2. 1H and 13C NMR Studies of the Z Isomers (2, 4–6, and 8)
Results elucidating the stereochemical features of monoacylated diaminoalkanes on the basis of the 1H- and 13C-NMR spectra are discussed. The Z isomers show characteristic infrared spectra. Characteristic chemical shifts (δH and δC) and wavenumbers (νmax) are embedded in the Experimental Section. The absence of the intramolecular N…H+…N hydrogen bonds (H+-bonding) strongly modifies the spectra of secondary amides 2 and 4-6, causing changes in the NMR signals (quintet multiplicity, no characteristic six-proton sharp singlet). Comparison between 5 (CH3N−H…O=CNH) and 6 (CON−H…NHCH3) has been made (Figure 1). The data presented here support the idea about the existence of two different types of interactions, stabilizing conformational states in the deprotonated products. The negative polarized group CONH can act as either a hydrogen-bond donor or acceptor. Intramolecular, nitrogen-to-oxygen migration from carbamoyl NH to carbamoyl CO allows the transfer of the polymethylene-bridged methylamino group in the secondary amide moiety to afford 5.
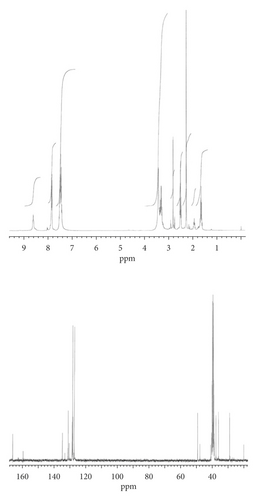
Due to the character of the CON−H…+NH2CH3 hydrogen bond, equivalence of two protons of the methylammonium group is seen in solution. Only the Z configuration can be stabilized by such bridges. Compounds 2 and 4 have strong structural resemblance owing to the arrangement of the +NH2CH3 groups as compared with N-methylaminopropane hydrochloride (7). The most probable explanation of the observed increase in the δCONH and decrease in the δNH2CH3 values of 4 is related to a change in the torsion angle Θ between the plane of the aromatic ring and the amide functional group [20] (larger value Θ for 2) whereas carbamoyl carbon resonances of 4 (165.6) are comparable with the corresponding spectrum of 2 (166.3); 166.1 observed for 8. Hydrogen atoms involved in the formation of hydrogen bonds are known to have decreased electron density. It results in an increase in the δH value for the CONH protons of 2, 4, and 6 with respect to 8: δCONH absorption exhibits a blue shift.
3.3. The Amide Linkage Extraordinary Infrared Absorptions
An additional insight into the interpretation of the IR spectra is gained through a comparison with (Z)-N-propylbenzamide (8). Amide 8 has been selected as the model compound for the Z structure. The Z isomers exhibit the amide I (νCO) and II (νCN + δNH) bands shifted towards lower wavenumbers. The appropriate E isomer (1650 cm−1 and 1537 cm−1) [3] compared with 2 (1640 cm−1 and 1531 cm−1, respectively) exhibits these amide bands at higher wavenumbers. The C=O stretching vibrations (amide I band) in the Z case are hypsochromically shifted towards 8 (1634 cm−1). The value of the blue shift and the strength of the intramolecular H-bond are correlated. The formation of an H-bond in a CON−H…+NH2 system is accompanied by strengthening of the covalent N-H bond.
The amide II band (1570–1515 cm−1) resulted from interaction between the N-H bending and the C-N stretching of the C-N-H group is inapplicable (This band broadens at 1550 cm−1 and 8 absorbs at higher wavenumbers with respect to both isomers probably because of side effects caused, on the other hand, the red shift of the frequency) for observed physical phenomenon although a second weaker band near 1250 cm−1 supports it (also derived from interaction between the N-H bending and C-N stretching: 2, 1255 cm−1; 4, 1259 cm−1; 6, 1262 cm−1; and 8, 1244 cm−1 as a reference point). Furthermore the model, which took into account the out-of-plane N-H wagging (a broad medium band in the 726–699 cm−1 region in the spectra), has been exploited in order to check for unusual shifts (Figure 2). As a support for the above explanation, the shift to higher frequency by 9–28 cm−1 confirmed the results.
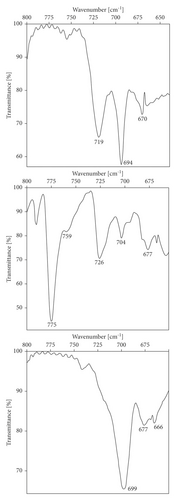
Blue-shifting CON−H…+NH2CH3 H-bond is characterized by the strengthening of the N-H bond which causes the shortening of the proton-donor N-H bond (blue shift of the stretch frequency [21, 22]). Intramolecular bond formation is accompanied by a lowering of the band intensity (substantial change in the bond dipole moment). The shift in N-H stretching frequency and the electronic nature of this type of hydrogen bonding differ from the red shift [23]. The knowledge of this specific feature has been extended to the other vibration bands and can also be used to probe both the strength of the interaction made with the amide element and its conformational disposition. Open-chain secondary amides absorb near 1640 cm−1 when examined in the solid state. The position of absorption depends on the environmental factors as the stereoelectronic effects (potential for intramolecular hydrogen bonding included in the structure).
Comparisons of multiple combination bands of medium intensity arising from stretching in the CON−H…+NH2CH3 group in the IR spectra (2, 2505 cm−1, 2446 cm−1; 4, 2492 cm−1, 2442 cm−1; and an increase in intensity for 7, 2494 cm−1, 2418 cm−1) support the features which are essential for the blue-shifting H-bond formation. The aforementioned E isomer [3] (stronger bonding) provided a blue shift of 20 cm−1 and 53 cm−1 (11 cm−1 and 28 cm−1 for 2). The remarkable stretching effect associated with the N…H+…N group has been previously observed by the author [2] during his detailed examination of IR properties. The most important features were opposite to those characteristics of classical H-bonds (red shift [4]). The range of study of blue-shifting H-bonds is here extended to other bonding types. A much more informative 1H-15N 2D NMR study of these compounds is described in the next section.
3.4. 1H-15N Spin-Spin Interactions of 2, 4, 7, and 8
The importance of 15N-NMR spectroscopy is recently increasing, since it is attractive in the context of the spin-spin interactions and this strategy makes it a potentially powerful tool (as a basic research tool). [2–4, 24] Since then not much attention has been paid to other possible developments of that concept. The two-dimensional correlation experiments presented here have a qualitative value for confirming the existence of geometrical stereoisomers of secondary amides. The pertinent GHSQC and GHMBC spectra of 2, 4, 7, and 8 are placed in Figure 3. The spectral parameter, the nitrogen-proton one-bond coupling constant indicates the presence of an intramolecular hydrogen bond.
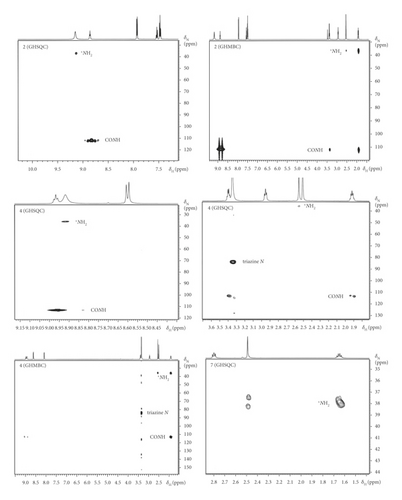
New gradient selected heteronuclear correlation sequences are introduced for the Z forms. One of them is assigned to the intramolecular CON−H…+NH2CH3 hydrogen bond in 2. The amide hydrogen forms an H-bond to the charged nitrogen atom. This model fits well with the experimental data, indicating the horizontal signal of triplet multiplicity (distinctive CON−H…+NH2CH3 couplings) in the GHSQC spectrum of 2. The most remarkable feature is that regarding the direct +NH2CH3 correlation. The E isomers are unable to form such a type of patterns because the hydrogen atoms are engaged in hydrogen- and dihydrogen-bonded systems (N…H+…N and CH2…HN unusual bonds). This explains the characteristic H−N…H+…NCH3 correlations (perpendicular triplet) observed in the previous study [2].
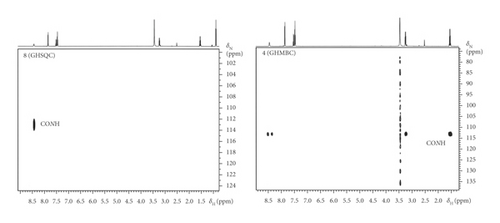
Analogous correlations to 2 are observed for 4. In addition, the GHSQC spectrum is useful for characterizing the electronic situation of the triazine residue at the ring nitrogen atoms. It is confirmed that there exist correlations to the N(CH3)2 protons in 4. The analysis of the GHMBC spectra allows separating several spin systems and permits determination of the cyclic array (an intramolecular hydrogen bond character). Two reference compounds 7 and 8 are presented for comparison in order to confirm the kind of interaction. The absence of the +NH2CH3 correlation was anticipated in 7 and supports the assignments of 2 and 4. The link between stereoselectivity and spin selectivity in stereoselective syntheses of the intramolecular cationic species has been established.
In secondary benzamide 8, the CONH signal is influenced by the solvent effect. A strong intermolecular interaction with water molecules is seen in the GHMBC spectrum of 8, leading to complete disappearance of the doublet in the GHSQC spectrum (presumably because of additional interaction of the carbamoyl nitrogen with H2O). This is not surprising since the formation of the above intramolecular complexes is due to the release of H2O from polar functionalities that form the hydrogen bonds. It is shown that the release of water molecules from the amide CO and NH groups is involved in the amide-amide hydrogen bond formation upon protein folding. [25] Similar factors are accepted for the water-mediated hydrogen bonds in RNA-ligand interactions [26, 27] and DNA bases. [28] In this way, the multidimensional NMR spectroscopy seems to be universal and might provide a tool for the spectroscopic probing of intermolecular potentials for more complex systems.
4. Conclusions
The most important intramolecular bonding phenomena (main driving forces towards the E/Z isomeric form) of the HB stabilized acylation reactions could be rationalized in terms of the nature of substrates. The reactions with thioamide 1 and nitrile 3 described here lead to the Z isomers as the most stable exclusive forms. Structure 2 has been additionally confirmed by the preparative transformation to the respective conformational isomers 5 and 6. Interestingly, the new conformational rearrangement 2 → 5 occurs during deprotonation involving facile migration into the sterically accessible carbamoyl oxygen. Herein the contrast between notable examples E [2, 3] and Z isomers is evident. The 1H-15N heteronuclear correlation spectroscopy has proved to be decisive in determining the intramolecular CON−H…+NH2CH3 hydrogen bonding essential character of the two Z stereoisomers 2 and 4, implying close approach. Stereochemical and mechanistic studies on the direct reactions of nitriles to secondary amides and cyclic amidines, [2] classified mistakenly as the Thio-Pinner reaction, [29] provide a vital piece of information about the importance of structural requirements in intermediates and the influence of reactant interactions (multicomponent hydrogen-bonded systems including H+-bonding and dihydrogen-bonded stereoisomers, organocatalysts containing H-donor-acceptor groups, mechanistic insights, and empirical rules for chemical shift differences and hydrogen bonding strength). The reaction gives different results depending on the structure of the starting materials. The configurational and conformational preferences of the intramolecular CON−H…+NH2CH3 positively charged linkage in the Z benzamides are particular. Principal results and major conclusions provide novel insight into the course of chemical reactions.
Conflicts of Interest
The author declares that he has no conflicts of interest.
Acknowledgments
This study did not receive any funds but is a part of employment (Department of Chemistry, Adam Mickiewicz University).
Open Research
Data Availability
All data used to support the findings of this study are included within the article.




